Abstract
Aims
Pharmacokinetic variability is likely to be a significant factor contributing to the interindividual differences in dose requirements, anti-inflammatory response and side-effects with inhaled corticosteroids (ICS), but there is limited information about the disposition of ICS during regular dosing with a pressurized metered dose inhaler (pMDI). This study uses a mixed effects modelling approach to quantify and compare the interindividual variability in pharmacokinetics of epimeric budesonide (BUD) and fluticasone propionate (FP) after repeat-dose inhalation.
Methods
This pharmacokinetic substudy was part of a previously published open-label, randomised, placebo-controlled, 7-period crossover study to evaluate the short-term effects on plasma cortisol levels of inhaled BUD (400, 800, 1600 µg twice daily) and FP (375, 750, 1000 µg twice daily) via pMDI in a group of healthy male volunteers. On the fifth day of each high-dose treatment period (BUD 1600 µg twice daily and FP 1000 µg twice daily), venous blood samples were collected in nine subjects prior to the last dose and at 15 min, 30 min, 1, 2, 4, 6 and 8 h postdose for measurement of plasma drug concentrations to determine the pharmacokinetics of epimeric BUD and FP following inhalation. Non-compartmental analysis and a mixed effects model were used to characterize the disposition profiles.
Results
Both drugs had a rapid absorption half-life (BUD 10 min vs FP 11.3 min), but quite different elimination half-lives (BUD 2.4 h vs FP 7.8 h). Although there were intraindividual differences in the handling of the 22R-and 22S-epimers of BUD, there were no consistent pharmacokinetic differences between the two enantiomers in the group as a whole. Consistent with previous reports of FP’s higher volume of distribution (V) and lower systemic bioavailability (F), the V/F ratio was lower for BUD than FP (498 l vs 8100 l). The parameter with the greatest interindividual variability for both BUD and FP was the rate of systemic absorption from the lung.
Conclusions
This is the first report describing the pharmacokinetics of epimeric BUD and FP after repeat dose inhalation via pMDI. Three observations may be of clinical relevance: (1) there is considerable intersubject variability in the rate of absorption of both drugs from the lung; (2) in some individuals there was a long t½,z for BUD, resulting in higher and more sustained plasma drug levels in the 4–12 h postdose period than would be predicted from single-dose pharmacokinetic data; and (3) there is evidence of diurnal variation in FP pharmacokinetics, with higher-than-expected plasma drug concentrations in the morning compared with the evening.
Keywords: budesonide, fluticasone propionate, population pharmacokinetics
Introduction
The development of inhaled corticosteroids (ICS) has been a major advance in the treatment of asthma. When beclomethasone dipropionate (BDP) first became available, laboratory assays for measuring drug concentrations in plasma were relatively insensitive and there was some justification for believing that the inhaled route of delivery would avoid significant systemic drug absorption provided the dose of BDP was kept below 2000 µg daily [1]. More recently, the development of sensitive gas chromato-graphy – mass spectrometry (GC-MS) techniques has made it possible to quantify corticosteroids in plasma following inhalation of doses that are well within the therapeutic range [2], and that these concentrations may be clinically significant is supported by case reports of Cushingoid features developing in patients treated with ICS [3].
It was eventually recognized that systemic drug absorption does occur even with modest doses of BDP given by inhaler, but this was initially attributed as being mainly due to that part of the dose that was inadvertently swallowed. This interpretation prompted the development of two new ICS that (in contrast to BDP) are subject to high first-pass hepatic metabolism, budesonide (BUD) (a 50 : 50 mixture of two epimers) and fluticasone propionate (FP), in the hope that ICS with low oral bioavailability would substantially reduce the risk of systemic side-effects. In practice, however, it transpired that both drugs exhibit significant systemic activity following inhalation [4]; for example, even though orally administered FP is 99% inactivated on first-pass through the liver there is considerable systemic activity when FP is administered by inhalation [4, 5]. These findings emphasize that the lung is a very efficient route for systemic delivery of ICS and that gastrointestinal absorption of drug that is inadvertently swallowed does not make a major contribution to the overall systemic bioavailability of ICS [6].
Clinical experience with ICS therapy shows that there is considerable intersubject variability in dose requirements, anti-inflammatory response and side-effects, but the extent to which this clinical variability is due to differences in pharmacodynamic sensitivity and/or differences in pharmacokinetics is unclear. Since the emphasis with BUD and FP has been on the investigation of oral bioavailability [7, 8], few studies have adequately characterized the pharmacokinetics of BUD and FP following inhalation. There are limited single-dose data [9, 10], but the intersubject and intrasubject variability in drug absorption and elimination have not been previously investigated after repeat-dose inhalation. The purpose of this study was to compare the pharmacokinetics of epimeric BUD and FP after repeat-dose inhalation using a new highly sensitive GC-MS assay and a mixed effects pharmacokinetic model analysis.
Methods
Study design
Twenty-eight healthy male volunteers (age range 18–35 years) gave written informed consent to participate in this study which was approved by the institutional Ethics committees of Royal Prince Alfred Hospital and St Vincents Hospital, Sydney. The experimental design and plasma cortisol results have been published elsewhere [4]. Briefly, this was an open-label, randomised, placebo-controlled 7-period crossover study to compare the short-term effects of different doses of BUD (400, 800, 1600 µg twice daily) and FP (375, 750, 1000 µg twice daily) via pressurized metered dose inhaler (pMDI); each treatment period lasted 5 days and was separated by washout periods of 10 days. For the last 24 h of each 5 day treatment period (i.e. 22.00 h on day 4–22.00 h on day 5), subjects were admitted to the clinical research centre for blood sampling and direct supervision of the last two doses of inhaled medication. On the 5th day of each high-dose treatment (BUD 1600 µg twice daily and FP 1000 µg twice daily), 6 ml venous blood samples were collected in a random subgroup of nine individuals at 10.00 h (immediately prior to the scheduled dose), then at 15, 30 min, and 1, 2, 4, 6 and 8 h postdose for direct assay of BUD and FP concentrations in plasma.
GC-MS assay of BUD and FP concentrations in plasma
Highly sensitive and specific methods have been developed previously by our group for the simultaneous quantification of BUD 22R-and 22S-epimers [11] and FP [12] in human plasma. For the present study, these methods were further refined to decrease the lower limits of quantification (LOQ) to 0.05 ng ml−1 for BUD and 0.02 ng ml−1 for FP.
Model independent analysis
The observed Cmax and tmax were tabulated for each subject. The terminal slope (k) was estimated using log-linear regression of the terminal portion of each curve. The area under the concentration vs time curve for one dosing interval (AUC(0,τ)) was calculated for each patient using linear trapezoids when concentrations were increasing and log-linear trapezoids when concentrations were decreasing. Clearance (CL/F) was calculated as:
| (1) |
The first moment curve (concentration × time vs time) was calculated for each data set and the area under the moment curve (AUMC) calculated using an interpolation-integration method. After multiple dosing at steady state, the observed tmax is related to k and ka according to:
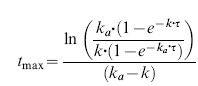 |
(2) |
The estimated k and the observed tmax were then used to obtain an estimate of the absorption rate constant (ka) for each individual. These ‘noncompartmental’ estimates of k and ka were then used to calculate the observed mean residence time (MRTobs) from the multiple dose data.
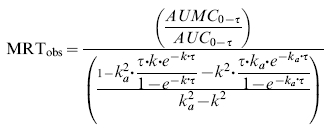 |
(3) |
An estimate of the volume of distribution at steady state (Vdss/F) was calculated as:
| (4) |
where mean residence time after intravenous administration (MRTiv) was calculated as:
| (5) |
Pharmacokinetic modelling
Concentration data for BUD and FP were plotted against time. Samples below the LOQ for BUD and FP were not included in the analysis. Four basic pharmacokinetic models were explored for both BUD and FP: (1) one compartment absorption + one compartment disposition; (2) one compartment absorption + two compartment disposition; (3) two compartment absorption + one compartment disposition; and (4) one compartment absorption + one/two compartment disposition ± diurnal variation. An exploratory two-stage pharmacokinetic analysis was performed to determine which model best described each subject’s data. The pharmacokinetic models were parameterized as V/F (i.e. central volume of distribution, V, divided by bioavailability, F, which was estimated as a single parameter) and first order rate constants (e.g. k12 represents the first order rate constant between compartments one and two, with units ‘per hour’).
The ratio of BUD 22R-to 22S-concentrations in each individual was plotted over time to look for systematic differences between the two epimers. Since BUD is a 50 : 50 mixture of the 22R-epimer and 22S-epimer, the dose of each epimer was assumed to be 50% of the administered dose. The 22R-and 22S-epimer concentrations were analysed simultaneously by modelling the parameter for the 22R-epimer (PR) and 22S-epimer (PS) as shown:
| (6) |
If the addition of θ2 to the model was not statistically significant, the model parameter was assumed to be the same for both epimers. A pharmacokinetic model was implemented to evaluate possible differences in BUD and FP pharmacokinetic parameters (P) between day and night as shown:
| (7) |
As for (6), if the addition of θ2 to the model was not statistically significant, the model parameter was assumed to be the same for night and day. Non-linear mixed effects pharmacokinetic models for BUD and FP were developed. The interindividual error on each of the model parameters was modelled using a log-normal variance model
| (8) |
where P1i is the first parameter in the ith individual, θ1 is the typical value of parameter one in the population, and η1 is a random variable with mean zero and variance σ11. A ‘constant c.v.’ model was used to describe the variance of the residual intraindividual error
| (9) |
where Yj is the jth observation, and ε is a random variable with mean zero and variance ω11.
Data analysis and statistical analysis
An ordinary differential equation (ODE) integrator was employed using an embedded Runge Kutta algorithm with adaptive step size control [13]. This function was compiled using Microsoft Visual C + + as a dynamic link library for Microsoft Excel and was used for model development and individual ‘fits’. All mixed effects modelling was performed with NONMEM (ADVAN6, Version V, 1.0) [25], using the ‘first order method’. The statistical significance of each parameter was assessed by evaluation of the log likelihood objective function, examination of the standard errors of parameter estimates, and graphs of weighted residuals vs time. Model bias (MDWR, median weighted residual) and precision (MDAWR, median absolute weighted residual, and 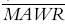 ) were calculated as described by Billard et al.[14]. Two case deletion diagnostics, the Cook score [15] and the covariance ratio [16], were calculated to detect influential subjects. Final results for the population modelling approach were tabulated.
) were calculated as described by Billard et al.[14]. Two case deletion diagnostics, the Cook score [15] and the covariance ratio [16], were calculated to detect influential subjects. Final results for the population modelling approach were tabulated.
Results
Plasma drug concentrations
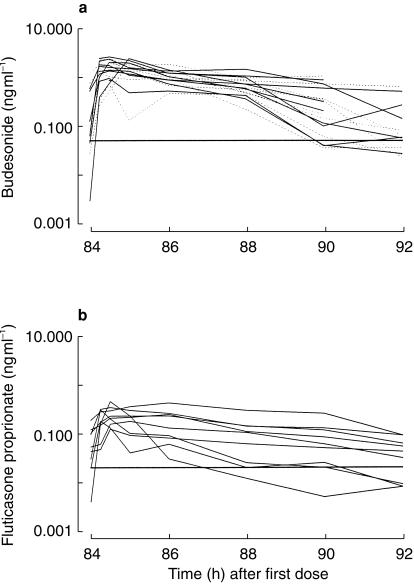
A total of 72 samples were available (eight samples from each of nine individuals) for each drug. Figure 1a shows the measured concentrations of BUD (22R and 2S) in each volunteer. 10/72 samples of BUD 22R, and 11/72 samples of BUD 22S were below the LOQ. Figure 1b shows the measured concentrations of FP in each volunteer. 6/72 samples of FP were below the LOQ. The results of model-independent analysis of the steady-state plasma pharmacokinetics of R-and S-BUD and FP are shown in Table 1.
Figure 1.
a) Individual concentration data for BUD 22R-and 22S-epimers (solid line and dashed line, respectively) on the 5th day of 1600 µg twice daily via pMDI. The horizontal line represents the LOQ at 0.05 ng ml−1. b) Individual concentration data for FP on the 5th day of 1000 µg twice daily via pMDI. The horizontal line represents the LOQ at 0.02 ng ml−1. The peak concentrations obtained after BUD were greater than after FP.
Table 1.
Model independent analysis of the steady state plasma pharmacokinetics of R-and S-budesonide (BUD) and fluticasone (FP) after repeat dose inhalation. Geometric mean (95% confidence intervals).
| R-BUD | S-BUD | FP | |
|---|---|---|---|
| Cmax (ng ml−1) | 1.8 (1.4–2.3) | 1.7 (1.2–2.2) | 0.26 (0.18–0.36) |
| tmax (h) | 0.46 (0.34–0.64) | 0.43 (0.30–0.61) | 0.63 (0.35–1.14) |
| t½,elim* (h) | 2.3 (1.3–4.1) | 2.3 (1.3–4.0) | 2.7 (1.4–5.2) |
| AUC (ng ml−1.h) | 6.1 (4.4–8.5) | 5.5 (3.7–8.3) | 0.85 (0.49–1.48) |
| AUC (% under data) | 85 (76–94) | 85 (78–92) | 86 (83–90) |
| CL/F (l h−1) | 163 (118–225) | 182 (121–273) | 1171 (677–2025) |
| t½,abs* (min) | 6.3 (3.8–10.3) | 5.7 (3.3–9.8) | 9.8 (4.0–24) |
| MRTobs (h) | 3.90 (2.41–6.32) | 3.85 (2.40–6.18) | 4.2 (2.40–7.36) |
| Vdss/F (l) | 600 (400–900) | 660 (453–963) | 4150 (2094–8225) |
Absorption and elimination half-life, calculated as natural log(2)/ka and natural log(2)/k, respectively.
Pharmacokinetic modelling of budesonide
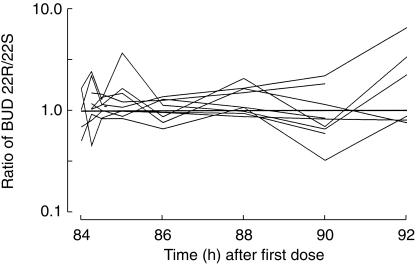
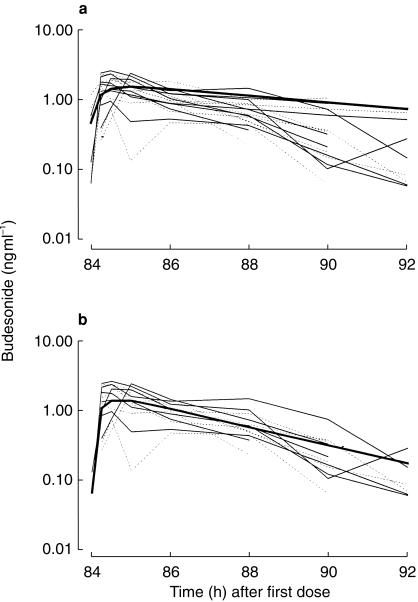
Figure 2 shows the ratio of BUD 22R-to 22S-epimers in each individual. Although there were differences in the 22R-and 22S-epimer concentrations within individuals, mixed effects modelling revealed no statistically significant differences in the 22R-and 22S-model parameters in the group as a whole (Table 2). Figure 3a shows the result of the simultaneous fit of the one compartment absorption and one compartment disposition pharmacokinetic model through the 22R and 22S BUD data from all nine subjects. This model was very biased (22R-epimer MDWR=−20.4%, 22S-epimer MDWR=−24.1%). The two compartment disposition model was equally unsatisfactory. Case deletion diagnostics revealed that two individuals had very small covariance ratios, suggesting that they were incompatible with the rest of the data. Figure 3b shows the improvement when these two individuals were removed from the data set. There was a marked improvement in the bias (22R-epimer MDWR=+ 4%, 22S-epimer MDWR=−1.9%), although the precision was only moderate (22R-epimer MDAWR=+ 38%,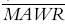 = + 46%, 22S-epimer MDAWR=+ 33%,
= + 46%, 22S-epimer MDAWR=+ 33%,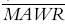 = + 38%). Thus, in seven individuals, a one compartment absorption (t½ = 10 min) and one compartment disposition (t½ = 2.4 h) model described the data best (Table 2). Two individuals required a two compartment disposition model to adequately describe their pharmacokinetics (t½,β = 24 h). There were no statistically significant differences to suggest a difference between night and day model parameters ((7)).
= + 38%). Thus, in seven individuals, a one compartment absorption (t½ = 10 min) and one compartment disposition (t½ = 2.4 h) model described the data best (Table 2). Two individuals required a two compartment disposition model to adequately describe their pharmacokinetics (t½,β = 24 h). There were no statistically significant differences to suggest a difference between night and day model parameters ((7)).
Figure 2.
Ratio of BUD 22R-to 22S-epimers vs time for each individual. A ratio of 1 : 2 and 2 : 1 would appear equidistant from the line of unity, as the data are shown on a logarithmic scale.
Table 2.
Population pharmacokinetic parameters for BUD after repeated dose administration from a pMDI in seven men using mixed effects model.
| Parameter | Estimate | Standard error |
|---|---|---|
| k12 (h−1) | 3.82 | 0.598 |
| V/F (l) | 498 | 62.4 |
| k20 (h−1) | 0.284 | 0.0134 |
| ω11* | 1.34 | 0.833 |
| ω22* | 0.076 | 0.0465 |
| ω33* | – | – |
| σ11 | 0.148 | 0.0152 |
The variances of η (Equation 8) refer to the random effects on k12 (absorption rate constant), V/F, and k20 (elimination rate constant), respectively. ω33 could not be estimated with confidence.
Figure 3.
a) Individual concentration for BUD 22R-and 22S-epimers (solid line and dashed line, respectively) for all nine subjects. The bold line shows the biased fit of the mono-exponential absorption mono-exponential disposition model. b) Shows the less biased description of the data with two subjects removed. These two subjects require a two compartment disposition model to accurately characterize their pharmacokinetics, resulting in a more sustained budesonide concentration between 4 and 12 h postdose.
Pharmacokinetic modelling of fluticasone propionate
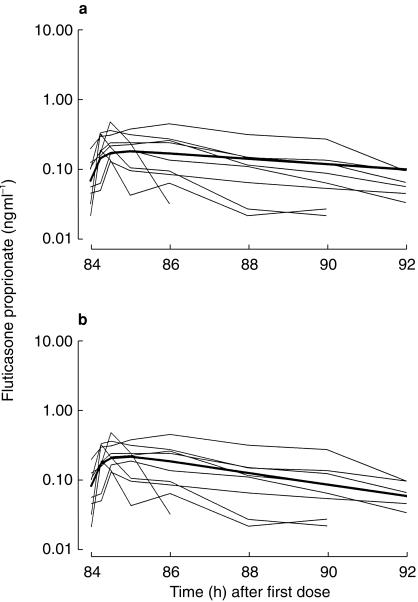
Figure 4a shows the fit of the one compartment absorption and one compartment disposition pharmacokinetic model to the FP data from all nine subjects. This model was unbiased (MDWR=−0.8%), although the precision was less than obtained for BUD (MDAWR=+ 47%, 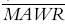 = + 57%). The two compartment disposition model offered no statistically significant improvement. Thus, in all nine individuals, a one compartment absorption (t½ = 11.3 min) and one compartment disposition (t½ = 7.76 h) model described the data best (Table 3). The interindividual variability in the FP absorption rate was greater than for BUD.
= + 57%). The two compartment disposition model offered no statistically significant improvement. Thus, in all nine individuals, a one compartment absorption (t½ = 11.3 min) and one compartment disposition (t½ = 7.76 h) model described the data best (Table 3). The interindividual variability in the FP absorption rate was greater than for BUD.
Figure 4.
a) Individual concentration for FP for all nine subjects. The bold line shows the fit of the mono-exponential absorption mono-exponential disposition model. b) shows the fit of the pharmacokinetic model, which permitted a diurnal variation in the rate of elimination (a more rapid elimination half-life during the day).
Table 3.
Pharmacokinetic parameters for FP after repeated dose administration from a pMDI in nine men using a mixed effects model.
| Parameter | Estimate | Standard error |
|---|---|---|
| k12 (h−1) | 3.67 | 0.846 |
| V/F (l) | 8100 | 1240 |
| k20 (h−1) | 0.0893 | 0.0085 |
| ω11* | 4.73 | 3.49 |
| ω22* | 0.261 | 0.108 |
| ω33* | – | – |
| σ11 | 0.167 | 0.0583 |
The variances of η (Equation 8) to the random effects on k12 (absorption rate constant), V/F, and k20 (elimination rate constant), respectively. ω33 could not be estimated with confidence.
Inspection of Figure 4a reveals evidence of diurnal variation in the pharmacokinetics. The first sample (at 84 h) is taken 12 h after the previous 22.00 h FP dose, and the last sample (at 92 h) is taken only 8 h after the 10 am FP dose. We would expect the 12 h postdose sample to be less than the 8 h postdose sample. However, in all but one subject, the 12 h postdose FP concentration is greater than the 8 h postdose concentration. We developed a model that permitted a step increase in the elimination rate constant during the day compared with the night, and the result is shown in Figure 4b. This model provided a statistically significant improvement in the fit (difference in −2 times log likelihood = 31.1, P < 0.01), with different estimates of the elimination t½ during the day and night (3.54 vs 8.9 h, respectively).
Discussion
There are significant differences in the pharmacokinetic properties of the various ICS currently used in the treatment of asthma [17], but previous pharmacokinetic analyses of BUD and FP have focused mainly on single-dose i.v. and inhaled data [7–10]. This study is the first to describe the pharmacokinetics of epimeric BUD and FP after repeat dose inhalation via pMDI.
The area under the curve (AUC) after a single dose is equivalent to that after multiple doses at steady-state during one dosing interval (τ). However, this relationship is not true for the area under the first moment curve (AUMC). We have used a method recently described by Rohatagi et al.[18] to analyse our multiple dose data. The determination of MRTobs from multiple dose data can only be done correctly if either the remaining area under the curve after the end of the last dosing interval is known, or if correction factors (equation 3) are used. Although equation 3 is based on a one-compartment model, it permits the estimation of ka without the use of a compartmental curve fitting approach [18]. However, the estimate of ka is dependent on the estimate of the terminal slope, and any error is carried forward to the MRT and Vdss calculation.
Although the data were well described by monoexponential absorption and monoexponential disposition functions, these parameter estimates should be interpreted with caution. In particular, possible misassignment of absorption and disposition rate constants cannot be identified without intravenous data. Nevertheless, a mixed effects modelling approach was used to characterize for both drugs the rate and interindividual variability of systemic absorption from the lung, and the volume of distribution (V/F) and elimination t½. BUD and FP are rapidly absorbed into the circulation, although the interindividual variability for absorption of FP was greater than that for BUD. The much larger estimate of V/F for FP compared with BUD is consistent with previous reports showing that FP has a greater volume of distribution (V) and lower systemic bioavailability (F) [8]. The elimination t½ for BUD is typically much shorter than FP [7, 8].
Three observations derived from this analysis may be of some clinical relevance. Firstly, these results confirm that there is considerable interindividual variability in the rate of systemic absorption of both BUD and FP through the lung. It is well recognized that only 15–20% of the inhaled dose via pMDI reaches the lungs, and there are inconsistencies in drug delivery between subjects and within the same subject during repeated dosing, but the absorption rate constants derived from this study suggest that, in addition to the variability in drug delivery, there may be additional variability in the rate of transfer of drug across the mucosal barrier into the systemic circulation, especially for FP. Thus, improvements in inhaler device technology to reduce the variability in drug delivery may have only a partial effect on the intersubject variability in systemic bioavailability of ICS.
Secondly, in some individuals there was evidence of a long t½,β after repeat dosing with inhaled BUD, resulting in higher and more sustained plasma drug levels in the latter half of the dosing interval. There have been no previous estimates of BUD absorption half-life following pMDI administration. A one-compartment disposition model (plasma t½ 2.4 h) accurately characterized the repeat-dose data in seven out of the nine individuals, but two volunteers had distinctly higher and more sustained plasma levels of BUD between 4 and 12 h postdose than would be predicted on the basis of previous single-dose pharmacokinetic data. The profiles for these two subjects were more appropriately described by a two-compartment disposition model with a much longer terminal half-life (t½,β 24 h). The significance of these findings is not entirely clear, but single-dose parameters may not accurately reflect the pharmacokinetics of BUD during chronic dosing. Presumably those subjects with a prolonged elimination half-life and higher plasma BUD concentrations might, in clinical practice, be more susceptible to the adverse effects such as adrenal suppression. This would require confirmation by further studies to examine the concentration-effect relationships, but the present study has highlighted intersubject differences in elimination half-life and raised the possibility that circulating levels of BUD during repeat dose inhalation may be significantly higher than anticipated from previous single-dose data.
The parameters derived for repeat-dose BUD in this study are broadly consistent with previous pharmacokinetic results from single-dose studies. For example, Ryrfeldt et al.[7] reported a plasma half-life (t½) of 2.8±1.1 h, distribution volume (Vd) of 301.3±41.7 l and plasma clearance (CL) of 83.7±27.5 l h−1. In addition, Thorsson et al.[19] have recently compared the pulmonary and systemic availability of BUD after inhalation from a dry powder inhaler and pMDI. Following intravenous administration, the estimated pharmacokinetic parameters were plasma t½ 2.3 h (range 1.7–3.4 h), Vss 2.69 l kg−1 (1.41–5.02 l kg−1), and CL 1.34 l min−1 (0.94–1.98 l min−1). The measurements of systemic availability were 38% (23–62%) and 26% (15–53%) following inhalation of the metered-dose from a dry powder inhaler and pMDI, respectively.
The pharmacokinetics of the 22R-and 22S-epimers of BUD were first reported in six healthy male subjects [20]. The mean pharmacokinetic parameters for the 22R-epimer after a single intravenous dose of BUD were as follows: plasma t½ 2.66±0.57 h, Vd 425±100 l and CL 117±40 l h−1, and correspondingly for the 22S-epimer: t½ 2.71±0.69 h, Vd 245±38 l, and CL 67±19 l h−1. The differences in plasma t½ and Vd for the two epimers achieved statistical significance. In particular, the higher Vd noted for the 22R-epimer was thought to be the result of a higher tissue affinity. Pedersen et al.[21] have also reported the pharmacokinetics of single-dose epimeric BUD in six children with asthma. After single-dose i.v. and inhaled administrations the plasma levels of 22S were consistently higher than 22R (ratio 1.2–1.4) at all sampling times in all individuals [21]. In addition, the systemic bioavailability, volume of distribution and total plasma clearance estimates were all significantly higher for the 22R-epimer. The half-life (approximately 1.5 h) did not differ between the two epimers, but was significantly shorter than that reported in adults [20]. In contrast to these reports of enantiomer-selective disposition, in the present study there were no consistent differences in the pharmacokinetics of the 22R- and 22S-epimers after 5 days of repeat-dose inhalation.
The third observation from the present study that may have clinical significance relates to the apparent diurnal variation in FP pharmacokinetics during repeat dose inhalation, with higher-than-expected FP concentrations in the morning compared with the evening. Without measured concentration data during the nocturnal phase of the study, we cannot be certain why this has occurred. Possible explanations include a difference in the rate or extent of lung absorption, or a difference in the volume of distribution or rate of elimination, during the night compared with the day. It should also be acknowledged that the dose of FP used in this study is higher than that recommended for routine practice and our estimate of FP absorption may only apply to an initial rapid phase of short duration.
The parameters derived for FP in this study are worthy of comparison with previous pharmacokinetic data from single-dose studies. In a study of healthy males given single i.v. doses of FP, Mackie et al.[8] found extensive distribution (Vd 318 l), rapid clearance (CL 1.1 l min−1) and a t½ of 7.8 h. In a study by Rohatagi et al.[22], who reported the pharmacokinetics of FP in 12 healthy volunteers after single inhaled doses of FP 500, 1000 or 2000 µg via pMDI, the disposition profiles were characterized using a one-compartment absorption and one-compartment elimination model, with a t½ of 7.7–8.3 h. These authors reported rapid absorption rate constants of 10.3±5.6 h−1, 9.1±3.5 h−1, and 6.8±1.2 h−1 (t½ of 4.03–6.11 min) for the three dosing groups, and noted that the intersubject variability was very high only in the case of the absorption rate constant.
Thorsson et al.[9] also studied 12 healthy subjects administered a single dose of FP 1000 µg via Diskhaler and repeated inhalations (1000 µg twice daily) for 7 days. They found that the average plasma concentration of FP was about 1.7 times higher after multiple inhalations than after a single dose, attributing the accumulation to a slow elimination half-life. They performed a simultaneous analysis of their single dose intravenous and single dose inhalation data with NONMEM, enabling accurate characterization of a three compartment model for FP pharmacokinetics, with distribution, intermediate and terminal t½ values of 2.7 min, 1.3 h and 14.4 h, respectively [9]. The volume of distribution at steady state was large (693–1045 l) and the absorption t½ for FP administered via Diskhaler was 1.6–2.5 h (much slower than that reported with a pMDI), and the authors estimated the systemic availability to be 13.6–18.0% of the nominal dose [9]. Although a prediction of the steady state concentrations was made based on the single-dose model, the authors did not attempt to model their repeat dose inhalation data [9]. Möllman et al.[10] have also studied the pharmacokinetics of single doses of FP 250, 500, 1000 and 3000 µg administered by inhalation. They reported an average absorption rate constant of 3.79±2.16 h−1 (absorption t½ = 11 min) and an elimination rate constant of 0.11±0.01 h−1 (elimination t½ = 6.3 h).
Falcoz et al. administered FP by both inhaled and intravenous routes to 12 healthy male volunteers in a two-way crossover study [23]. They performed a deconvolution analysis, which showed that the absorption of FP is initially rapid, then prolonged, consistent with a biexponential absorption process from the lung. The authors reported that 50% of the dose was absorbed from the lung in 1.1 h (95% CI 0.9–1.4 h), whereas the time for absorption of 90% of FP was 7.4 h (95% CI 5.3–10.4 h). A biexponential absorption process might therefore explain a short initial t½ and a longer mean absorption time [10].
Thus, the present study is consistent with several previous analyses of single dose data in showing a rapid absorption t½ (11.3 min) and long elimination t½ (7.76 h) after repeat-dose inhalation of FP.
This study characterized the kinetics of BUD and FP in healthy volunteers, but the data may not necessarily reflect the disposition characteristics of ICS in the airways and lungs of patients with asthma or chronic obstructive pulmonary disease [24]. Further studies should address the impact of lung disease on the variability in systemic absorption of BUD and FP via pMDI.
Acknowledgments
We thank Dr S. Edsbacker, Astra AB, Lund, Sweden and GlaxoWellcome Australia for the gifts of budesonide and fluticasone propionate, respectively.
References
- 1.Smith MJ, Hodson ME. Effects of long term inhaled high dose beclomethasone dipropionate on adrenal function. Thorax. 1983;38:676–681. doi: 10.1136/thx.38.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edsbacker S, Andersson KE, Ryrfeldt A. Nasal bioavailability and systemic effects of the glucocorticoid budesonide in man. Eur J Clin Pharmacol. 1995;29:477–481. doi: 10.1007/BF00613465. [DOI] [PubMed] [Google Scholar]
- 3.Chisholm D, Chalkley S. Cushing’s syndrome from an inhaled glucocorticoid. Med J Aust. 1994;161:232. [PubMed] [Google Scholar]
- 4.Donnelly R, Williams KM, Baker AB, Badcock CA, Day RO, Seale JP. Effects of budesonide and fluticasone on 24-hour plasma cortisol. A dose–response study. Am J Resp Crit Care Med. 1997;156:1746–1751. doi: 10.1164/ajrccm.156.6.9703003. [DOI] [PubMed] [Google Scholar]
- 5.Grahnen A, Eckernas RM, Brundin RM, Ling-Anderson A. An assessment of the systemic activity of single doses of inhaled fluticasone in healthy volunteers. Br J Clin Pharmacol. 1994;38:521–525. doi: 10.1111/j.1365-2125.1994.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seale JP, Donnelly R. Carticosteroid treatment of asthma: now at the crossroads. Resp Med. 1999;93:141–146. doi: 10.1016/s0954-6111(99)90306-2. [DOI] [PubMed] [Google Scholar]
- 7.Ryrfeldt A, Andersson P, Edsbacker S, Tonnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Resp Dis. 1982;122(Suppl.):86–95. [PubMed] [Google Scholar]
- 8.Mackie AE, Ventresca GP, Fuller RW, Bye A. Pharmacokinetics of intravenous fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:539–542. doi: 10.1046/j.1365-2125.1996.36110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorsson L, Dahlstrom K, Kallen A, Paulson J, Wiren JE. Br J Clin Pharmacol. Vol. 43. 1997. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects; pp. 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollmann H, Wagner M, Meibohm B, et al. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53:459–467. doi: 10.1007/s002280050407. 10.1007/s002280050407. [DOI] [PubMed] [Google Scholar]
- 11.Li YN, Tattam B, Brown KF, Seale JP. Determination of epimers 22R and 22S of budesonide in human plasma by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatog Biomed Applic. 1996;683:259–268. doi: 10.1016/0378-4347(96)00111-9. [DOI] [PubMed] [Google Scholar]
- 12.Li YN, Tattam BN, Brown KF, Seale JP. A sensitive method for the quantification of fluticasone propionate in human plasma by high-performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J Pharmac Biomed Analysis. 1997;16:447–452. doi: 10.1016/s0731-7085(97)00073-3. [DOI] [PubMed] [Google Scholar]
- 13.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C. The Art of Scientific Computing. 2. 16. Cambridge University Press; 1992. Integration of ordinary differential equations; pp. 707–752. [Google Scholar]
- 14.Billard V, Gambus PL, Barr J, et al. The pharmacokinetics of 8-methoxypsoralen following i.v. administration in humans. Br J Clin Pharmacol. 1995;40:347–360. doi: 10.1111/j.1365-2125.1995.tb04557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook RD, Weisberg S. Residuals and influence in regression. New York: Chapman & Hall; 1982. [Google Scholar]
- 16.Christensen R, Pearson LM, Johnson W. Case deletion diagnostics for mixed models. Technometrics. 1992;34:38–45. [Google Scholar]
- 17.Derendorf H, Hochhaus G, Meibohm B, Mollmann H, Barth J. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. J Allergy Clin Immunol. 1998;101:S440–S446. doi: 10.1016/s0091-6749(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 18.Rohatagi S, Kan S, Derendorf H. Non-compartmental analysis of pharmacokinetic data after multiple dose intravenous and oral administration. Pharmazie. 1997;52:529–532. [PubMed] [Google Scholar]
- 19.Thorsson L, Edsbacker S, Conradson TB. Lung deposition of budesonide from turbuhaler is twice that from a pressurised metered-dose inhaler P-MDI. Eur Resp J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 20.Ryrfeldt A, Edsbacker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther. 1884;35:525–530. doi: 10.1038/clpt.1984.71. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen S, Steffensen G, Ekman I, Tonnesson M, Borga O. Pharmacokinetics of budesonide in children with asthma. Eur J Clin Pharmacol. 1987;31:579–582. doi: 10.1007/BF00606634. [DOI] [PubMed] [Google Scholar]
- 22.Rohatagi S, Bye A, Falcoz C, et al. Dynamic modeling of cortisol reduction after inhaled administration of fluticasone propionate. J Clin Pharmacol. 1996;36:938–941. doi: 10.1002/j.1552-4604.1996.tb04761.x. [DOI] [PubMed] [Google Scholar]
- 23.Falcoz C, Brindley C, Mackie A, Bye A. Input rate into the systemic circulation of fluticasone propionate after a 1000 µg inhaled dose from the diskhaler. J Clin Pharmacol. 1998;8:927. [Google Scholar]
- 24.Hallett C. Cortiscosteroid treatment of asthma: now at the crossroads. Resp Med. 1999;93:292. doi: 10.1016/s0954-6111(99)90030-6. [DOI] [PubMed] [Google Scholar]
- 25.Beal SL, Sheiner LB. NONMEM user’s guide. San Francisco: University of California San Francisco; 1979. [Google Scholar]