Abstract
Aims
To examine the tolerability and disposition of i.v. adenosine (SUNY4001) in healthy male Japanese volunteers.
Methods
SUNY4001 was infused i.v. for 6 min at 0 (placebo), 60, 100, 120 and 140 µg kg−1 min−1 in a dose-escalating manner in 30 healthy subjects. Adenosine and its metabolites were determined in the plasma and urine.
Results
Only plasma hypoxanthine was increased from 3 min during until 5–10 min after SUNY4001 infusion at the higher rates without any significant dose-related changes in plasma adenosine, inosine, xanthine or uric acid, or in urinary adenosine and all metabolites compared with the placebo. There was a dose-related increase in the incidence of subjective symptoms such as heat sensation, flushed face, dyspnoea, chest discomfort, etc. Transient and self-subsiding episodes of second-degree atrioventricular block were found in two subjects each at the higher doses.
Conclusions
Adenosine infusion at ≤ 140 µg kg−1 min−1 was concluded to be generally well tolerated.
Keywords: adenosine, healthy volunteer, metabolite, pharmacokinetics, tolerability
Introduction
Thallium-201 myocardial perfusion imaging during coronary vasodilation induced by pharmacological agents has been accepted as an alternative to exercise stress imaging for the diagnosis of coronary disease [1]. Dipyridamole, which exerts a vasodilative effect indirectly by blocking the uptake of endogenous adenosine and by inhibiting adenosine degradation [2, 3], has been used for pharmacologic stress imaging. Recently, intravenous infusion of adenosine has also been used for that purpose since it can produce maximal coronary hyperaemia. Adenosine, like dipyridamole, increases the coronary blood flow in myocardial regions perfused by normal coronary arteries, whereas the myocardial blood flow remains unaltered in regions perfused by stenotic arteries [4]. Moreover, because of its negative chronotropic and dromotropic effects, adenosine has been successfully used in the treatment of selected supraventricular tachycardias [5].
The safety and potential usefulness of intravenous adenosine have been confirmed in clinical trials [6, 7]. However, the pharmacokinetic properties of intravenous adenosine have not been investigated except in the report of Blardi et al. and need to be fully clarified [8]. In the present study, adenosine and its metabolites were measured in association with its intravenous infusion in healthy male adult Japanese volunteers to explore the pharmacokinetics and tolerability.
Methods
The research protocol was approved by the local Ethics Committee prior to the study.
Subjects
A total of 30 healthy male volunteers, aged 27.9 ± 6.3 (mean ± s.d.; range: 20–43) years and weighing 64.7 ± 9.5 kg, participated in the study after giving their written informed consent.
Study design
6-Amino-9-β-d-ribofuranosyl-9H-purine (adenosine, SUNY4001), was dissolved in saline to a final volume of 30 ml. Six subjects each received 6 min adenosine infusions (5 ml min−1) with use of an infusion pump (TE-311, Terumo, Tokyo, Japan) at 60, 100 and 140 µg kg−1 min−1 in a dose-escalating manner after confirming the safety of the previous dose. Five volunteers received a 6 min adenosine infusion at 120 µg kg−1 min−1 since further increase of the dose up to 180 µg kg−1.min−1 could not be tolerated by the subjects due to the adverse events mentioned later. At each infusion rate, one or two subjects received a placebo (saline only) single-blind, and the data obtained from these subjects (n = 7) were pooled for use as a control.
Cardinal symptoms
All subjective and objective symptoms were monitored. Holter electrocardiograms (ECG) were continuously monitored on the display and recorded on a tape from before the start until after the termination of infusion.
Laboratory tests
Vital signs, including body temperature and blood pressure were monitored just before and periodically up to 24 h after administration. Blood biochemistry and haematology tests, urinalysis and electrocardiography were also performed.
Sample collection and preparation for analysis
Venous blood samples (5 ml each) were collected in ice-cold tubes containing 100 mg of 500 mm ethylene diamine tetra-acetic acid (EDTA)-2Na and 0.05% 2′-deoxycofolmycin, respectively, before (0 min) and at 3, 4.5, 6, 6.5, 7, 8, 9, 11 and 16 min after the beginning of the 6 min intravenous infusion. After sampling, 100 mg of 0.5% dipyridamole was immediately added to the blood while shaking, and the plasma was separated by centrifugation at 1000 g for 15 min while cooling. An aliquot (0.1 ml) of the plasma prepared for measurement of the adenosine concentration was held at room temperature for succinylation for 10 min and stored at −20 °C until it was analysed. The remaining plasma, intended for measurement of adenosine metabolites, inosine, xanthine, hypoxanthine and uric acid, was stored similarly. Urine was collected at intervals of −12–0 h before and 0–2, 2–4, 4–8, 8–12 and 12–24 h after the beginning of adenosine infusion. The volume of time-block urine samples was accurately measured. An aliquot (10 ml) was stored in a similar manner to that of the plasma samples.
Detection of adenosine and metabolites
For adenosine assay, succinylated plasma and urine specimens were subjected to radioimmunoassay (r.i.a.) using a double-antibody technique (Adenosine RIA Kit, Yamasa) [9]. The limit of detection was 0.00625 nmol ml−1.
A nonsuccinylated plasma specimen was appropriately diluted with purified water and deproteinized in a pressurized ultrafiltration unit (UFP1LCC Molcut II, Japan Millipore Industry). An aliquot of the filtrate was then injected on h.p.l.c. column, and each metabolite was separated and detected by u.v. absorption (254 nm). The detection limits for each adenosine metabolite in plasma were as follows: inosine 0.02–0.07 nmol ml−1; hypoxanthine 0.30–1.22 nmol ml−1; xanthine 0.22–1.15 nmol ml−1; and uric acid 10.0–100 nmol ml−1.
The frozen urine specimens were thawed, and an aliquot of each sample was warmed at 70°C for solubilization and homogenization of the components; this was then diluted 40-fold with purified water. The sample was deproteinized in the pressurized ultrafiltration unit and aliquots of the filtrate were injected on h.p.l.c. column in the same manner as the plasma specimens. Each metabolite was isolated and detected under the same conditions as described above for the plasma concentrations.
The concentration (nmol ml−1) of each adenosine metabolite in the test sample was calculated from a standard curve prepared by adding known amounts of each metabolite standard to the control urine and applying the same procedures as for the test sample. The detection limits for each adenosine metabolite in the urine were as follows: inosine 0.81–2.31 nmol ml−1; hypoxanthine 19.16–31.24 nmol ml−1; xanthine 12.70–30.81 nmol ml−1; and uric acid 0.80–251.71 nmol ml−1.
Results
Heat sensation of the extremities, face, head or whole body, chest discomfort and dyspnoea were the most frequent complaints of the subjects during the adenosine infusion, and their incidence seemed to increase dose-dependently. Each subject experienced at least one of these symptoms at the infusion rates of 120 and 140 µg kg−1 min−1, although these symptoms were transient and disappeared immediately after termination of the infusion. Palpitation was complained of by two of five subjects at the highest rate of adenosine infusion.
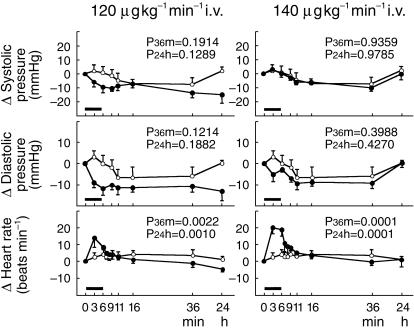
The heart rate increased during infusion of the drug and up to 30 min after termination of the infusion, depending on the dose: The heart rate was significantly increased by 10–20 beats min−1 at infusion rates of 120 (P = 0.0010) and 140 µg kg−1 min−1 (P = 0.0001) (Figure 1). The systolic and diastolic blood pressures and the diastolic blood pressure tended to be decreased at infusion rates of 120 and 140 µg kg−1 min−1, respectively, although those changes were not statistically significant.
Figure 1.
Changes in systolic and diastolic blood pressures and heart rate in healthy volunteers at adenosine infusion rates of 120 and 140 µg kg−1 min−1. ○ placebo; • SUNY4001. SUNY4001 infusion for 6 min using an infusion pump. P36m Differences in time dependency between placebo and dose level were tested by analysis of variance (from 3 min to 36 min). P24h Differences in time dependency between placebo and dose level were tested by analysis of variance (from 3 min to 24 h).
The Holter ECG revealed second-degree atrioventricular (AV) block in two subjects each at infusion rates of 120 and 140 µg kg−1 min−1. This kind of ECG change was observed at 2 - 5 min after beginning the adenosine infusion and immediately recovered without any treatment except in one subject who received 140 µg kg−1 min−1 adenosine infusion, in whom atrial fibrillation followed transient second-degree AV block. In this subject an atrial rhythm recovered spontaneously without any treatment about 3 h after termination of the infusion.
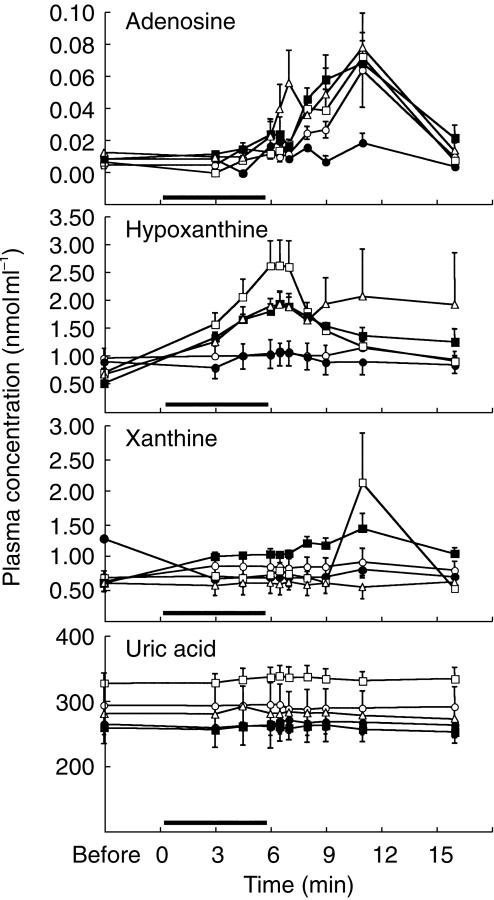
Figure 2 shows plots of plasma adenosine concentrations before and after its infusion in healthy subjects. The predose, endogenous adenosine in the plasma was below the detection limit (0.00625 nmol ml−1) in more than half of the subjects in each dose group except for the placebo (0.005 ± 0.002 nmol ml−1, mean ± s.e. mean, n = 7) and 120 µg kg−1 min−1 (0.009 ±0.001nmol ml−1, n = 5) groups. The concentration ranged, even when detectable, between 0.005 and 0.013 nmol ml−1. Although the plasma concentration of adenosine increased during and after infusion, no dose-related change was observed.
Figure 2.
Plasma concentrations of adenosine, hypoxanthine, xanthine and uric acid before, during and after adenosine infusion. SUNY4001 infusion for 6 min using an infusion pump. ○ saline; • 60 µg kg−1 min−1; □ 100 µg kg−1 min−1; ▪ 120 µg kg−1 min−1; ▵ 140 µg kg−1 min−1.
Plasma hypoxanthine, xanthine and uric acid were detectable before and after adenosine infusion in all subjects involved in the study (Figure 2). Only plasma hypoxanthine, the predose value of which was 0.53–0.99 nmol ml−1 (about 60–100 times higher than endogenous adenosine), significantly increased from 3 min after beginning of the infusion until 5, 10 and 3 min after termination of the infusion at infusion rates of 100, 120 and 140 µg kg−1 min−1, respectively, although no clear dose-dependency was found. In comparison with the control group, plasma hypoxanthine increased significantly from 4.5 min after the beginning of infusion until 2, 3 and 2 min after termination of the infusion at the upper three infusion rates, respectively. The plasma inosine concentration was below the detection limit in most of the subjects throughout the study except one subject each at infusion rates of 100 and 140 µg kg−1 min−1, i.e. 0.1–0.15 nmol ml−1 and 0.23–0.74 nmol ml−1 at 4.5–7 min and 9–16 min after the beginning of adenosine infusion, respectively.
Adenosine and its metabolites were also determined in urine samples collected at −12–0 h before, and 0–12 and 12–24 h after the beginning of adenosine infusion and expressed by normalization relative to the urinary creatinine concentration. These compounds showed no significant dose-related changes.
Discussion
Since the usually recommended dose of adenosine is 140 µg kg−1 min−1 for 6 min in the United States, Canada and the United Kingdom, the maximal tolerable dose had been expected to be 180 µg kg−1 min−1 or more for 6 min in the present study. However, the incidences of symptoms such as flushed face, dyspnoea, and chest discomfort appeared to increase dose-dependently. Moreover, two of six subjects experienced second-degree AV block during in continuous ECG monitoring at an infusion rate of 140 µg kg−1 min−1. Although ECG changes were transient and immediately recovered to normal, it was followed by atrial fibrillation which continued for 3 additional h in one subject. Therefore, the plan to increase the infusion rate up to 180 µg kg−1 min−1 was abandoned, and the lower infusion rate, i.e. 120 µg kg−1 min−1, was examined. Nevertheless, two of five subjects also experienced transient and self-resolving second-degree AV block.
In association with adenosine infusion, first-, second- or third-degree AV block has been observed in 7.6% of 9256 patients [10], and one subject each experienced second-and third-degree AV block, in 20 healthy subjects [6]. This effect of adenosine on AV conduction is predictable from the electrophysiological action of this substance to suppress conduction in the sinoatrial and AV nodes of the heart by binding to adenosine, especially A1, receptors.
At the higher rates of adenosine infusion, the heart rate was significantly increased, probably through a sympathetic reflex due to the vasodilative effects of adenosine since the atrial blood pressure tended to be decreased. These changes were not significant. No marked decrease in blood pressure was observed in our subjects due to many compensatory mechanisms, including an increase in sympathetic tone. However, a decrease in blood pressure of ≥ 10 mmHg has been reported to be frequently encountered in patients with ischemic heart disease [4, 11]. Although the increase in the heart rate and the decrease in the blood pressure were also transient and self-resolving in the present study, caution should be taken especially in elderly patients with ischaemic heart disease.
In vivo, suppression of the AV node or the vascular tone by adenosine would be expected to be quite transient since the duration of action is very short. Adenosine is easily removed from the extracellular space either through phosphorylation to adenosine monophosphate by adenosine kinase or through deamination by adenosine deaminase mainly after its transport into the cell [12]. Adenosine and its metabolites in the peripheral venous plasma showed no significant dose-related changes except for hypoxanthine which prevented us from determining the pharmacokinetic parameters of exogenous adenosine. Pharmacokinetic parameters were clearly determined over a similar adenosine dose range by Blardi et al.[8]. For blood collection and sample preparation, sufficient amounts of EDTA and 2′-deoxycofolmycin were added beforehand to the sampling tubes to inhibit degradation and transport of adenosine into erythrocytes, and dipyridamole was added to the blood sample immediately after collection and before plasma separation. Even in the plasma collected from the subjects administered the placebo, adenosine tended to increase after the termination of infusion. This suggests that the procedures used for blood sampling, such as use of a tourniquet and muscle clamping to facilitate sampling, might have caused release of endogenous adenosine from the surrounding tissues. Accordingly, adenosine intravenously infused at the rate of ≤ 140 µg kg−1 min−1 can be concluded to be rapidly degraded to hypoxanthine or subjected to cellular uptake, thereby preventing its recirculation in the blood.
In one subject in the highest dose group, second-degree AV block was followed by atrial fibrillation which continued for 3 h. Strong suppression of atrial conduction or shortening of the atrial action potential duration may provoke atrial fibrillation through the mechanism of multifocal re-entry [12, 13]. If atrial fibrillation is induced by rapid atrial pacing, it usually persists for only a few seconds after termination of the pacing. In some apparently healthy young people, however, it may persist for many hours, perhaps due to genetic variation in the electrical coupling of atrial cells.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (No. 10044258).
References
- 1.Albro PC, Gould KL, Westcott RJ, Hamilton GW, Ritchie JL, Williams DL. Noninvasive assessment of coronary stenosis by myocardial imaging during pharmacologic coronary vasodilation. III. Clinical trial. Am J Cardiol. 1978;42:751–760. doi: 10.1016/0002-9149(78)90094-2. [DOI] [PubMed] [Google Scholar]
- 2.Klabunde RE. Dipyridamole inhibition of adenosine metabolism in human blood. Eur J Pharmacol. 1983;21:21–26. doi: 10.1016/0014-2999(83)90026-2. [DOI] [PubMed] [Google Scholar]
- 3.Knabb RM, Gidday JM, Ely SW, Rubio R, Berne RM. Effects of dipyridamole on myocardial adenosine and active hyperemia. Am J Physiol. 1984;247:H804–H810. doi: 10.1152/ajpheart.1984.247.5.H804. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–1606. doi: 10.1161/01.cir.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 5.DiMarco JP, Miles W, Akhtar M, et al. Adenosine for paroxysmal supraventricular tachycardia: dose ranging and comparison with verapamil. Assessment in placebo-controlled, multicenter trials. Ann Intern Med. 1990;113:104–110. doi: 10.7326/0003-4819-113-2-104. [DOI] [PubMed] [Google Scholar]
- 6.Siffring PA, Gupta NC, Mohiuddin SM, et al. Myocardial uptake and clearance of T1–201 in healthy subjects: comparison of adenosine-induced hyperemia and exercise stress. Radiology. 1989;173:769–774. doi: 10.1148/radiology.173.3.2813784. [DOI] [PubMed] [Google Scholar]
- 7.Abreu A, Mahmarian JJ, Nishimura S, Boyce TM, Verani MS. Tolerance and safety of pharmacologic coronary vasodilation with adenosine in association with thallium-201 scintigraphy in patients with suspected coronary artery disease. J Am Coll Cardiol. 1991;18:730–735. doi: 10.1016/0735-1097(91)90796-c. [DOI] [PubMed] [Google Scholar]
- 8.Blardi P, Laghi Pasini F, Urso R, et al. Pharmacokinetics of exogenous adenosine in man after infusion. Eur J Clin Pharmacol. 1993;44:505–507. doi: 10.1007/BF00315554. [DOI] [PubMed] [Google Scholar]
- 9.Yamane R, Nakamura T, Matsuura E, Ishige H, Fujimoto M. A simple and sensitive radioimmunoassay for adenosine. J Immunoassay. 1991;12:501–519. doi: 10.1080/01971529108053277. [DOI] [PubMed] [Google Scholar]
- 10.Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry. J Am Coll Cardiol. 1994;23:384–389. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura S, Mahmarian JJ, Boyce TM, Verani MS. Equivalence between adenosine and exercise thallium-201 myocardial tomography: a multicenter, prospective, crossover trial. J Am Coll Cardiol. 1992;20:265–275. doi: 10.1016/0735-1097(92)90090-a. [DOI] [PubMed] [Google Scholar]
- 12.Bertlet BD, Hill JA. Adenosine: diagnostic and therapeutic uses in cardiovascular medicine. Chest. 1993;104:1860–1871. doi: 10.1378/chest.104.6.1860. [DOI] [PubMed] [Google Scholar]
- 13.Belhassen B, Pelleg G, Shoshani D, Laniado S. Atrial fibrillation induced by adenosine triphosphate. Am J Cardiol. 1984;53:1405–1406. doi: 10.1016/0002-9149(84)90104-8. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli L, Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983;244:H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]