Abstract
Aims
Topical sunscreens are routinely applied to the skin by a large percentage of the population. This study assessed the extent of absorption of a number of common chemical sunscreen agents into and through human skin following application of commercially available products.
Methods
Sunscreen products were applied to excised human epidermis in Franz diffusion cells with the amount penetrating into and across the epidermis assessed by h.p.l.c. for 8 h following application.
Results
All sunscreen agents investigated penetrated into the skin (0.25 g m−2 or 14% of applied dose), but only benzophenone-3 passed through the skin in significant amounts (0.08 g m−2 or 10% of the applied dose). With one exception, suncreen agents in corresponding products marketed for adults and children had similar skin penetration profiles.
Conclusions
Whilst limited absorption across the skin was observed for the majority of the sunscreens tested, benzophenone-3 demonstrated sufficiently high penetration to warrant further investigation of its continued application.
Keywords: skin penetration, sunscreen, topical application
Introduction
Systemic absorption of the commonly used sunscreen benzophenone-3 (or oxybenzone) after topical application to human volunteers has recently been reported [1]. Between 1 and 2% of the common ultraviolet A (UVA) absorber applied in a commercial sunscreen product, was excreted in the urine over a 48 h period following a single application. Little has been published about the chronic toxicity and disposition of topical sunscreen agents, although available data suggest low acute toxicity profiles [2]. The present study examined the skin penetration of benzophenone-3 and three other common sunscreen agents in a range of commercial products. An in vitro technique was used to allow accurate quantification of the amount of sunscreen which penetrated into and remained within the skin, and also the amount which penetrated through the skin and would therefore be available for systemic absorption. A disadvantage of in vivo assessment is that it uses measurement of urinary excretion to estimate skin penetration and may underestimate skin penetration as it cannot account for sunscreen which is distributed in the tissues, metabolized to unknown metabolites or excreted via other routes. In the case of benzophenone-3, studies in rats have reported substantial dermal metabolism, protein binding and excretion in both urine (67%) and faeces (21%) [3, 4], with a further study reporting excretion in breast milk [5].
Methods
Materials
The study was approved by the University of Queensland and Wesley Hospital Medical Ethics Committees (whose patients were advised of the research and donated skin following surgery). Human epidermis was obtained by heat separation [6] of skin obtained following abdominoplasty surgery. Briefly, this involves removal of the subcutaneous fat followed by immersion of the skin in water at 60° C for 2–3 min. The epidermis is then carefully peeled off the dermis at the dermal-epidermal junction. The six commercial sunscreen products tested, from three different pharmaceutical/cosmetic companies, consisted of three pairs (one each for children and adults) of milk/lotion products designated sun protection factor (SPF) 15+: Product A for toddler (AC) and adult (AA) each containing octyl methoxycinnamate (OM) 90 g l−1, benzophenone-3 (BP) 25 g l−1, titanium dioxide-(TD) 8 g l−1; Product B for toddler (BC) and adult (BA) each containing OM 75 g l−1, benzophenone-3 60 g l−1, octylsalicylate (OS) 50 g l−1, octocrylene (OC) 70 g l−1, titanium dioxide (TD) 34 g l−1; Product C for baby (CC) and adult (CA) each containing OM 75 g l−1, BP 20 g l−1, butyl methoxydibenzoylmethane (BM) 20 g l−1.
Study design
The study was performed using a Franz type diffusion cell which consists of a donor and receptor chamber separated by a membrane of human epidermis. The receptor chamber contained a receptor phase of 4% bovine serum albumin (BSA) in phosphate buffered saline (3.0–3.6 ml) to mimic physiological fluid. Following equilibration of the skin and receptor phase, a finite dose (20–25 g m−2) of the sunscreen product was applied to human epidermis mounted in a Franz type diffusion cell (skin surface area 1.13–1.23 cm2). The receptor fluid was stirred by a magnetic stirrer throughout the experimental period. After 8 h the receptor phase and epidermis were removed, and the sunscreen product remaining on the epidermal surface was wiped off with dry tissue, rinsed twice with distilled water and dried with tissue (this procedure had been previously validated). Sunscreen agents remaining in epidermal tissue were quantified by soaking the tissue in methanol, removal and soaking in a further methanol solution. These solutions were combined and sunscreen content assayed. This procedure was validated to provide a recovery over 95%. The concentration of all chemical sunscreen agents (BP, OS, OC and OM) in the receptor fluid samples and epidermis at 8 h following application was assayed by high performance liquid chromatography [7]. Seven to eight replicates were used for the study.
Stastical methods
Difference in the concentrations of sunscreen agents in receptor fluid and epidermis for each of the adult/child products was compared by t-test. The level of significance was set at 0.05.
Results
Of the five chemical sunscreens present in the products, benzophenone-3 was the only sunscreen found to have penetrated human skin to the receptor phase after the 8 h study period, with up to 0.08 g m−2 or 10% of the applied dose penetrating. Only one adult product tested (Product BA) had significantly greater skin penetration of benzophenone-3 than the corresponding product for children (BC: P < 0.05: 95% CI 0.0107, 0.0353); the other pairs of products exhibited similar penetration profiles (mean BP penetration in g m−2 and percentage applied at 8 h ranged from 0.03±0.01 to 0.08±0.01 g m−2 and 5.8±1.0–10.1±1.1%; 95% CI for differences AA vs AC–0.0125, 0.0065; CA vs CC–0.0091, 0.0031 (P > 0.05; Figure 1). Measureable concentrations of all sunscreen agents (up to 0.25 g m−2 or 14% of applied dose) remained within the epidermis at 8 h post application (Figure 2).
Figure 1.
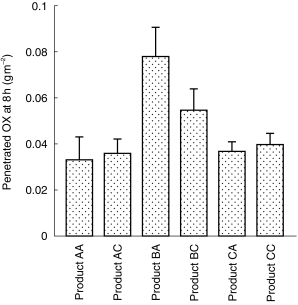
Skin penetration of benzophenone-3 following topical application of sunscreen products (mean±s.d., n = 7–8).
Figure 2.
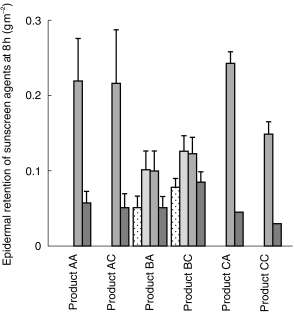
Epidermal retention of sunscreen agents following topical application of sunscreen products (mean ± s.d., n = 7–8).  OS,
OS,  OC,
OC,  OM,
OM,  OX.
OX.
Discussion
Of the sunscreen products evaluated in the current study, half were marketed specifically for use by children. Despite this marketing distinction, with the exception of BA, no significant difference between these products, and those designated for adult use with respect to sunscreen penetration was observed. It is salient to note that children exhibit a greater ratio of skin surface area to body weight [8], and their skin has been shown to be more permeable than that of the adult [9]. Therefore, with respect to BP, our data raise some concerns regarding the formulation of sunscreen products for specific application to children. The amount of BP penetration across excised human skin in vitro at 8 h was similar to that reported for urinary excretion of BP and metabolites following a single topical application of the same product to human volunteers (0.07 g m−2) [1], thus providing initial validation of the in vitro method. We are currently undertaking a preliminary study to assess skin tissue levels of BP using in vivo microdialysis which we hope will further validate the in vitro technique and provide information regarding tissue distribution and metabolism of BP in the skin. However, it must also be noted that none of the other sunscreen agents evaluated was appreciably absorbed. In conclusion, whilst limited absorption across the skin was observed for the majority of the sunscreens tested, benzophenone-3 demonstrated sufficiently high penetration to warrant further investigation of its continued application.
Acknowledgments
We acknowledge the support of the Queensland Cancer Fund and the NHMRC and the technical assistance of NV Hoffmann.
References
- 1.Hayden CGJ, Roberts MS, Benson HAE. Systemic absorption of sunscreen after topical application. Lancet. 1997;350:863–864. doi: 10.1016/S0140-6736(05)62032-6. [DOI] [PubMed] [Google Scholar]
- 2.Klein K. Encyclopedia of UV absorbers for sunscreen products. Cosmetics Toiletries. 1992;107:45–63. [Google Scholar]
- 3.El Dareer SM, Kalin JR, Tillery KF, Hill D. Disposition of 2-hydrox-4-oxybenzophenone in rats dosed orally, intravenously or topically. J Toxicol Environ Health. 1986;19:491–502. doi: 10.1080/15287398609530947. [DOI] [PubMed] [Google Scholar]
- 4.Okereke CS, Abdel-Rahman MS. Species differences in the disposition of benzophenone-3 after oral administration in rat and mouse. Toxic Substances J. 1994;13:239–251. [Google Scholar]
- 5.Hany J, Nagel R. Nachweis von UV-filtersubstanzen in muttermilch. Deutsche Lebensmittel-Rundschau. 1995;91:341–345. [Google Scholar]
- 6.Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–705. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- 7.Jiang R, Hayden CGJ, Prankerd RJ, Roberts MS, Benson HAE. High-performance liquid chromatographic assay for common sunscreening agents in cosmetic products, bovine serum albumin solution and human plasma. J Chromatogr B. 1996;682:137–145. doi: 10.1016/0378-4347(96)00063-1. [DOI] [PubMed] [Google Scholar]
- 8.Wester RC, Noonan PK, Cole MP, Maibach HI. Percutaneous absorption of testosterone in the newborn rhesus monkey: comparison to the adult. Pediatr Res. 1977;11:737–739. doi: 10.1203/00006450-197706000-00008. [DOI] [PubMed] [Google Scholar]
- 9.West DP, Worobec S, Solomon LM. Pharmacology and toxicology of infant skin. J Invest Dermatol. 1981;76:147–150. doi: 10.1111/1523-1747.ep12525553. [DOI] [PubMed] [Google Scholar]


