Abstract
Aims
To determine the kinetics of the formation of 6β-naltrexol from naltrexone in human liver cytosol, and to investigate the role of potential inhibitors.
Methods
The kinetics of the formation of 6β-naltrexol from naltrexone were examined in eight human liver cytosol preparations using h.p.l.c. to quantify 6β-naltrexol and, the extent of inhibition of 6β-naltrexol formation was determined using chemical inhibitors. The formation of 6β-naltrexol and the back reaction of 6β-naltrexol to naltrexone were also examined in a microsomal preparation.
Results
The Vmax, Km and CLint values for the formation of 6β-naltrexol from naltrexone were in the ranges of 16–45 nmol mg−1 protein h−1, 17–53 µm and 0.3–2.2 ml h−1 mg−1 protein, respectively. The steroid hormones testosterone (Ki = 0.3 ± 0.1 µm) and dihydrotestosterone (Ki = 0.7 ± 0.4 µm) were the most potent competitive inhibitors of 6β-naltrexol formation, with naloxone, menadione and corticosterone also producing > 50% inhibition at a concentration of 100 µm. The opioid agonists morphine, oxycodone, oxymorphone and hydromorphone, and a range of benzodiazepines showed < 20% inhibition at 100 µm. In the microsomal preparation, there was no formation of naltrexone from 6β-naltrexol nor any formation of 6β-naltrexol from naltrexone.
Conclusions
The intersubject variability in the kinetic parameters of 6β-naltrexol formation could play a role in the efficacy of and patient compliance with naltrexone treatment. This variability could be due in part to a genetic polymorphism of the dihydrodiol dehydrogenase DD4, one of the enzymes reported to be responsible for the formation of 6β-naltrexol from naltrexone. DD4 also has hydroxysteroid dehydrogenase activity which could account for the potent inhibition by the steroid hormones testosterone and dihydrotestosterone. The clinical significance of the latter finding remains to be established.
Keywords: 6β-naltrexol, human liver cytosol, inhibition, kinetics, naltrexone
Introduction
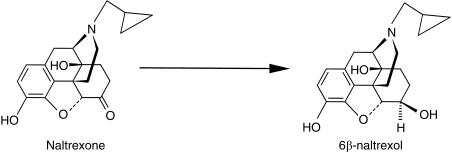
Naltrexone is an opioid antagonist used in the treatment of opioid and alcohol dependence. Compared with naloxone, naltrexone has a longer duration of action, allowing for once daily dosing. This long duration of action is considered to be due, in part, to its major human metabolite, 6β-naltrexol [1, 2] (Figure 1). Following an oral dose of naltrexone, 6β-naltrexol is present in much higher concentrations in plasma than the parent drug, and remains much longer in the systemic circulation due to its longer half-life of about 12 h compared with 4 h for naltrexone [3]. Although 6β-naltrexol has been known for many years to be quantitatively the major metabolite in humans [4], few studies have examined the enzyme(s) involved, and the kinetics of its formation. Ohara and coworkers, using purified enzyme preparations from autopsied human liver samples, showed stereospecific reduction of naltrexone to 6β-naltrexol by dihydrodiol dehydrogenases (DD1 pI 9.1; DD2 and DD4, pI 5.4), but not carbonyl reductase (EC 1.1.1.184), nor aldehyde reductase (EC 1.1.1.21) [5]. Using cloning techniques, DD4 was found to be identical to human 3α-hydroxysteroid dehydrogenase, which was identical to chlordecone reductase [6], but different from carbonyl reductase [7]. The family of dihydrodiol dehydrogenases (DDs 1–4) showed a broad range of substrate specificity in ketone reduction activity, indicating structurally distinct carbonyl-reducing enzymes in the liver [5]. However, in the above study, quantification of 6β-naltrexol was not performed, as formation was indirectly assessed by measuring the oxidation rate of NADPH at 340 nm, whilst reaction products were identified using thin layer chromatography by comparison with pure 6α-and 6β-naltrexol.
Figure 1.
Structural formulae of naltrexone and its main human metabolite 6β-naltrexol.
There are few drugs that undergo a similar metabolic pattern to naltrexone. The antipsychotic haloperidol has also been shown to be reduced by cytosolic carbonyl reductase, and to a lesser extent by DD1 and DD2 [5]. The reverse reaction (reduced haloperidol to haloperidol) has been shown to be carried out by microsomal cytochrome P450s [8], specifically CYP2D6 [9], and CYP3A4 [10, 11]. To date it has not been shown whether the reverse reaction of 6β-naltrexol to naltrexone can also occur in humans.
The aims of the current study were to (1) develop and validate an h.p.l.c. assay for 6β-naltrexol in human liver cytosol; (2) determine the enzyme kinetics for the formation of 6β-naltrexol from naltrexone in human liver cytosolic preparations; (3) characterize the possible enzyme(s) involved using chemical inhibitors, including likely concomitantly taken drugs, general reductase inhibitors, and some steroid compounds, and (4) determine the specificity of cytosolic compared to microsomal enzymes in the formation of 6β-naltrexol from naltrexone, and whether the reverse reaction (6β-naltrexol to naltrexone) occurs in microsomal preparations.
Methods
Chemicals
Naltrexone HCl, naloxone HCl, morphine HCl, oxycodone HCl, hydromorphone HCl, warfarin sodium, menadione sodium bisulphate, bovine serum albumin (fraction V), folin-ciocalteau reagent, DL-isocitric acid tri-sodium salt, isocitrate dehydrogenase and β-nicotinamide adenine dinucleotide phosphate (β-NADPH reduced form, and NADP type IV) were purchased from Sigma Aldrich (St Louis, Mo, USA). Racemic methadone was obtained from the National Institute on Drug Abuse (MD, USA), and oxymorphone HCl from Du Pont Pharmaceuticals (Wilmington, DE, USA). 6β-Naltrexol was synthesized as the free base in the Department of Chemistry, University of Adelaide by the method of Chatterjie et al.[12]. Its identity was confirmed by nuclear magnetic resonance, mass spectroscopy, infrared spectroscopy and thin layer chromatography. The yield was 73%, and purity was 97% in the β-form, and 3% in the α-form. Methanol was of h.p.l.c. grade (BDH Laboratory Supplies, Poole, UK). Other chemicals used were potassium dihydrogen orthophosphate (KH2PO4) (BDH Chemicals, Kilsyth, Australia) and triethylamine (TEA) (Prolabo, Paris, France). Additional compounds used in the inhibitor studies were haloperidol, chlorpromazine, finasteride and nicotine (all from Sigma Aldrich) and testosterone, dihydrotestosterone and corticosterone (gifts from Associate Professor Howard Morris, Department of Clinical Biochemistry, Institute of Medical and Veterinary Science, Adelaide, Australia). Temazepam, oxazepam and lorazepam were obtained from Wyeth Pharmaceuticals GmBH (Munster, Germany), flunitrazepam from Roche Products (Dee Why, Australia), diazepam was a gift from Professor John Miners, Flinders Medical Centre (Adelaide, Australia), and fluoxetine from Associate Professor Wayne Hooper, Department of Medicine, Royal Brisbane Hospital (Brisbane, Australia). Phenobarbitone sodium was purchased from Faulding Australia (Adelaide, Australia). All other chemicals and reagents were of analytical grade quality.
Preparation of cytosol
This study was approved by the Committee on the Ethics of Human Experimentation of the University of Adelaide and the Human Ethics Committee of the Royal Adelaide Hospital. Patients gave written informed consent for their liver tissue to be used following cholecystectomy or partial hepatectomy. The liver tissue samples used (HLS# 11, 15, 18, 19, 21, 22, 24 and 31) were from donors ranging in age from 41 to 73 years, of whom four were female, and four male. Pre-operative biochemistry and haematology levels were within the normal range for all patients, except patient #15 (serum alkaline phosphatase and alanine aminotransferase, four-and two-fold the upper reference limit, respectively); patient #19 (γ-glutamyl transferase three times the upper limit); patient #31 showed abnormal haematology with a decreased haemoglobin (4 g dl−1 below the male reference limit), and increased white cell and platelet counts (20% above normal). The kinetic parameters of 6β-naltrexol for these three patients were not dissimilar to the values from those patients who exhibited no biochemical or haematological abnormalities.
Cytosol was prepared as a by-product of the preparation of microsomes by differential centrifugation of liver homogenate [13]. Liver samples, cytosol and microsomes were stored at −80 °C until used. The cytosolic samples were analysed for protein concentration [14], and diluted in phosphate buffer to 0.5 mg protein ml−1 for use as stock for assays.
Cytosolic incubations
Incubations were performed in duplicate at 37 °C in a shaking water bath for 60 min. The incubates of 200 µl final volume contained 100 mm phosphate buffer (pH 7.4), 140 µm NADPH, substrate (naltrexone) in the concentration range 5–150 µm and 25 µg of cytosolic protein. The enzymatic formation of 6β-naltrexol was terminated by the addition of ice-cold methanol. The samples were then vortexed briefly, centrifuged for 10 min at 14000 rev min−1, and 100 µl of the supernatant was injected onto the h.p.l.c. system. The rate of formation of 6β-naltrexol from naltrexone (60 µm), was found to be linear up to 120 min and up to 100 µg cytosolic protein.
Microsomal incubations
Incubations were performed as above, and the incubates of 200 µl final volume contained 100 mm phosphate buffer (pH 7.4), NADPH-generating system (1 mm NADP, 1 unit ml−1 isocitrate dehydrogenase, 5 mm isocitric acid, and 5 mm MgCl2), substrate (naltrexone 15, 60 and 150 µm or 6β-naltrexol 20, 60, 150, 250, 500 and 1000 µm), and 25 µg of microsomal protein from one of the above livers (HLS #31).
6β-naltrexol quantification
6β-Naltrexol was quantified using a reversed phase h.p.l.c. system comprising an LC-6 A pump (Shimadzu, Kyoto, Japan) operating at a flow rate of 1.0 ml min−1, a Wisp 710B autoinjector (Waters, Milford, MA, USA), and a Radial-Pak Cartridge phenyl column packed with phenyl 5NVPH 4µ (Waters) which was inserted in a Radial Compression Module (RCM 8 × 10 mm), and operated at a pressure of approximately 2500 psi. A precolumn (Alltima C18 5 µm, Alltech Corporation, IL, USA) was preceded by a 4 µm inline filter. The mobile phase for optimal separation of analytes comprised 16% methanol, 0.2% triethylamine and 25 mm KH2PO4 with the final pH adjusted to 3.0 with orthophosphoric acid. Detection of analytes was achieved using an ultraviolet detector (Jasco UVIDEC-100, Tokyo, Japan) at 220 nm. Retention times for naltrexone and 6β-naltrexol, were 13 and 16 min, respectively. A Shimadzu C-R6A Integrator (Shimadzu Corporation) performed the integration of peak heights.
Quantification of 6β-naltrexol was performed with calibration curves consisting of eight standards over the concentration range 0.25–20 µm. Inter-assay precision was monitored with quality control (QC) samples prepared in duplicate at three concentrations: low (LQC 1.75 µm), medium (MQC 7.5 µm) and high (HQC 14 µm). Mean inter-assay precision (n = 8 for LQC and HQC, and n = 7 for MQC) values were 16.2%, 2.6%, and 3.1%, while mean inter-assay accuracy values (n = 8 for LQC and HQC, and n = 7 for MQC) were 90%, 98% and 96% of the absolute value for the LQC, MQC and HQC's, respectively. Similarly, mean intra-assay precision (n = 10) values were 14.4%, 1.2% and 1.2%, and mean accuracy values were 92%, 97% and 94% for the LQC, MQC and HQC, respectively. At the limit of quantification (0.25 µm), precision (n = 20) was 8.6%, with a mean absolute accuracy of 88%.
Inhibition studies with chemical inhibitors
Cytosolic preparations from three human liver samples (HLS# 11, 15 and 31) were used in triplicate to examine the inhibition of 6β-naltrexol formation. The naltrexone concentration for the initial inhibitor studies was 30 µm, the approximate Km value for the liver samples used. Inhibitors were incubated initially at 100 µm, and for those compounds that showed greater than 50% inhibition, Ki values were determined. This was performed by incubating the inhibitors at four different concentrations with three different substrate concentrations (25, 50 and 100 µm).
A variety of inhibitors was tested on the basis that they were general reductase inhibitors, or they were often coadministered to people for whom naltrexone was prescribed. Most inhibitors [nicotine, morphine, methadone, oxycodone, naloxone, hydromorphone and oxymorphone (pH 7.4), and warfarin (pH 9.0)] were dissolved in phosphate buffer. The benzodiazepines lorazepam, temazepam, oxazepam, diazepam and flunitrazepam as well as chlorpromazine, phenobarbitone, fluoxetine and menadione were dissolved in 5% methanol (final concentration 0.625% in assay). Haloperidol was dissolved in 25% methanol (final concentration in assay 3.125%). The steroid hormones testosterone, dihydrotestosterone and corticosterone and finasteride (a 5α-reductase inhibitor), were dissolved in 10% ethanol (1.25% in assay). Incubations containing equivalent amounts of diluent were always used as controls and, in addition, control incubations containing inhibitors alone were used to confirm that the inhibitors did not produce any chromatographic peaks that could interfere with the quantification of 6β-naltrexol. Assay conditions for the inhibitor studies did not differ from those of the kinetic study.
Data analysis
The rates of formation (V) of 6β-naltrexol from the substrate naltrexone concentration (C) were expressed as nmol mg−1 protein h−1, and Eadie-Hofstee (V/Substrate concentration vs V) plots were constructed. One-enzyme and two-enzyme Michaelis-Menten equations, with weighting 1/y, were fitted to the data using nonlinear least-squares regression analysis (Regression, Blackwell Scientific Publications, Oxford, UK). In all cases the one-enzyme fit was substantially superior to the two-enzyme model. Intrinsic clearance (CLint) was calculated as Vmax/Km. Inhibition was expressed as mean ± s.d., and Ki values for those inhibitors tested were determined by fitting different types of inhibitor models competitive, noncompetitive and uncompetitive to the data.
The choice of model was based on visual inspection of the goodness of fit of the observed data to those predicted, a significant reduction in the weighted sum of squared deviations, and random distribution of the scatter of observed data points about the fitted curve [15]. Paired t-tests comparing 6β-naltrexol formation in the presence of each inhibitor to the uninhibited formation were performed to determine if the differences in formation were significant. All data are expressed as mean ± s.d..
Results
Kinetics of formation of 6β-naltrexol
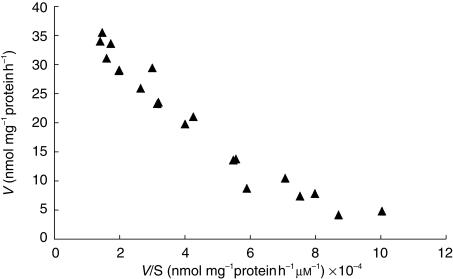
Eadie-Hofstee plots were found to be linear (Figure 2) and a single enzyme Michaelis-Menten kinetic equation was found to adequately fit the data.Table 1 shows the individual Vmax, Km, and CLint values for the formation of 6β-naltrexol from naltrexone for the eight human liver cytosol preparations. The variability in Vmax was 2.9-fold, Km 3.2-fold, and intrinsic clearance 7.7-fold.
Figure 2.
Eadie-Hofstee representation of the formation of 6β-naltrexol from naltrexone in human liver cytosol 31.
Table 1.
Enzyme kinetics of the formation of 6β-naltrexol from naltrexone using human liver cytosol preparations from eight patients.
| Human liver sample | Vmax (nmol mg−1protein h−1) | Km (µm) | CLint⋆ (ml h−1mg−1 protein) |
|---|---|---|---|
| 11 | 30.9 | 21.6 | 1.43 |
| 15 | 29.3 | 17.8 | 1.65 |
| 18 | 16.8 | 52.5 | 0.32 |
| 19 | 16.7 | 19.2 | 0.87 |
| 21 | 15.8 | 55.1 | 0.29 |
| 22 | 38.2 | 17.1 | 2.23 |
| 24 | 18.9 | 48.5 | 0.39 |
| 31 | 45.6 | 43.3 | 1.05 |
| Mean | 26.5 | 34.4 | 1.03 |
| s.d. | 11.3 | 16.9 | 0.70 |
| % CV | 42.6 | 49.1 | 70.0 |
CLint=Vmax/Km.
There was no formation of 6β-naltrexol from naltrexone in a microsome preparation, nor was there any naltrexone produced when 6β-naltrexol was incubated with microsomes and an NADPH-generating system.
Inhibition with chemical inhibitors
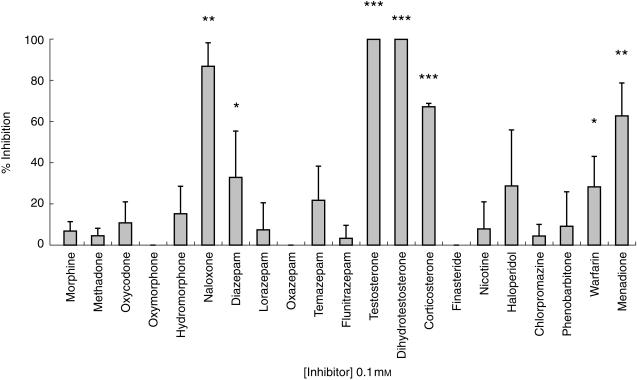
Figure 3 summarizes the data using 100 µm inhibitor concentrations. Naloxone, menadione, testosterone, dihydrotestosterone and corticosterone produced greater than 50% inhibition of 6β-naltrexol formation. Nicotine, morphine, methadone, oxycodone, oxymorphone, hydromorphone, lorazepam, flunitrazepam, oxazepam, chlorpromazine, phenobarbitone and finasteride produced less than 20% inhibition. Warfarin, haloperidol, diazepam and temazepam inhibition values were between 20 and 40%. Fluoxetine cochromatographed with naltrexone and 6β-naltrexol, and therefore its inhibition properties, if any, could not be tested in this system. At a range of concentrations, the steroids testosterone, dihydrotestosterone and corticosterone all showed concentration-dependent increases in inhibition of 6β-naltrexol formation. The competitive inhibition model was found to best describe the data, and Ki values for the most potent inhibitors (naloxone, corticosterone, menadione, testosterone and dihydrotestosterone) are shown in Table 2.
Figure 3.
The effect of chemical inhibitors (100 µm) as grouped into opioids (morphine to naloxone), benzodiazepines (diazepam to flunitrazepam), steroids (testosterone to corticosterone) and miscellaneous (finasteride to menadione), on the formation of 6β-naltrexol from naltrexone by human liver cytosolic enzymes prepared from three different livers. Values represent mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control incubations.
Table 2.
Ki values for the inhibition of 6β-naltrexol formation from naltrexone in human liver cytosol preparations from three different livers, determined from a competitive inhibition model. Values represent mean ± s.d.
| Inhibitor | Ki (µm) |
|---|---|
| Naloxone | 19.9 ± 5.3 |
| Menadione | 5.6 ± 2.8 |
| Corticosterone | 10.5 ± 4.7 |
| Testosterone | 0.28 ± 0.14 |
| Dihydrotestosterone | 0.73 ± 0.38 |
Discussion
This study showed that the hepatic enzymatic formation of 6β-naltrexol from naltrexone in human liver was confined to the cytosol and was not present in the microsomal fraction tested, exhibited considerable intersubject variability, and that the enzyme(s) involved could be inhibited by a number of compounds. The assay used in the current study to quantify the 6β-naltrexol in human liver cytosol preparations was shown to be both simple, precise and accurate. Unlike assays of naltrexone and 6β-naltrexol in plasma and urine [16, 17], no extraction was necessary, removing the need for an internal standard.
There was considerable intersubject variability in both the Vmax (2.9-fold) and Km (3.2-fold) values in human liver cytosol. Intrinsic clearance (CLint) values, which are suggestive of a drug of high hepatic extraction, showed even larger interindividual variation (7.7-fold) than Vmax or Km. Recently, Kume and coworkers demonstrated a variant allele of the DD4 enzyme (DD4(S145C/L311V)), with approximately one third the wild type catalytic activity towards naltrexone [18]. The authors suggest that this could account for the intersubject variabilities in Km and Kcat values found in an earlier study [5]. Such differences in metabolism between individuals could result in considerable variability in plasma and brain naltrexone and 6β-naltrexol concentrations.
Whereas the long duration of action of naltrexone has been attributed, in part, to persistence of its metabolite 6β-naltrexol [1, 2], it is not known whether the lack of positive outcomes in some patients [19], could be due to low circulating concentrations of 6β-naltrexol. King and coworkers showed that at 3 h post-naltrexone (50 mg orally), urine concentrations of 6β-naltrexol were up to 10 times higher than those of naltrexone [17]. Furthermore those subjects who reported subjective side-effects such as nausea, headache, anxiety and spontaneous erection had higher urine concentrations of 6β-naltrexol than the subjects who were free from these side-effects [17]. Therefore, patient compliance and in particular willingness to stay on therapy could be related to plasma 6β-naltrexol concentrations. Hence, knowledge of the factors causing variability in the formation of 6β-naltrexol could be of importance for individualizing therapy.
Inhibition of the formation of 6β-naltrexol by menadione is consistent with the involvement of ketone reductase in this reaction [20]. Additionally, 6β-naltrexol formation was not detected in the microsomal fraction, suggesting a lack of involvement of cytochrome P450 enzymes. The antipsychotic drug haloperidol is reduced in vivo by cytosolic ketone reductase [21]. The reduced metabolite can be converted back to the parent compound by microsomal cytochrome P450 enzymes [9]. In the present study, there was no back-conversion of 6β-naltrexol to naltrexone in the microsomal preparation tested. The conditions used were similar to those used by Pan and colleagues, who showed that CYP3A4 mediated the formation of haloperidol from reduced haloperidol with a Km of 51–59 µm and a Vmax of 190–334 pmol mg−1 min−1[11].
In order to further characterize the enzymes responsible for the formation of 6β-naltrexol from naltrexone, inhibitor studies were conducted. The androgenic steroid testosterone, and its major metabolite dihydrotestosterone were very potent inhibitors, with mean Ki values of 280 nm and 730 nm, respectively. The Ki of testosterone for the inhibition of 6β-naltrexol formation was 280 nm, which is approximately 10-fold the upper limit of normal circulating plasma concentrations in males. Therefore, this interaction is unlikely to be of clinical significance. However, it is reasonable to postulate that since these steroids are potent inhibitors of 6β-naltrexol formation, the reverse may also occur, such that long-term naltrexone use may have deleterious effects on steroid metabolism. Further work is needed to test this hypothesis, and to determine the effect of other steroid compounds that are present in plasma at relatively high concentrations.
In contrast to testosterone, finasteride, an inhibitor of 5α-reductase, the enzyme responsible for the formation of dihydrotestosterone from testosterone in vivo, did not affect the formation of 6β-naltrexol. The latter finding was anticipated, since 5α-reductase is a microsomal enzyme. However, steroid hormones are metabolized by a number of different enzyme systems, both cytosolic and microsomal, including reductases, hydroxysteroid dehydrogenases and the cytochrome P450 monooxygenases [22]. One such cytosolic enzyme (17β-hydroxysteroid dehydrogenase type I), has been shown to contain tyrosine and lysine residues which are found at the active sites of bacterial 3α,20β hydroxysteroid dehydrogenase, with similar secondary structural folds [23]. Although this enzyme is substantially more reactive towards oestradiol than testosterone [24], the dihydrodiol dehydrogenase responsible for the reduction of naltrexone to 6β-naltrexol may have sufficient homology with this and other forms of 17β-hydroxysteroid dehydrogenase to be subject to the potent inhibition by testosterone and dihydrotestosterone seen in the present study. Corticosterone, metabolized predominantly by adrenal microsomal 18-hydroxylase, was also a moderately potent inhibitor of 6β-naltrexol formation, but the significance of this is as yet unknown. The effects of other steroid compounds, including the components of oral contraceptive preparations and hormone replacement therapies, on this reaction have yet to be tested.
The inhibition of 6β-naltrexol formation by naloxone was not unexpected due to the similarity between the structures of naloxone (which also undergoes reduction) and naltrexone. Because of weak or absent inhibition of 6β-naltrexol formation by nicotine, the benzodiazepines (with the possible exception of diazepam), and other opioid compounds, there seems little likelihood of drug/drug interactions occuring in vivo between naltrexone and these drugs. Haloperidol did not produce significant inhibition of 6β-naltrexol formation tested in the present study at 100 µm, but this concentration is significantly lower than the reported Km of haloperidol of 0.5–0.6 mm[21].
In summary, the hepatic cytosolic formation of the pharmacologically active 6β-naltrexol from naltrexone showed considerable interpatient variability which could be a determinant of the efficacy of naltrexone in vivo. The potent inhibition of the reaction by androgenic steroids is unlikely to be of clinical significance. While naltrexone metabolism appears to be unaffected by the presence of drugs likely to be coadministered, pharmacokinetic and possible pharmacogenetic factors affecting the formation and elimination of 6β-naltrexol could influence the willingness of patients to remain on naltrexone treatment.
Acknowledgments
The authors would like to thank Associate Professor David Ward, and Dr Marc Kimber of the Department of Chemistry, University of Adelaide for their help in the synthesis of 6β-naltrexol. Susan Porter was a recipient of a Dawes Postgraduate Scholarship from the Royal Adelaide Hospital, Adelaide, Australia. Funding for this research was provided by the Faculty of Health Sciences of the University of Adelaide, and the Royal Adelaide Hospital Research Committee.
References
- 1.Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: Disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;30:315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- 2.Cone EJ, Gorodetzky CW, Yeh SY. The urinary excretion profile of naltrexone and metabolites in man. Drug Metab Dispos. 1974;2:506–512. [PubMed] [Google Scholar]
- 3.Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- 4.Dayton HE, Inturrisi CE. The urinary excretion profiles of naltrexone in man, monkey, rabbit, and rat. Drug Metab Dispos. 1976;4:474–478. [PubMed] [Google Scholar]
- 5.Ohara H, Miyabe Y, Deyashiki Y, Matsuura K, Hara A. Reduction of drug ketones by dihydrodiol dehydrogenases, carbonyl reductase and aldehyde reductase of human liver. Biochem Pharmacol. 1995;50:221–227. doi: 10.1016/0006-2952(95)00124-i. 10.1016/0006-2952(95)00124-i. [DOI] [PubMed] [Google Scholar]
- 6.Binstock JM, Iyer RB, Hamby CV, et al. Human hepatic 3α-hydroxysteroid dehydrogenase: possible identity with human hepatic chlordecone reductase. Biochem Biophys Res Comm. 1992;187:760–766. doi: 10.1016/0006-291x(92)91260-w. [DOI] [PubMed] [Google Scholar]
- 7.Wermuth B, Bohren KM, Heinemann G, von Wartburg J-P, Gabbay KH. Human carbonyl reductase: nucleotide sequence analysis of a cDNA and amino acid sequence of the encoded protein. J Biol Chem. 1988;263:16185–16188. [PubMed] [Google Scholar]
- 8.Korpi ER, Costakos DT, Wyatt RJ. Interconversions of haloperidol and reduced haloperidol in guinea pig and rat liver microsomes. Biochem Pharmacol. 1985;34:2923–2927. doi: 10.1016/0006-2952(85)90017-6. [DOI] [PubMed] [Google Scholar]
- 9.Tyndale RF, Kalow W, Inaba T. Oxidation of reduced haloperidol to haloperidol: involvement of human P450 2D6 (sparteine/debrisoquine monooxygenase) Br J Clin Pharmacol. 1991;31:655–660. doi: 10.1111/j.1365-2125.1991.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo S, Odomi M. Involvement of human cytochrome P450 in reduced haloperidol oxidation. Eur J Clin Pharmacol. 1998;54:253–259. doi: 10.1007/s002280050455. 10.1007/s002280050455. [DOI] [PubMed] [Google Scholar]
- 11.Pan LP, De Vriendt C, Belpaire F. In-vitro characterization of the cytochrome P450 isoenzymes involved in the back oxidation and N-dealkylation of reduced haloperidol. Pharmacogenetics. 1998;8:383–389. doi: 10.1097/00008571-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjie N, Inturrisi CE, Dayton HB, Blumberg H. Stereospecific synthesis of the 6β-hydroxy metabolites of naltrexone and naloxone. J Med Chem. 1975;18:490–492. doi: 10.1021/jm00239a010. [DOI] [PubMed] [Google Scholar]
- 13.Zanger UM, Vilbois F, Hardwick JP, Meyer UA. Absence of hepatic cytochrome P450bufI causes genetically deficient debrisoquine oxidation in man. Biochem. 1988;27:5447–5454. doi: 10.1021/bi00415a010. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Mikus G, Somogyi AA, Bochner F, Eichelbaum M. Codeine O-demethylation: rat strain differences and the effects of inhibitors. Biochem Pharmacol. 1991;41:757–762. doi: 10.1016/0006-2952(91)90077-i. [DOI] [PubMed] [Google Scholar]
- 16.Davidson AF, Emm TA, Pieniaszek HJ Jr. Determination of naltrexone and its major metabolite, β-naltrexol, in human plasma using liquid chromatography with electrochemical detection. J Pharm Biomed Anal. 1996;14:1717–1725. doi: 10.1016/0731-7085(96)01794-3. 10.1016/0731-7085(96)01794-3. [DOI] [PubMed] [Google Scholar]
- 17.King AC, Volpicelli JR, Gunduz M, O'brien CP, Kreek MJ. Naltrexone biotransformation and incidence of subjective side effects – a preliminary study. Alcohol Clin Exp Res. 1997;21:906–909. [PubMed] [Google Scholar]
- 18.Kume T, Iwasa H, Shiraishi H, et al. Characterisation of a novel variant (S145C/L311V) of 3α-hydroxysteroid/dihydrodiol dehydrogenase in human liver. Pharmacogenetics. 1999;9:763–771. [PubMed] [Google Scholar]
- 19.O'malley SS, Jaffe AJ, Chang G, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- 20.Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem. 1981;256:1206–1213. [PubMed] [Google Scholar]
- 21.Inaba T, Kovacs J. Haloperidol reductase in human and guinea pig livers. Drug Metab Dispos. 1989;17:330–333. [PubMed] [Google Scholar]
- 22.Schanzer W. Metabolism of anabolic androgenic steroids. Clin Chem. 1996;42:1001–1020. [PubMed] [Google Scholar]
- 23.Labrie F, Luu-The V, Lin S-X, et al. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 24.Blomquist CH, Bealka DG, Hensleigh HC, Tagatz GE. A comparison of 17β-hydroxysteroid oxidoreductase Type 1 and Type 2 activity of cytosol and microsomes from human term placenta, ovarian stroma and granulosa-luteal cells. J Steroid Biochem Mol Biol. 1994;49:183–189. doi: 10.1016/0960-0760(94)90009-4. [DOI] [PubMed] [Google Scholar]