Abstract
Two factors secreted from the nerve terminal, agrin and neuregulin, have been postulated to induce localization of the acetylcholine receptors (AChRs) to the subsynaptic membrane in skeletal muscle fibers. The principal function ascribed to neuregulin is induction of AChR subunit gene expression and to agrin is the aggregation of AChRs. Here we report that when myoblasts engineered to secrete an agrin fragment were placed into the nerve-free region of denervated rodent muscle, the host muscle fibers expressed AChR ɛ-subunit gene transcripts, characteristic of the neuromuscular synapse in adult muscle. Transcripts were colocalized with agrin deposits and AChR clusters that were resistant to electrical muscle activity. More directly, single innervated muscle fibers injected intracellularly with agrin expression plasmids in their extrasynaptic region developed a functional ectopic postsynaptic membrane with clusters of adult-type AChR channels and acetylcholinesterase and accumulation of myonuclei. The results demonstrate that agrin is the principal neural signal that induces the formation of the subsynaptic apparatus in the muscle fiber and controls locally, either indirectly or directly, the transcription of AChR subunit genes and the aggregation of AChRs.
Keywords: endplate, innervation
Innervation of skeletal muscle fibers induces the redistribution of preexisting fetal acetylcholine receptors (AChRs), composed of α2βγδ subunits, to the subsynaptic muscle membrane. Clustering of fetal AChRs is followed by the nerve-induced expression of the AChR ɛ-subunit gene selectively in myonuclei underlying the synapse (1), leading to the expression of adult-subtype AChRs with a composition of α2βɛδ in the synaptic membrane (2). The signaling molecules from the nerve are thought to be agrin for AChR clustering and AChR-inducing activity (ARIA) for induction of AChR gene expression. Agrin, upon release from the nerve terminals, binds stably to the synaptic portion of the muscle fiber’s basal lamina (BL; ref. 3). Agrin is encoded by a single gene from which several isoforms arise by alternative mRNA splicing, each differing in their AChR-clustering activities (4, 5).
The local synthesis of new AChRs at the synapse is induced by a BL-bound factor that activates transcription of AChR subunit genes in the myofiber nuclei at the synapse (6, 7). This induction is thought to be mediated by ARIA. ARIA, originally isolated from chicken brain (8), is synthesized by motor neurons (9), is present in synaptic BL (10, 11), and increases the level of AChR ɛ-subunit mRNA (12) in cultured mouse myotubes by activating gene transcription (13). It is encoded by a gene (9), rat and human homologs of which encode Neu differentiation factor and heregulin, respectively (14, 15), as well as glial growth factor (GGF; ref. 16), each arising by alternative mRNA splicing. The products derived from this gene have collectively been named neuregulins (NRGs). Muscle cells express several members of the epidermal growth factor receptor family, ErbB-2, ErbB-3, and ErbB-4, that are localized at the synapse and are tyrosine-phosphorylated upon NRG binding (9, 17–19). Based on these findings, ARIA/NRG has been proposed to be the nerve-supplied factor stimulating the switch in synaptic AChR composition from the fetal α2βγδ type to the adult α2βɛδ type (9, 12).
Recently, we found that recombinant agrin, deposited by transfected cells onto substrate, induces the expression of AChR ɛ-subunit in aneural cultured muscle cells via transcriptional activation in the absence of nerve-supplied NRGs (20). Consistent with this, transcriptional specialization of subsynaptic nuclei was not observed in mice in which the genes encoding agrin (21) or MuSK, a putative receptor for agrin, had been deleted (22). In both cases, however, presynaptic nerve terminals failed to develop. Thus, the absence of subsynaptic specializations in these animals could be due to the failure of undifferentiated nerve endings to secrete molecules mediating nerve-evoked postsynaptic differentiation.
We show that agrin alone, in the absence of nerve terminals and of nerve-derived NRGs, is able to induce the formation of a functional postsynaptic-like membrane in the extrasynaptic region of denervated and innervated muscle fibers in vivo. Agrin-induced ectopic expression of the AChR ɛ-subunit gene and adult-type AChR channels (containing an ɛ subunit) were used as an indicator for formation of an endplate-like membrane in a previously synapse-free fiber segment. Some of these results have appeared in abstract form (23).
MATERIALS AND METHODS
Cell Transfection, Injection of Cells and Plasmids into Muscle, and Electrophysiology.
Rat L6 myoblasts and mouse C2C12 myoblasts were transfected by using the calcium phosphate method with 18 μg of plasmids pCBA-1 and 2 μg of pMCNeo at a final total concentration of 3 μg of DNA per ml (24). Stably transfected, agrin-secreting clones were selected with G418 and ELISA (25). Male Sprague–Dawley rats (100–200 g) or Swiss White mice (40–50 g) were anesthetized with isoflurane or nembutal (1 ml/kg of body weight). Soleus muscles were denervated bilaterally by cutting the soleus nerve. Three to 4 days (d) after denervation, 8000–12,000 stably transfected cells or control cells, suspended in 10 μl of PBS, were injected via a 34-gauge needle into the proximal endplate-free region of the soleus muscle, or sham injections were made. Starting on the day before cell injection, animals were force-fed daily with cyclosporine at 150 mg/kg of body weight dissolved in olive oil. Muscles were excised 4–5 d after cell injection and divided into endplate-free and endplate-containing segments for transcript analysis. Some muscles were stimulated in vivo electrically with 100-Hz trains, 1 s in duration, once every 100 s, via implanted electrodes for 4–6 d, beginning 3–4 d after intramuscular injection of cells (26). In the intracellular injection experiments, the expression plasmids for agrin were injected into single muscle fibers via fine-tipped glass pipettes. Intracellular penetration of pipettes was monitored by recording resting membrane potentials of −60 to −70 mV. The cDNAs encoding full-length agrin7A4B8 (27) and green fluorescent protein (GFP) cloned into pcDNA1 or pRK expression vectors were dissolved at 200 ng/μl in 150 mM KCl containing Fast Green FCF (Sigma; 25 mg/ml), back-filled into the pipette, and then pressure-ejected into the proximal endplate-free region (5–10 mm away from the endplate region) of single fibers of exposed soleus. Brief pressure pulses (1–2 bar; 1 bar = 100 kPa) were applied to the end of the injection pipette and injection was monitored visually by the injected dye. Animals were anesthesized as described above.
Electrophysiological characterization of AChR channel properties by fluctuation analysis of ACh-evoked membrane currents at endplates and at agrin-induced ectopic AChR clusters was done as described (6).
In Situ and Northern Blot Hybridizations.
Frozen cross-sections 12–16 μm thick were prepared. In situ hybridization used 35S-labeled cRNA specific for rat AChR ɛ- and δ-subunit mRNAs and autoradiography for detection (1). Fiber diameters were measured while viewing sections in Nomarski interference-contrast optics.
Total RNA was extracted from synaptic or extrasynaptic segments of soleus muscle, resolved in a 1.4% formaldehyde agarose gel, and transferred to nylon membrane. Northern blot hybridization analysis was done with a digoxygenin-labeled cRNA rat muscle AChR ɛ probe (20). Densitometric data were normalized to levels of actin mRNA.
Staining.
Agrin deposits were stained with an anti-chicken agrin polyclonal antiserum, AChRs were stained with rhodamine labeled α-bungarotoxin, AChR ɛ subunit was stained with mAb 168 (28). Acetylcholinesterase (AChE) activity was visualized histochemically (29), and nuclei were stained with bisbenzimide.
RESULTS
Agrin Delivered by Transfected Myoblasts to Rat Muscle Fibers Induces Ectopic Expression of AChR ɛ-Subunit Gene.
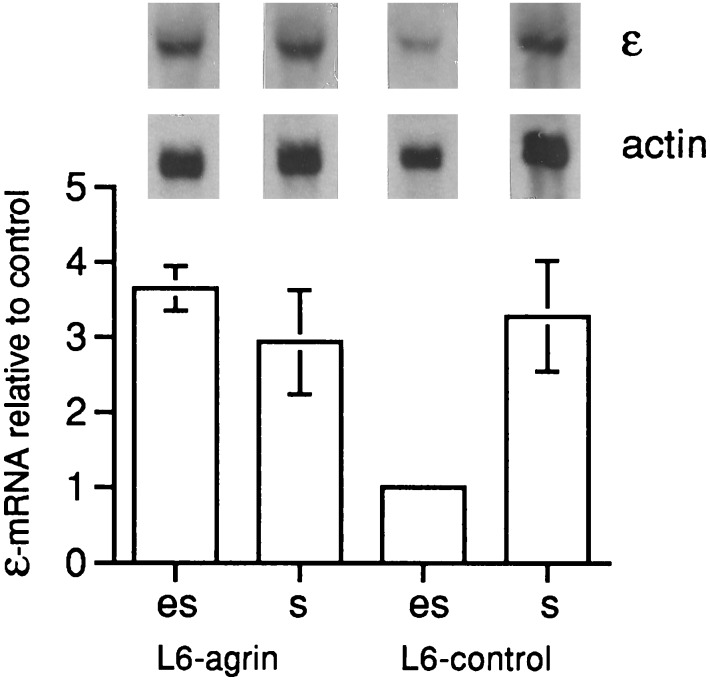
We examined whether agrin induces AChR expression in muscle fibers in vivo. Myoblasts of the L6 and C2C12 cell lines stably transfected to secrete the CBA-1 fragment (20) of chicken agrinA4B19 were injected into the proximal endplate-free region of denervated rat or mouse soleus muscles, respectively. Five to 6 d after the injection, the level of ɛ-subunit mRNA in extrasynaptic rat muscle segments containing either of two agrin-secreting L6 cell clones was 2.0 ± 0.2 and 3.8 ± 0.8 times higher (Fig. 1), than in contralateral control muscle segments injected with control L6 cells (mean ± SEM, four to six pairs of muscles were analyzed for each clone). The difference was statistically significant (P < 0.05, Student’s t test).
Figure 1.
CBA-1 fragment of agrin A4B19 delivered by stably transfected rat L6 myoblasts injected into the nerve-free region (es) of denervated soleus muscle increases the level of AChR ɛ-subunit transcripts as observed in Northern blot hybridization. Test muscles were injected with stably transfected cells (L6-agrin); contralateral control muscles were injected with the same number of nontransfected L6 cells. Bar diagrams indicate levels of transcripts (mean ± SEM; n = 4 to 6) upon injection of agrin-secreting cells of one positive L6 clone (es, L6-agrin), relative to those injected with control L6 cells (es, L6-control); bars marked s give data for the muscle segments that contained synapses. Injection of another transfected clone of L6 cells and of transfected C2C12 cells into mouse soleus muscle gave similar results. Injection of L6 control cells or sham injection with saline into an endplate-free region did not affect AChR ɛ-subunit mRNA levels compared with noninjected muscles (data not shown). Levels of actin-specific mRNA are shown for control.
The ratio of transcript levels in extrasynaptic test and control muscle segments was similar to that of synaptic and extrasynaptic segments in uninjected muscles, which averaged 3.8 ± 0.7 (P < 0.01). Similarly, a 2.7 ± 0.5 times increase in AChR ɛ-subunit RNA was found in extrasynaptic segments of mouse soleus muscles injected with stably transfected C2C12 cells compared with control muscles (P < 0.05). In contrast, transcript levels in muscles injected with control cells or sham-injected with saline were not different from one another (P > 0.4; data not shown). Transcript levels in myotube cultures derived from the transfected clones were only slightly (1.2 ± 0.1 times, n = 3) higher than those derived from untransfected control L6 and C2C12 cell cultures. Hence, agrin delivered by myoblasts induced ectopic expression of the ɛ-subunit gene in adult muscle.
One characteristic property of nerve-induced synapse-specific AChR expression is its resistance to electrical impulse activity in the muscle fiber even after section of the motor nerve (30). In contrast, extrasynaptic AChR expression in denervated muscle is down-regulated by exogenous electrical stimulation. We examined cross-sections of denervated muscle injected with agrin secreting cells for colocalization of chicken agrin, ectopic AChR clusters, and AChR ɛ-subunit mRNA accumulations in denervated and subsequently electrically stimulated muscle. Injected muscles were electrically stimulated for 4–6 d with a stimulation pattern that is sufficient to abolish extrasynaptic AChRs in denervated muscle (26).
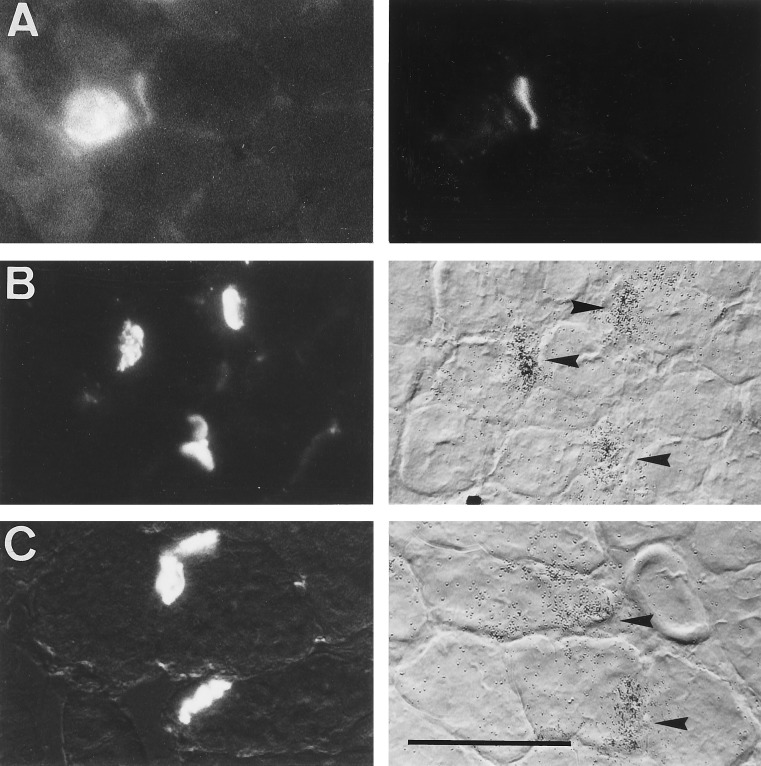
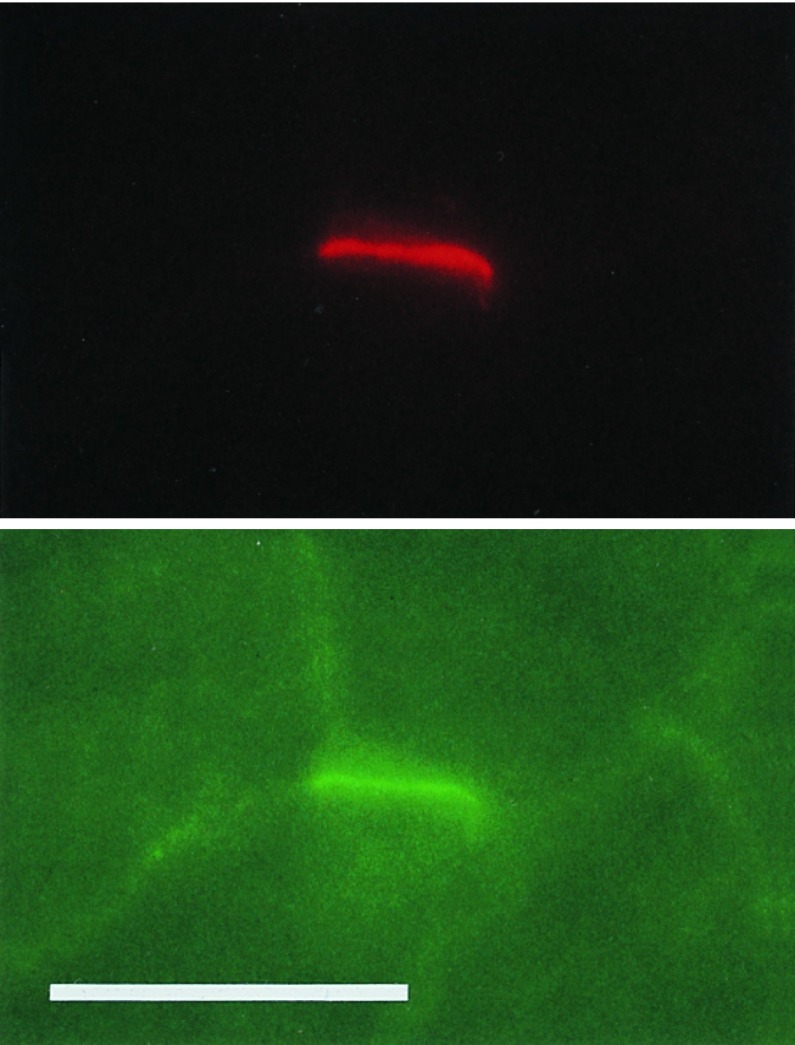
In cross-sections of stimulated muscle previously injected with agrin-secreting L6 cells, but not in control muscles, staining with rhodamine-labeled α-bungarotoxin showed AChR clusters in extrasynaptic fiber segments. This labeling occurred around and within the injection site, which could be identified by its characteristic content of small cell profiles, most likely representing L6 myoblasts or newly formed myotubes. Clusters of AChRs were observed both on small and large cell profiles, the latter of which had diameters similar to that of host muscle fibers outside the injection region. These large fibers located at the periphery of the injection region had been electrically stimulated, since they did not contain diffusely distributed AChRs in the surface membrane, unlike the small cells and the fibers from contralateral denervated but nonstimulated muscle. Staining with anti-chicken agrin antibodies showed agrin expressed in the small cells (Fig. 2A Left). Some of the agrin-immunoreactive cells were colocalized with focal deposits of agrin and with AChR clusters on the surface of apposed large muscle fibers (Fig. 2A Right). Forty-one AChR clusters in three injected muscles were examined for colocalization with agrin, and at 37 fibers (90%) agrin deposits were resolved.
Figure 2.
CBA-1 AgrinA4B19 delivered by injected L6 cells induces ectopic AChR clusters and colocalized accumulations of AChR ɛ-subunit mRNA in denervated but electrically stimulated rat soleus muscle. (A) Colocalization of agrin (Left) and AChR cluster (Right) in the endplate-free region of denervated and 5-d stimulated muscle. Shown are an agrin-positive cell and apposed agrin deposit (Left) that follows the outline of an adjacent fiber of the host muscle. (B) Colocalization of activity-resistant ectopic AChR clusters (Left) and ɛ-subunit mRNA accumulation (Right, arrowheads) in endplate-free region of denervated and 5-d stimulated muscle. (C) Original endplates of denervated muscle processed in parallel to that in B. Note that densities of silver grains at original endplates are comparable to those at ectopic sites (as shown in B). [Bar = 70 μm (A) and 100 μm (B and C).]
AChR clusters (Fig. 2B Left) were colocalized, in 43 of 47 sites examined, with focal accumulations of AChR ɛ-subunit gene transcripts (Fig. 2B Right, arrowheads). These ectopic ɛ-subunit gene transcripts were expressed by electrically stimulated fibers of the host muscle since histograms of diameters of cells expressing ɛ-subunit mRNA in the injection region and of host muscle fibers outside the injection region showed that the majority of fibers (88 of 95 examined) with ectopic ɛ-subunit mRNA accumulations had diameters (35.5 ± 0.7 μm) similar to fibers of the host muscle (37.2 ± 0.8 μm). The smallest profiles expressing transcripts, representing injected agrin-secreting cells, were only a small minority of all ɛ-subunit-mRNA-positive cells (5 of 95; diameter, 15.8 ± 1.4 μm). The possibility that AChR transcripts were expressed by fibers regenerating from damaged muscle, which express AChR ɛ-subunit mRNA constitutively (6, 31), seems unlikely for the following reasons. (i) Unlike in the present experiments, extrasynaptic expression of ɛ-subunit mRNA in regenerating fibers is abolished by electrical muscle activity (6). (ii) In 65 of 67 fiber profiles expressing ɛ-subunit mRNA, nuclei were located at their periphery rather than in the center, which would have indicated the presence of regenerating fibers (6). Silver grain density at L6 cell-induced ectopic sites did not obviously differ from that at the original endplates of muscle processed in parallel (Fig. 2C), suggesting that the level of AChR ɛ-subunit gene expression at the ectopic sites was not significantly different from that at the original synapses.
Thus, these observations suggest that the ɛ-subunit-specific transcripts were induced by the agrin secreted from the L6 cells injected into the muscle and that the secreted agrin presumably was bound to extracellular matrix of the host muscle fibers.
Ectopic Injection of Agrin Expression Plasmids into Innervated Muscle Fibers Induces Postsynaptic-Like Membranes.
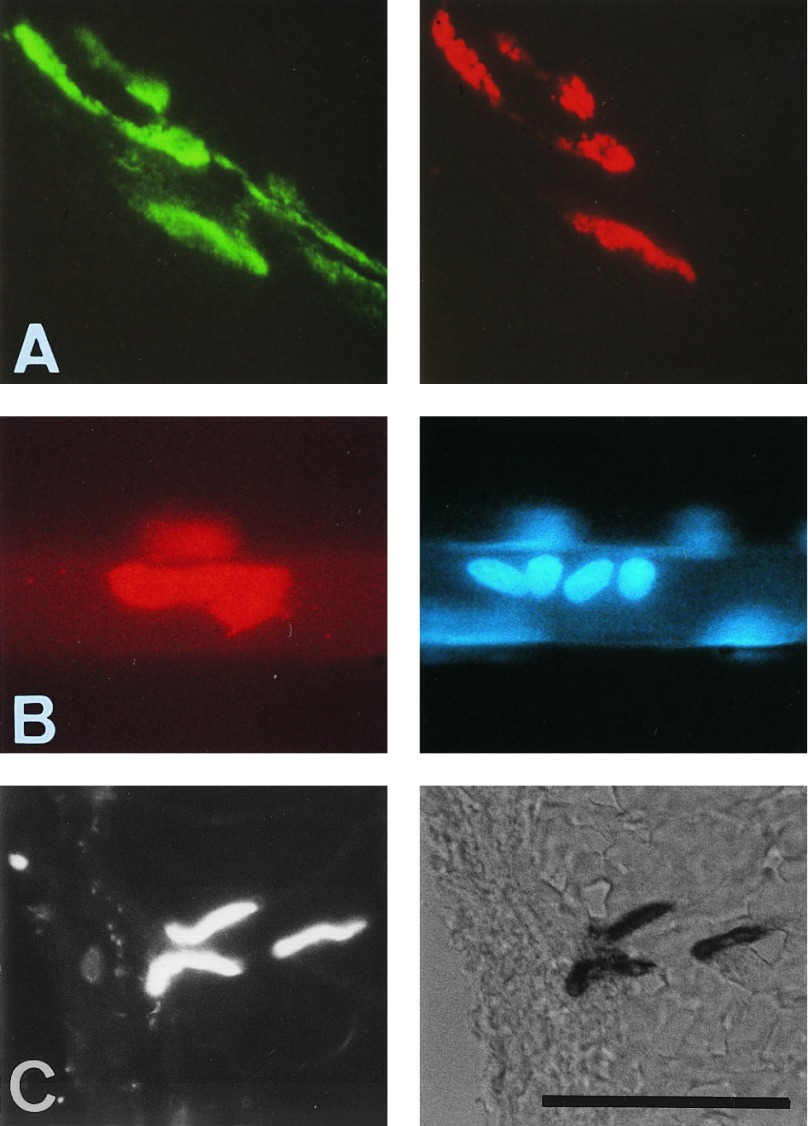
In the experiments described above, the induction of ectopic AChR clusters and ɛ-subunit gene transcript accumulations could have been influenced by cosecretion with agrin of an unidentified factor specific for the myoblasts injected into the host muscle. Furthermore, most ectopic AChR clusters were induced on fibers located deep inside the muscle, thus preventing analysis of their functional properties. We circumvented these problems by intracellular injection of an expression plasmid for full-length chicken agrin cAgrin7A4B8 (27) into extrasynaptic regions of individual, normally innervated fibers of the adult rat soleus. Innervated fibers were used in these experiments to test the resistance of agrin-induced ectopic postsynaptic structures to the physiological pattern of muscle activity and to see whether the effects of agrin depended on the innervation state. Coinjection of a GFP expression plasmid allowed identification of injected fibers days to weeks later. Five days after intracellular injection, about 20% of the injected fibers were GFP-positive and showed dense deposits of agrin at the site of the injection. Agrin immunoreactivity was observed along several fiber diameters and agrin was also deposited on fibers adjacent to the injected one. Clusters of AChRs were present in these fibers and were colocalized with deposits of agrin on the surface of the muscle. In contrast, AChR clusters were not observed in fibers injected with GFP expression plasmid alone. Agrin-injected sites analyzed 12–16 d after injection shared a number of structural and functional properties with the subsynaptic membrane of normal neuromuscular synapses. (i) AChR clusters were colocalized with agrin deposits both in the injected and neighboring fibers (Fig. 3A). These clusters were resistant to muscle activity, since the injected fibers were innervated. (ii) Clustered myonuclei (Fig. 3B) and AChE activity (Fig. 3C) were colocalized with the agrin-induced AChR clusters. (iii) In situ hybridization of fiber profiles showed ectopic accumulation of AChR ɛ-subunit (Fig. 4) and δ-subunit (data not shown) gene transcripts that were colocalized with the agrin-induced AChR clusters. These agrin-induced ɛ-subunit transcripts were translated into AChR ɛ subunit as indicated by the staining with a specific antibody (Fig. 5; mAB168; ref. 28). Agrin-induced AChR clusters and AChR gene transcript accumulations were stable for at least 28–32 d after intracellular injection of the agrin expression plasmid.
Figure 3.
Agrin7A4B8 ectopically expressed by rat soleus fibers upon intracellular injection of agrin expression plasmids induced formation of ectopic “postsynaptic-like” membrane. (A and C) Taken at 14–16 d. (B) Taken at 28 d after plasmid injection from fibers that were innervated at their neuromuscular synapses. (A) Confocal micrographs show deposits of agrin (Left) around injected fiber and colocalized AChR clusters (Right). (B) Colocalization of agrin-induced ectopic AChR clusters (Left) and accumulation of myonuclei (Right). (C) Colocalization of agrin-induced ectopic AChR clusters (Left) and ectopic AChE (Right); cross-section is shown. (Bar = 100 μm.)
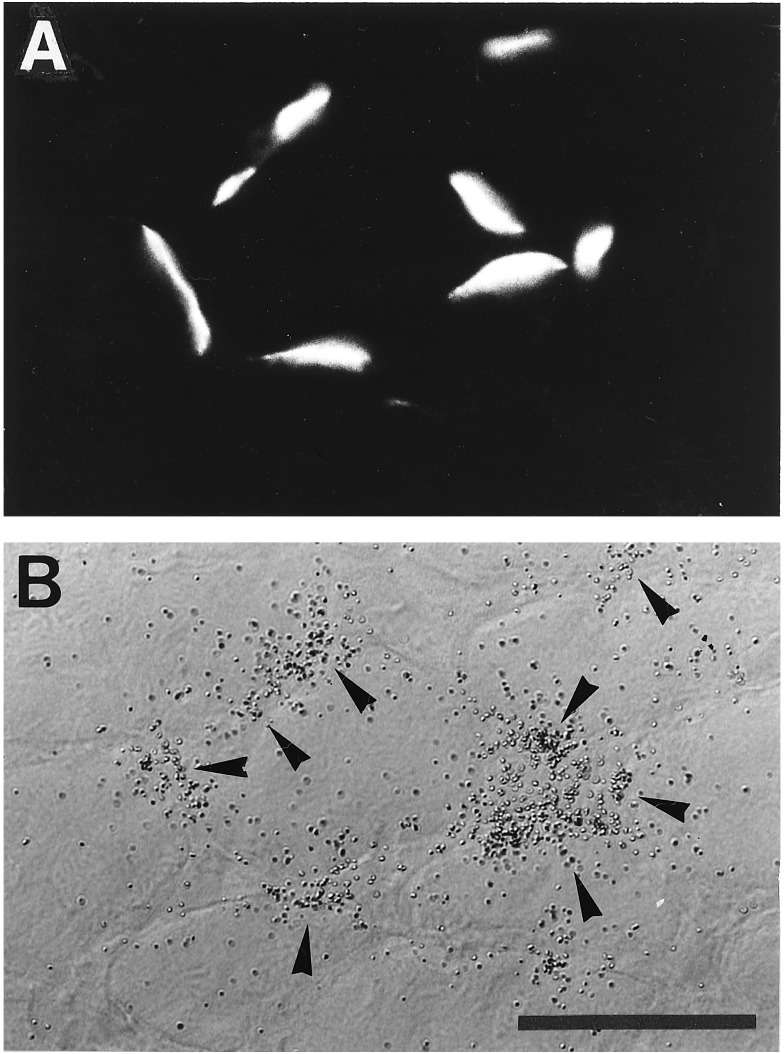
Figure 4.
Colocalization of agrin-induced AChR cluster and ɛ-subunit mRNA at ectopic sites of innervated muscle fibers. (A) Cross-section through surface fibers of innervated soleus, 32 d after intracellular injection of agrin expression plasmid (Agrin7A4B8) into extrasynaptic region of single muscle fibers. Agrin expressed by muscle fiber induced ectopic AChR clusters. (B) Same section as shown in A after hybridization with 35S-labeled cRNA probe specific for AChR ɛ-subunit mRNA and autoradiography. Note colocalization of silver grains (arrowheads) with AChR clusters. (Bar = 70 μm.)
Figure 5.
AChR ɛ subunit in ectopic sites. (Upper) Agrin-induced ectopic AChR cluster in innervated soleus fiber as visualized by rhodamine-labeled α-bungarotoxin. (Lower) This AChR cluster contains AChR ɛ subunit stained with mAb 168. (Bar = 70 μm.)
AChRs in Ectopic Postsynaptic-Like Membrane Have Functional Properties Similar to Those in Normal Endplates.
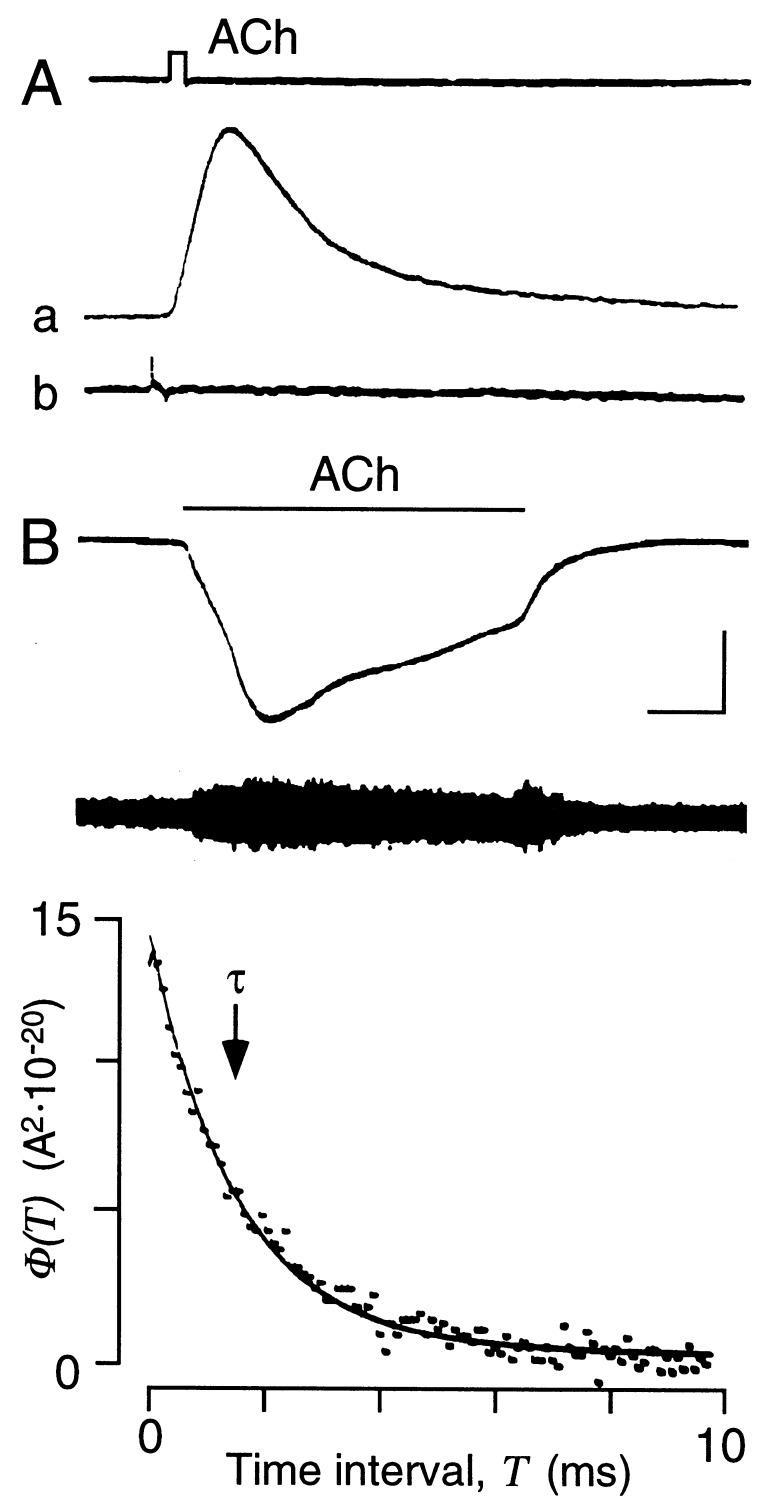
AChRs in injected fibers expressing agrin ectopically were analyzed functionally by mapping the acetylcholine (ACh) sensitivity. Injected sites showed focal high sensitivity to iontophoretically applied ACh. Fibers responded with brisk depolarization, which was not seen in membrane segments a few tens of microns removed from the sensitive sites (Fig. 6A). These ectopic, ACh-sensitive sites were agrin-induced, since they were colocalized with AChE activity as indicated by staining subsequent to physiological characterization. Fluctuation analysis of ACh-induced currents indicated that the apparent open time of AChR channels induced by the agrin expression plasmid injection was short, as in normal endplates. The autocorrelation function of ACh-induced currents was characterized by a decay time constant (τ) of 1.63 ± 0.06 ms (mean ± SEM; n = 8, at −70 mV, 18–19°C; Fig. 6B). This time constant is similar to that measured at the endplates analyzed in the same muscles (τ = 1.73 ± 0.10 ms, n = 5), and it is 3–5 times shorter than that measured at neonatal muscle endplates before the γ/ɛ-subunit switch in endplate channel properties has occurred (32).
Figure 6.
Adult-type AChR channels in ectopic postsynaptic-like membrane. (A) Application of short pulses of ACh (upper trace) at ectopic site of innervated fiber produces rapid membrane depolarization (trace a). No response was detected at a site 100 μm away (trace b). Membrane potential was −62 mV. Calibration bars (see B) represent 5 nA, 2 mV, and 10 ms. (B) Functional properties of ectopic AChR channels. Upper trace shows membrane current evoked by prolonged application of ACh (bar above trace) at same sensitive site as shown in A. The fiber was voltage-clamped locally to −70 mV. Lower trace shows ac-coupled current record as in upper trace to illustrate increase in membrane current fluctuations during ACh application. Calibration bars represent 20 nA, 2.5 nA, and 10 s. (Lower) Autocorrelation function of ACh-induced current fluctuations shown above. Data points represent value of autocorrelation function φ(T) for different time intervals, T. Exponential function φ(T) = σ2·exp(−T/τ) is fitted to data points where σ2 represents the total variance and τ represents the decay time constant. The decay of the autocorrelation function is described by a single exponential with τ = 1.5 ms (marked by arrow; −70 mV, 18°C), indicating short AChR channel open bursts as measured for AChRs in normal adult neuromuscular junctions.
DISCUSSION
The main result of the experiments reported is that agrin, a BL factor deposited by the motor nerve terminal, can induce the formation of an ectopic “synapse-like” muscle membrane with mature functional properties, as observed in normal synapses. This formation occurs in vivo in the extrasynaptic fiber segments in the absence of motor nerve terminals. Like nerve-induced end-plates, the agrin-induced ectopic postsynaptic-like site showed myonuclei accumulation, focal AChE activity, and adult-type AChR channels (32) that contain an ɛ subunit (2). Unlike extrasynaptic AChRs in denervated muscle, the agrin-induced AChR clusters in extrasynaptic muscle regions are resistant to electrical muscle stimulation or normal muscle activity. The induction of ɛ-subunit gene transcript accumulation indicates that the ectopic clusters of adult AChRs do not merely reflect accumulations of preexisting AChRs that are present at low density even in innervated soleus muscle (33). Thus, these observations imply that the motor nerve terminal may control functional subsynaptic specialization in the muscle fiber via the release of a single signaling molecule, agrin. The release from the terminal of another factor, ARIA/NRG, does not appear to be a requirement. However, the results do not exclude participation of ARIA/NRG further downstream in the agrin-induced signal cascade. If ARIA/NRG is required, it must be present in the surface of nonneuronal cells. Indeed, ARIA/NRG-like immunoreactivity is present in muscle cells (34). Furthermore, cultured muscle cells can express ARIA/NRG-like biological activity that induces the phosphorylation of ErbB-2 in primary myotubes as do NRGs (T.M. and H.R.B., unpublished results). Therefore, agrin in the absence of nerve terminals could induce ARIA/NRG-dependent AChR gene expression by concentrating or locally presenting muscle-derived ARIA/NRG or another factor with ARIA-like activity in the myofiber surface from where it could activate AChR expression in the myofiber by an autocrine mechanism. Supporting this view, autocrine control of proliferation via secretion of NRG and activation of ErbB-2 has been observed recently in neoplastic fibroblasts (35).
Participation of muscle-derived ARIA/NRG-like activity in synaptic AChR expression would reconcile differences in the developmental expression patterns of AChR ɛ-subunit mRNA and of ARIA/NRG and ErbB-3 at neuromuscular synapses. During the formation of the neuromuscular synapse, the continued supply of neural factor can be interrupted by denervation at birth (postnatal day 0; P0) without affecting the normal development of AChR ɛ-subunit gene expression for at least up to P8 (1), demonstrating that the neural signal in the basal lamina is already fully developed at P0. In rat hind limb muscles, clusters of AChRs at developing neuromuscular synapses, indicative for agrin action, begin to form as early as embryonic day 16 (E16), ɛ-subunit mRNA can be resolved at E18 (36), and agrin immunoreactivity can be resolved perinatally (37). On the other hand, NRG-like immunoreactivity and ErbB-3, although present at P0, become concentrated at synapses only 1–2 weeks after birth and continue to increase in the following weeks (18, 34). Thus, the localization of ARIA/NRG activity and its receptors at the synapse appears to lag about 10 d behind the expression of the neural factor that is adequate for full stimulation of adult-type AChR expression. This apparent discrepancy in the time course of expression in developing synapses would be resolved if ARIA/NRG in the synaptic BL was derived from muscle under the control of a neural factor such as agrin.
Although our observations are consistent with synaptic AChR gene expression induced by agrin via muscle-derived ARIA/NRG, one cannot exclude, at present, a different more direct pathway via a cognate agrin receptor that is independent of ARIA/NRG. Involvement of MuSK (22, 38) in agrin-induced expression of AChRs is not supported, however, since only isoforms of agrin that cluster AChRs also cause phosphorylation and activation of MuSK. In contrast, the ability of substrate-bound agrin isoforms to activate AChR gene expression is independent of their AChR-clustering activity (20).
To understand agrin-controlled AChR subunit gene expression, it will be important to elucidate, whether ARIA/NRG secretion and/or localization of such factors to the synaptic BL is affected by BL-bound agrin. This could explain our previous finding in muscle cultures that the induction of AChR gene expression by agrin depends on its binding to the extracellular matrix (20).
Acknowledgments
We thank Dr. M. Ruegg (Biozentrum, University of Basel) for anti-chicken agrin antibodies and agrin cDNA clones, Dr. M. Ruegg, Dr. A. R. Martin (University of Colorado Health Sciences Center, Denver), and Dr. Lonnie Wollmuth (Max-Planck-Institut für Medizinische Forschung, Heidelberg, Germany) for comments on earlier versions of the manuscript, Dr. J. Lindstrom (University of Pennsylvania Medical Center, Philadelphia) for a gift of mAb 168, Dr. L. Landmann (Institut für Anatomie, University of Basel, Switzerland) for confocal laser micrographs, and Dr. G. Engel, Sandoz, Basel, Switzerland, for a gift of cyclosporine. This work was supported by grants from the Swiss National Science Foundation, the Swiss Foundation for Research on Muscle Disease, the Ott-Fonds of the Swiss Academy of Medical Sciences, and the Freie Akademische Gesellschaft, Basel.
ABBREVIATIONS
- ACh
acetylcholine
- AChR
ACh receptor
- ARIA
AChR-inducing activity
- AChE
acetylcholinesterase
- BL
basal lamina
- NRG
neuregulin
- d
day(s)
- GFP
green fluorescent protein
References
- 1.Brenner H R, Witzemann V, Sakmann B. Nature (London) 1990;344:544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- 2.Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Nature (London) 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- 3.McMahan U J. Cold Spring Harbor Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Ruegg M A, Tsim K W K, Horton S E, Kröger S, Escher G, Gensch E M, McMahan U J. Neuron. 1992;8:691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- 5.Ferns M, Hoch W, Campanelli J T, Rupp F, Hall Z W, Scheller R H. Neuron. 1992;8:1079–1086. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H R, Herczeg A, Slater C R. Development (Cambridge, UK) 1992;116:41–53. doi: 10.1242/dev.116.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Jo S A, Burden S J. Development (Cambridge, UK) 1992;115:673–680. doi: 10.1242/dev.115.3.673. [DOI] [PubMed] [Google Scholar]
- 8.Jessell T M, Siegel R E, Fischbach G D. Proc Natl Acad Sci USA. 1979;76:5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 10.Jo S A, Zhu X, Marchionni M A, Burden S J. Nature (London) 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- 11.Goodearl A D J, Yee A G, Sandrock A W, Jr, Corfas G, Fischbach G D. J Cell Biol. 1995;130:1423–1434. doi: 10.1083/jcb.130.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinou J C, Falls D L, Fischbach G D, Merlie J P. Proc Natl Acad Sci USA. 1991;88:7669–7673. doi: 10.1073/pnas.88.17.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu G C, Moscoso L M, Sliwkowski M X, Merlie J P. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 14.Holmes W E, Sliwkowski M X, Akita R W, Henzel W J, Lee J, Park J W, Yansura D, Abadi N, Raab H, Lewis G D, Shepard M H, Kuang W-J, Wood W I, Goeddel D V, Vandlen R L. Science. 1992;256:1205–1209. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 15.Wen D, Peles E, Cupples R, Suggs S V, Bacus S S, Lou Y, Trail G, Hu S, Silbiger S M, Ben Levy R, Koski R A, Lu H S, Yarden Y. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 16.Marchionni M A, Goodearl A D J, Chen M S, Bermingham-McDonogh O, Kirk C, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 17.Corfas G, Falls D L, Fischbach G D. Proc Natl Acad Sci USA. 1993;90:1624–1628. doi: 10.1073/pnas.90.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Lai C, Thomas S, Burden S J. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altiok N, Bessereau J L, Changeux J P. EMBO J. 1995;14:4258–4266. doi: 10.1002/j.1460-2075.1995.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones G, Herczeg A, Ruegg M A, Lichtsteiner M, Kröger S, Brenner H R. Proc Natl Acad Sci USA. 1996;93:5985–5990. doi: 10.1073/pnas.93.12.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautam M, Noakes P G, Moscoso L, Rupp F, Scheller R H, Merlie J P, Sanes J R. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 22.DeChiara T M, Bowen D C, Valenzuela D M, Simmons M V, Poueymirou W T, Thomas S, Kinetz E, Compton D L, Rojas E, Park J S, Smith C, DiStefano P S, Glass D J, Burden S J, Yancopoulos G D. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 23.Brenner H R. Eur J Neurosci Suppl. 1996;9:3. (abstr.). [Google Scholar]
- 24.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gesemann M, Denzer A J, Ruegg M A. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lømo T, Westgaard R H. J Physiol (London) 1975;252:603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denzer A J, Gesemann M, Schumacher B, Ruegg M A. J Cell Biol. 1995;131:1547–1560. doi: 10.1083/jcb.131.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson S, Shelton D G, Lei S, Lindstrom J M, Conto-Tronconi B. J Neuroimmunol. 1992;36:13–27. doi: 10.1016/0165-5728(92)90027-i. [DOI] [PubMed] [Google Scholar]
- 29.Koelle G B, Friedenwald J S. Proc Soc Exp Biol Med. 1949;70:617–622. doi: 10.3181/00379727-70-17013. [DOI] [PubMed] [Google Scholar]
- 30.Witzemann V, Brenner H R, Sakmann B. J Cell Biol. 1991;114:125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman D, Carlson B M, Staple J. Neuron. 1991;7:649–658. doi: 10.1016/0896-6273(91)90377-c. [DOI] [PubMed] [Google Scholar]
- 32.Sakmann B, Brenner H R. Nature (London) 1978;276:401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- 33.Miledi R, Zelena J. Nature (London) 1966;210:855–866. doi: 10.1038/210855a0. [DOI] [PubMed] [Google Scholar]
- 34.Moscoso L M, Chu G C, Gautam M, Noakes P G, Merlie J P, Sanes J R. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- 35.Avila M A, Velasco J A, Cho C, Lupu R, Wen D, Notario V. Oncogene. 1995;10:963–971. [PubMed] [Google Scholar]
- 36.Brenner H R, Rotzler S, Kues W, Witzemann V, Sakmann B. Dev Biol. 1994;165:527–536. doi: 10.1006/dbio.1994.1272. [DOI] [PubMed] [Google Scholar]
- 37.Hoch W, Ferns M, Campanelli J T, Hall Z W, Scheller R H. Neuron. 1993;11:479–490. doi: 10.1016/0896-6273(93)90152-h. [DOI] [PubMed] [Google Scholar]
- 38.Glass D J, Bowen D C, Stitt T N, Radziejewewski C, Bruno J, Ryan T E, Gies D R, Shah S, Mattsson K, Burden S J, DiStefano P S, Valenzuela D M, DeChiara T M, Yancopoulos G D. Cell. 1996;85:1–20. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]