Abstract
Aims
To determine whether repeated once daily administration of grapefruit juice altered the pharmacokinetics or pharmacodynamics of the calcium antagonist amlodipine.
Methods
The effects of grapefruit juice on the pharmacokinetics and pharmacodynamics of oral and intravenous amlodipine were assessed in 20 healthy men in a placebo-controlled, open, randomized, four-way crossover study using single doses of amlodipine 10 mg. For 9 days beginning with the day of administration of amlodipine, grapefruit juice (or water control) was given once daily, and blood samples, blood pressure and heart rate measures were obtained. Plasma concentrations of amlodipine and its enantiomers were determined in separate assays by GC-ECD.
Results
Oral amlodipine had high systemic availability (grapefruit juice: 88%; water: 81%). Pharmacokinetic parameters of racemic amlodipine (AUC, Cmax, tmax, and kel) were not markedly changed with grapefruit juice coadministration. Total plasma clearance and volume of distribution, calculated after intravenous amlodipine, were essentially unchanged by grapefruit juice (CL 6.65 ml min−1 kg−1, juice vs 6.93 ml min−1 kg−1, water; Vdss 22.7 l kg−1, juice vs 21.0 l kg−1, water). Grapefruit juice coadministration did not greatly alter the stereoselectivity in amlodipine oral or intravenous kinetics. The sum of S(–) and R(+) enantiomer concentrations correlated well with total racemic amlodipine concentration (r2 = 0.957; P = 0.0001). Coadministration of grapefruit juice with either route of amlodipine administration did not significantly alter blood pressure changes vs control.
Conclusions
Grapefruit juice has no appreciable effect on amlodipine pharmacodynamics or pharmacokinetics, including its stereoselective kinetics. Bioavailability enhancement by grapefruit juice, noted with other dihydropyridine calcium antagonists, does not occur with amlodipine. Once daily grapefruit juice administration with usual oral doses of amlodipine is unlikely to alter the profile of response in clinical practice.
Keywords: amlodipine, enantiomers, grapefruit juice, pharmacokinetics
Introduction
Grapefruit juice is known to interact with drugs in many different therapeutic classes [1–4], including certain dihydropyridine calcium channel antagonists. Of the dihydropyridine calcium channel antagonists evaluated to date for enhanced oral bioavailability by grapefruit juice, nisoldipine and felodipine are affected to the greatest extent, with mean increases in AUC of 2-and 3-fold, respectively [1, 2, 5].
Amlodipine, prescribed in the management of angina and hypertension, has a pharmacokinetic profile that differs from other dihydropyridines [6, 7]. Specifically, amlodipine has a lower hepatic extraction ratio, hence its higher oral bioavailability. With its high tissue affinity, oral amlodipine is taken up by hepatic tissue and then redistributed back into the systemic circulation [8, 9]. These properties result in a later time to peak plasma concentration and a longer plasma elimination half-life compared with other dihydropyridines. It undergoes extensive oxidative metabolism to pyridine derivatives [10–12].
The purpose of this study was to evaluate the potential interaction of grapefruit juice with amlodipine by studying pharmacokinetics and pharmacodynamics after both oral and intravenous administration. Since most dihydropyridines, including amlodipine, contain one or more chiral centres and may display different pharmacologic activity among the enantiomers [13], the stereoselective disposition of amlodipine when coadministered with grapefruit juice was also investigated.
Methods
Study population
Twenty males (mean age, 31.5 years; range, 20–45 years), 2 White and 18 Hispanic, participated in the study. Body weight was within 10% of ideal (mean, 70 kg; range, 62–85 kg). Each subject was judged to be healthy from the results of a detailed medical history, physical examination, 12-lead resting ECG, serum biochemistry, haematology and routine urinalysis. Each individual provided written informed consent for the study, that was approved by the committee on human research, the Independent Investigational Review Board Inc. Plantation, Florida.
Experimental protocol
The design was a placebo (water)-controlled, open, randomized, 4-way crossover study. Subjects received on separate occasions single doses of either racemic amlodipine besylate 10 mg immediate release tablet, or intravenous amlodipine maleate; amlodipine was given with and without grapefruit juice. The oral formulation, Norvasc™ (Pfizer, Inc., Groton CT), was supplied by Pfizer. The same brand (Old South; Lykes Pasco, Dade City FL) and lot number of frozen concentrate white grapefruit juice was used throughout the study.
Each subject was to receive all four treatments (A-D), with at least 2 weeks intervening: A – intravenous amlodipine and grapefruit juice; B – intravenous amlodipine and water; C – oral amlodipine and water; D – oral amlodipine and grapefruit juice. Subject assignment to one of four treatment sequence groups was through computer-generated randomization.
Subjects fasted for 8 h prior to and 4 h following drug administration at approximately 08.00 h. The water or grapefruit juice (240 ml) was taken at the same time as the oral medication or just prior to the commencement of the intravenous amlodipine infusion. On each of the 8 days following amlodipine administration, 200 ml of grapefruit juice (treatments A and D) or water (treatments B and C) were ingested with breakfast.
Blood was collected in heparinized tubes just prior to oral and intravenous dosing, at the end of the 10 min infusion, and at the following times after dosing: 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 10, 12, 14, 16, 24, 36, 48, 72, 96, 120, 144, 168, 192 and 216 h. Additional blood samples were collected midway through the infusion (−5 min), at the completion of the infusion (0 min), and at 5 and 15 min after the end of the infusion. During the first 4 h after amlodipine dosing, subjects refrained from drinking caffeinated beverages and from lying down, except for vital sign measurements.
Pharmacodynamic measures were supine and standing blood pressure and heart rate. They were measured in duplicate just prior to oral dosing or at the end of the infusion of amlodipine and at 5 min, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 24, 36, 48, 72, 96, 120, 144, 168, 192 and 216 h after amlodipine administration.
Sample analysis
Plasma samples, obtained by centrifugation, were stored at −20 °C until analysis. Unresolved amlodipine and the enantiomers of amlodipine were determined in separate assays, both using capillary gas chromatography with electron capture detection (GC-ECD).
The analysis of unresolved amlodipine in plasma has been described elsewhere [14]. Briefly, amlodipine samples were prepared as follows. Plasma (1 ml), containing internal standard UK52829–42 (Pfizer, Groton CT), was buffered (∼pH 9, borate buffer) and then extracted with methyl t-butyl ether (MTBE). The organic phase was back-extracted into citric acid. The organic layer was discarded and the aqueous layer was made alkaline, derivatized with trimethylacetyl chloride (TMA), and then underwent a second extraction. The organic solvent was evaporated and the residue reconstituted with ethyl acetate for subsequent GC analysis.
For enantiomers, the sample preparation procedure differed primarily in that the second extraction preceded the derivatization with α-methoxy-α-trifluoromethyl-phenylacetyl chloride in the presence of dimethylaminopyridine. Silylated glassware was used.
Amlodipine and the enantiomers were analysed in separate assays using a gas chromatograph (Hewlett-Packard model 5890) equipped with an 63Ni EC detector and a DB-5 (J & W Scientific, Folsom CA) capillary column (13 m × 0.32 mm i.d., 0.25 µm film thickness).
The limits of quantification were 0.2 ng ml−1 of plasma for amlodipine and 0.5 ng ml−1 for R(+)-amlodipine and S(–)-amlodipine. Correlation coefficients of 0.9980 or better were achieved from the regression equations of the six or seven concentration point calibration curves. All amlodipine quality control samples were within 10% of the nominal concentration. The LOQ pool of samples for R(+)-amlodipine, analysed in seven separate runs, displayed a difference of 7.5% difference from theoretical, while that for S(–)-amlodipine was 8.1%; all other quality control samples displayed accuracy within 16.6% of theoretical. Intraday and interday coefficients of variation for amlodipine were 11.6% (at 0.2 ng ml−1) or better; precision for the enantiomers ranged from 4.1 to 9.1%.
Data analysis and statistics
Amlodipine pharmacokinetic parameters were determined as follows. The values of maximum plasma concentration (Cmax) of amlodipine and its enantiomers and the time of first occurrence of Cmax for amlodipine (tmax) were obtained directly from the observed data. The Cmax values were natural log transformed prior to analysis.
The terminal phase elimination rate constant kel was estimated by least squares regression analysis during the terminal log-linear phase of the concentration-time curve. It was used to determine terminal half-life (t½ = ln 2/kel). Absolute systemic bioavailability (F) of the oral tablet formulation was calculated by the ratio of AUCoral to AUCintravenous. AUC(0,∞) was estimated as the sum of AUC(0,τ) and the plasma concentration at time τ estimated from the aforementioned regression, divided by kel, where τ is the latest blood sampling time which had a quantifiable concentration. Total plasma clearance was estimated as the ratio of the dose and AUCintravenous. Finally, Vdss, the steady state volume of distribution, was calculated as the product of dose and AUMC(0,∞)/[AUC(0,∞)]2 following intravenous dosing, where AUMC(0,∞) was the area under the first moment curve.
For amlodipine R(+) and S(–) enantiomers, the following pharmacokinetic parameters were evaluated: AUC(0,96 h) and Cmax following intravenous administration of amlodipine, and AUC(0,24 h) after oral dosing. Following oral amlodipine, the enantiomer plasma concentration observed at 8 h (C8) was statistically evaluated, rather than a Cmax value for the enantiomers. For each of these four pharmacokinetic parameters, the S(–)/R(+) ratios were calculated as indices of stereoselectivity in amlodipine pharmacokinetics.
For haemodynamic measures, area under the effect-time curves [AUEC(0,24 h)] were calculated for individual subjects using the linear trapezoidal method. Maximum changes in systolic blood pressure, diastolic blood pressure, and heart rate were also tabulated for individual subject.
These areas and maximum AUEC(0,24 h) values, as well as that for the pharmacokinetic parameters of AUC(0,∞), Cmax and kel, were statistically evaluated using an analysis of variance (anova) model in SAS. The anova model contained sequence, subject within sequence, period, treatment, and treatment × period effect. Adjusted mean differences between treatment effects were determined, as well as the sequence effect and the period and treatment effects. The pharmacokinetic parameters of F, tmax(oral), CLtotal and Vdss only involved two treatments; consequently, the anova model for their analysis did not include sequence or period effects. All testing was conducted at a 5% level of significance.
Results
Pharmacokinetics of racemic amlodipine
Grapefruit juice did not significantly alter the systemic availability of amlodipine (Table 1). The mean systemic availability of oral amlodipine was 81% with water (control) and 88% with juice. The ratio of juice/water point estimate and 95% confidence interval was 107.8% (95% CI: 98.4, 118.4), which was not statistically significant.
Table 1.
Effect of grapefruit juice coadministration on the pharmacokinetics of amlodipine.
| Pharmacokinetic variable for amlodipine | Amlodipine + grapefruit juice | Amlodipine + water (control) | Ratio juice/water Point estimate (%) and 95% CI |
|---|---|---|---|
| Oral amlodipine | |||
| Cmax (ng ml−1) | 6.2 ± 1.1 | 5.8 ± 1.1 | 107.1 (91.7, 125.0) |
| AUC(0,∞) (ng ml−1 h) | 315 ± 76 | 293 ± 58 | 107.8 (99.7, 116.5) |
| tmax (h after dose) | 7.6 ± 1.4 | 7.9 ± 1.7 | 0.2 (−0.9, 0.5) |
| kel (h−1) | 0.0166 ± 0.0034 | 0.0177 ± 0.0034 | −0.0010 (–0.0026, 0.0007) |
| F | 0.88 ± 0.15 | 0.81 ± 0.14 | 107.9 (98.4, 118.4) |
| Intravenous amlodipine | |||
| Cmax (ng ml−1) | 30.1 ± 12.1 | 34.8 ± 13.7 | 85.9 (73.6, 100.3) |
| AUC(0,∞) (ng ml−1 h) | 374 ± 88 | 358 ± 88 | 102.5 (94.8, 110.9) |
| kel (h−1) | 0.0161 ± 0.0029 | 0.0181 ± 0.0041 | −0.0022 (–0.0039, −0.0005)⋆ |
| CL (ml min−1 kg−1) | 6.65 ± 1.99 | 6.93 ± 1.72 | –0.2 (–1.1, 0.8) |
| Vdss (l kg−1) | 22.7 ± 5.1 | 21.0 ± 3.8 | 1.9 (–0.7, 4.5) |
Mean ± s.d. values. Point estimates (and 95% CI) are either the ratio of the adjusted geometric means of treatments (Cmax, AUC), arithmetic means (F), or the difference in the adjusted arithmetic means of treatment (all other kinetic variables).
P < 0.05.
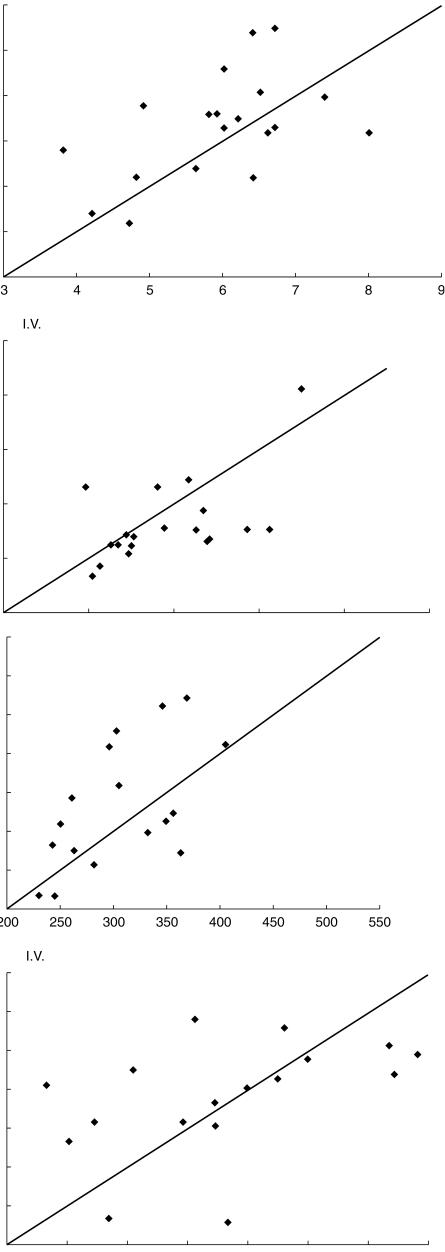
Mean amlodipine pharmacokinetic parameters of AUC, Cmax, tmax, and kel during grapefruit juice compared with water are summarized in Table 1. For both routes of administration, except for kel in the intravenous study, these parameters were not significantly changed with grapefruit juice coadministration. As shown in Figure 1a for Cmax, and Figure 1b for AUC(0,∞), paired treatment data for the individual subjects were distributed approximately equally about the line of identity. The AUC(0,∞) ratio of grapefruit juice to water for oral amlodipine was 107.8% (95%CI: 99.7–116.5); the Cmax ratio was 107.1% (95% CI: 91.7–125). The confidence intervals for AUC(0,∞) and Cmax for oral, and intravenous amlodipine did not achieve significance (Table 1). The mean tmax value following oral administration with juice (7.6 h) was similar to that with water (7.9 h).
Figure 1.
a) Relationship between amlodipine Cmax (ng ml−1) following consumption of grapefruit juice and water in 20 healthy males after oral (upper panel) or intravenous (lower panel) drug administration. b) Relationship between amlodipine AUC(0,∞) (ng ml−1 h) following consumption of grapefruit juice and water in 20 healthy males after oral (upper panel) or intravenous (lower panel) amlodipine.
The values of total plasma clearance and volume of distribution, calculated for intravenous amlodipine, were essentially unchanged by grapefruit juice (Table 1). Mean total clearance was 6.65 ml min−1 kg−1 with grapefruit juice and 6.93 ml min−1 kg−1 with placebo. Mean Vdss was 22.7 l kg−1 with grapefruit juice and 21.0 l kg−1 with placebo.
Plasma elimination half-life was calculated from kel after both routes of administration. After all four treatments, mean kel values ranged from 0.0161 h−1−0.018 h−1, corresponding to half-lives of 43.1 h to 38.3 h. For the intravenous route, the kel value was slightly smaller for amlodipine with grapefruit juice compared with water; this difference was statistically significant. For the oral route, kel after grapefruit juice was not significantly different than after water.
Sequence effects, period effects, and treatment by period effects were not significant for Cmax and kel; AUC(0,∞) showed no significant sequence effects but statistically significant period and treatment by period effects (P = 0.0104 and P = 0.0053, respectively). Taken alone, this significant period effect does not alter conclusions regarding treatment comparisons since periods and treatments can be estimated independently in this design.
Pharmacokinetics of amlodipine enantiomers
The analyses for AUC and peak plasma concentration of amlodipine enantiomers were performed on the subset of subjects with complete data for each route of administration. The numbers of subjects used in the pharmacokinetic parameter summary statistics are listed, along with the corresponding mean ± s.d. values, in Table 2.
Table 2.
Effect of grapefruit juice coadministration on the stereoselective pharmacokinetics of amlodipine.
| Pharmacokinetic variable | Enantiomer | Amlodipine + grapefruit juice | Amlodipine + water (control) | Ratio juice/water Point estimate (%) and 95% CI |
|---|---|---|---|---|
| Oral amlodipine | ||||
| AUC(0,24h) (ng ml−1 h) | ||||
| (n = 10) | S(–) | 44.9 ± 8.3 | 42.7 ± 7.3 | 102.9 (96.6, 109.6) |
| R(+) | 41.2 ± 10.6 | 40.9 ± 7.7 | 99.2 (93.1, 105.8) | |
| S/R | 1.08 | 1.04 | 103.7 (99.2, 108.3) | |
| C8 (ng ml−1) | ||||
| (n = 14) | S(–) | 2.7 ± 0.4 | 2.6 ± 0.4 | 103.7 (96.4, 111.5) |
| R(+) | 2.5 ± 0.4 | 2.5 ± 0.5 | 101.1 (94.7, 108.0) | |
| S/R | 1.06 | 1.03 | 102.5 (100.2, 104.9)⋆ | |
| Intravenous amlodipine | ||||
| AUC(0,96h) (ng ml−1 h) | ||||
| (n = 15) | S(–) | 138.9 ± 35.3 | 137.0 ± 37.6 | 100.2 (88.2, 113.8) |
| R(+) | 131.4 ± 33.8 | 133.2 ± 37.6 | 98.1 (86.7, 110.9) | |
| S/R | 1.06 | 1.03 | 102.2 (95.9, 108.8) | |
| Cmax (ng ml−1) | ||||
| (n = 11) | S(–) | 12.4 ± 3.4 | 16.7 ± 6.7 | 74.4 (57.8, 95.6)⋆ |
| R(+) | 13.0 ± 3.6 | 17.2 ± 7.0 | 75.6 (58.8, 97.3)⋆ | |
| S/R | 0.95 | 0.97 | 98.3 (94.4, 102.4) | |
For subjects (n) with complete data for both enantiomers (i.e. after both routes and with both juice and control treatments). S/R ratios are geometric means; all others are arithmetic means ± s.d. C8 is plasma concentration at 8 h after oral dosing
P < 0.05.
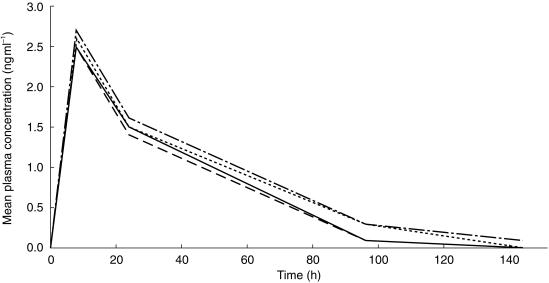
Figure 2 shows the plasma enantiomer concentration vs time curves following oral dosing. The sum of concentrations of the two individual enantiomers correlated well with the concentration of total racemic amlodipine (r2 = 0.957; P = 0.0001).
Figure 2.
Mean plasma concentrations of R(+) and S(–)-amlodipine after a 10 mg oral dose to 20 healthy male subjects consuming either grapefruit juice or water (placebo). ― placebo, R-enantiomer; - - - grapefruit juice, R-enantiomer; · · · · placebo, S-enantiomer; - · - · - grapefruit juice, S-enantiomer.
Overall, grapefruit juice coadministration did not greatly alter the stereoselectivity in amlodipine kinetics. As is suggested by the plasma concentrations in Figure 2 for oral amlodipine, the mean AUC(0,24 h) for the S(–) and R(+) enantiomers showed no statistically significant change with concomitant grapefruit juice administration. This was supported by similar observations from the S/R ratios for C8(oral) and Cmax(intravenous), and for oral AUC(0,24 h) and intravenous AUC(0,96 h) data. Paired treatment data for the individual subjects were distributed about equally above and below the line of identity. The corresponding plots for the individual enantiomers showed a similar distribution (data not shown).
For oral amlodipine, the mean concentration at 8 h (C8) for the S(–) and R(+) enantiomers showed no statistically significant change with concomitant grapefruit juice administration. The S/R ratio of C8 was slightly but significantly larger (3%) during the juice compared with the placebo.
For intravenous amlodipine, the mean AUC(0,96 h) for the S(–) and R(+) enantiomers and the S/R ratio showed no statistically significant change with concomitant grapefruit juice administration (Table 2). With grapefruit juice, the absolute values of Cmax for both the S(–) and R(+) enantiomers significantly declined by the same extent (i.e. 24% and 26%, respectively), generating an S/R ratio for Cmax that was nearly the same after juice and placebo.
Haemodynamics and adverse effects
Overall, there were no statistically significant treatment effects on blood pressure for either route of amlodipine administration. For pulse rate, only one statistically significant treatment effect was observed, and that was during intravenous amlodipine.
Grapefruit juice coadministration had no significant effect after either oral or intravenous amlodipine in either the maximum change from baseline or the AUEC(0,24 h) for the following parameters: standing systolic and diastolic blood pressure, supine systolic and diastolic blood pressure. There also were no significant differences between treatments after either intravenous or oral amlodipine in the AUEC(0,24 h) for standing and supine pulse rate, and for maximum change in supine pulse rate. The mean maximum changes from baseline in blood pressure and pulse rate, both standing and supine, are listed in Table 3.
Table 3.
Maximum change in blood pressure (mmHg) and pulse rate (beats min−1).
| Parameter | Intravenous amlodipine with: Grapefruit juice | Water (control) | Oral amlodipine with: 95% CI | Grapefruit juice | Water (control) | 95%CI |
|---|---|---|---|---|---|---|
| Supine | ||||||
| Systolic BP (mmHg) | −15.9 ± 13.4 | −11.8 ± 6.7 | −10.4, 2.1 | −11.7 ± 8.8 | −9.2 ± 5.9 | −8.7, 3.7 |
| Diastolic BP (mmHg) | −10.1 ± 7.3 | −11.5 ± 6.2 | –3.1, 6.0 | −11.5 ± 5.0 | −13.2 ± 7.2 | −2.9, 6.3 |
| Pulse (beats min−1) | 9.0 ± 4.2 | 12.7 ± 7.0 | –7.7, 0.4 | 14.4 ± 7.8 | 13.5 ± 6.5 | −3.2, 4.9 |
| Standing | ||||||
| Systolic BP (mmHg) | −20.9 ± 11.8 | −18.8 ± 8.8 | –8.2, 3.9 | −14.8 ± 9.4 | −11.9 ± 6.1 | −8.9, 3.1 |
| Diastolic BP (mmHg) | −11.7 ± 5.4 | −13.3 ± 6.7 | –2.5, 5.6 | −13.3 ± 6.6 | −13.0 ± 5.8 | −4.4, 3.7 |
| Pulse (beats min−1) | 20.1 ± 8.8 | 25.1 ± 11.0 | –9.8, −0.2⋆ | 15.2 ± 6.5 | 15.8 ± 9.5 | −5.4, 4.2 |
Adjusted mean ± s.d. values Treatment difference P < 0.05, intravenous amlodipine plus grapefruit juice vs intravenous amlodipine plus water.
A statistically significant difference between treatments, during intravenous amlodipine but not oral amlodipine, was observed for the maximum increase in standing pulse rate, but not the supine pulse rate. The difference in standing pulse rates between intravenous amlodipine with grapefruit juice and intravenous amlodipine with water (adjusted means ± s.d.) was −5.0 ± 2.4 beats min−1 (95% CI−9.8, −0.2) (Table 3).
One subject discontinued study participation due to a mild tension headache following the second treatment sequence which consisted of oral amlodipine with grapefruit juice. Mild tension headache was reported in another subject during intravenous amlodipine and grapefruit juice; the subject completed the study.
Discussion
In this study of 20 normal men, we found that amlodipine pharmacokinetics and pharmacodynamics were virtually unaffected by grapefruit juice administration. There is no simple explanation for the significant difference in the elimination rate constant kel, in the intravenous study since neither total clearance nor the volume of distribution was altered by grapefruit juice. Unlike felodipine, that interacts with cimetidine and with grapefruit juice, and nitrendipine, that displays significant changes in its pharmacokinetics with the coadministration of food, cimetidine or grapefruit juice [15], amlodipine does not appear to interact with any of the latter [6, 7]. With its lower first pass metabolism and higher oral bioavailability relative to other dihydropyridine calcium antagonists such as felodipine and nitrendipine, amlodipine is less subject to such interactions.
Amlodipine, like other 1,4-dihydropyridine calcium channel antagonists is a substrate for CYP3A4 [12, 16]. However, unlike the other drugs in its class, grapefruit juice which inhibits CYP3A4 failed to alter the pharmacokinetics of amlodipine. This would seem consistent with the known effect of grapefruit juice constituents on intestinal CYP3Aand the minimal presystemic metabolism of amlodipine. Moreover experimental evidence indicates that the human CYP3A family comprises a number of closely related genes [16] and this may contribute to the differential inhibition of this enzyme system. An alternative mechanism by which grapefruit juice may alter the metabolism of coadministered drugs is by inhibiting P-glycoprotein activity [17–19]. However, available evidence indicates that amlodipine may not be a substrate for P-glycoprotein transport. For example, whereas verapamil, a potent inhibitor of P-glycoprotein [20, 21] elevates digoxin concentrations and inhibits digoxin renal elimination [22, 23], coadministrtation of amlodipine is not associated with any alteration in digoxin steady-state concentrations or renal excretion [24].
Josefsson et al.[25] studied the interaction between a single glass of grapefruit juice and amlodipine 5 mg in 12 young, healthy men. They reported a significant increase in AUC and peak concentrations of amlodipine. There were no changes in systolic or diastolic blood pressure or heart rate accompanying the pharmacokinetic changes. Our study found no clinically important change in the pharmacokinetics of either oral or intravenous amlodipine 10 mg or of its R(+) and S(–) enantiomers, and consequently no effect on blood pressure or heart rate. With a single 5 mg oral dose, it is likely that the analytical method used by Josefsson et al.[25] was operating near the detection limit for amlodipine concentration, which may have increased the variability in their results.
The administration of amlodipine and grapefruit juice in this study was similar to the pattern in actual practice. Amlodipine 10 mg once daily is a common dosage regimen for the treatment of hypertension or angina. A glass of grapefruit juice may be typically consumed daily in the morning. Although this study evaluated only single doses of amlodipine, the conditions of the study in which amlodipine was coadministered with grapefruit juice had the potential for observing any alteration in plasma amlodipine concentrations [5, 26, 27]. While there may be some limitations on the ability to extrapolate results to women, patients with disease states, and those taking concomitant medications, it is likely that our study results are predictive of outcomes in a patient setting.
Most dihydropyridines, except nifedipine, have a chiral centre on the carbon at position 4 and generally are formulated as racemic mixtures. It has been demonstrated that the pharmacological effects differ between enantiomers of nitrendipine, felodipine, nimodipine, nilvadipine, manidipine and benidipine [13], with the higher potency residing in the S-enantiomer. Their S/R ratios are greater than one for plasma or serum concentrations for all except nimodipine, which is less than one, indicating stereoselective pharmacokinetics. In most cases, the S-enantiomer displays mean Cmax and AUC values that exceed those of its optical antipode by as much as three-fold [28].
In contrast to other dihydropyridines evaluated to date, the concentrations of amlodipine enantiomers are approximately equal after administration of the racemic drug, mean oral S/R ratios 1.04 for AUC(0,24 h) and 1.03 for C8. However, like oral nitrendipine, grapefruit juice does little to alter this stereochemical picture, causing only a slight but statistically significant increase in the amlodipine S/R ratio for the AUC(0,24 h) value to 1.08 and the C8 to 1.06.
A route of administration-dependent difference in stereoselective pharmacokinetics has been reported for nisoldipine in humans [13], nitrendipine in humans [15] and nilvadipine in dogs [13]. Our study demonstrates that the disposition of amlodipine enantiomers in humans is essentially independent of the route of amlodipine administration and grapefruit juice does not alter this property.
In conclusion, this study demonstrated that the pharmacokinetics of both racemic amlodipine and its enantiomers and their haemodynamic effects were essentially unaltered by grapefruit juice. A 240 ml glass of grapefruit juice taken daily with a single oral dose of amlodipine did not change the pharmacokinetics or haemodynamic effects of the drug and therefore, would not be expected to alter the profile of response to amlodipine in clinical practice.
Acknowledgments
This study was supported by a grant from Pfizer Inc. It was presented in part at the Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, 1997. We acknowledge Dr Barbara Ameer for valuable editorial assistance during the preparation of this manuscript.
References
- 1.Ameer B, Weintraub RA. Drug interactions with grape fruit juice. Clin Pharmacokin. 1997;33:103–121. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice–drug interactions. Br J Clin Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivisto KT, Lilja JJ, Backman JT, Neuvonen PJ. Repeated consumption of grapefruit juice considerably increases plasma concentrations of cisapride. Clin Pharmacol Ther. 1999;66:448–453. doi: 10.1016/S0009-9236(99)70007-X. [DOI] [PubMed] [Google Scholar]
- 4.Van Agtmael MA, Gupta V, van der Graaf CAA, van Boxtel CJ. The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999;66:408–414. doi: 10.1053/cp.1999.v66.a101946. [DOI] [PubMed] [Google Scholar]
- 5.Takanaga H, Ohnishi A, Murakami H, et al. Relationship between time after intake of grapefruit juice and the effect on pharmacokinetics and pharmacodynamics of nisoldipine in healthy subjects. Clin Pharmacol Ther. 2000;67:201–214. doi: 10.1067/mcp.2000.104215. [DOI] [PubMed] [Google Scholar]
- 6.Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118:1100–1103. doi: 10.1016/0002-8703(89)90834-x. [DOI] [PubMed] [Google Scholar]
- 7.Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokin. 1992;22:22–31. doi: 10.2165/00003088-199222010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey MJ, Smith DA. Hepatic uptake is the main factor in the apparent slow absorption of amlodipine (Abstract) Br J Clin Pharmacol. 1991;33:219P. [Google Scholar]
- 9.Walker DK, Humphrey MJ, Smith DA. Importance of metabolic stability and hepatic distribution to the pharmacokinetics of amlodipine. Xenobiotica. 1994;24:243–250. doi: 10.3109/00498259409043236. [DOI] [PubMed] [Google Scholar]
- 10.Stopher DA, Beresford AP, Macrae PV, Humphrey MJ. The metabolism and pharmacokinetics of amlodipine in humans and animals. J Cardiovasc Pharmacol. 1988;12(Suppl 7):S55–S59. doi: 10.1097/00005344-198812007-00012. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22:21–25. doi: 10.1111/j.1365-2125.1986.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beresford AP, Macrae PV, Alker D, Kobylecki RJ. Biotransformation of amlodipine. Arzneim-Forsch/Drug Res. 1989;39:201–209. [PubMed] [Google Scholar]
- 13.Tokuma Y, Noguchi H. Stereoselective pharmacokinetics of dihydropyridine calcium antagonists. J Chromatog A. 1995;694:181–193. doi: 10.1016/0021-9673(94)00832-t. [DOI] [PubMed] [Google Scholar]
- 14.Beresford AP, Macrae PV, Stopher DA, Wood BA. Analysis of amlodipine in human plasma by gas chromatography. J Chromatogr Biomed App. 1987;420:178–183. doi: 10.1016/0378-4347(87)80170-6. [DOI] [PubMed] [Google Scholar]
- 15.Soons PA, Vogels BAPM, Roosemalen MCM, et al. Grapefruit juice and cimetidine inhibit stereoselective metabolism of nitrendipine in humans. Clin Pharmacol Ther. 1991;50:394–403. doi: 10.1038/clpt.1991.156. [DOI] [PubMed] [Google Scholar]
- 16.Guengerich FP, Brian WR, Iwasaki M, et al. Oxidation Dihydropyridine Calcium Channel Blockers Analogues Human Liver Cytochrome P-450 IIIA4. J Med Chem. 1991;34:1838–1844. doi: 10.1021/jm00110a012. [DOI] [PubMed] [Google Scholar]
- 17.Edwards DJ, Fitzsimmons ME, Schuetz EG, et al. 6′,7′-Dihydroxybergamottin in grapefruit juice and Seville orange juice: Effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin Pharmacol Ther. 1999;65:237–244. doi: 10.1016/S0009-9236(99)70102-5. [DOI] [PubMed] [Google Scholar]
- 18.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P4503A and P–glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 19.Takanaga H, Ohnishi A, Yamada S, et al. Effect of grapefruit juice and other juices in P-glycoprotein function of drug absorption in the intestine. Xenobiotic Metab Dispos. 1998;13(Suppl):S110–S111. [Google Scholar]
- 20.Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doppenschmitt S, Spanhn-Langguth H, Regardh CG, Langguth P. Radioligand-binding assay employing P-glycoprotein-overexpressing cells: Testing drug affinities to the secretory intestinal multidrug transporter. Pharm Res. 1998;15:1001–1006. doi: 10.1023/a:1011965707998. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen KE, Dorph-Pedersen A, Hvidt S, Klitgaard NA, Nielsen-Kudsk F. Digoxin–verapamil interaction. Clin Pharmacol Ther. 1981;30:311–316. doi: 10.1038/clpt.1981.165. [DOI] [PubMed] [Google Scholar]
- 23.Klein HO, Lang R, Weiss E, Di Segni E, Libhaber C, Guerrero J, Kaplinsky E. The influence of verapamil on serum digoxin concentration. Circulation. 1982;65:998–1003. doi: 10.1161/01.cir.65.5.998. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JB. Effects of amlodipine on steady-state digoxin concentrations and renal digoxin clearance. J Cardiovasc Pharmacol. 1988;12:1–5. doi: 10.1097/00005344-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Josefsson M, Zackrisson A-L, Ahlner J. Effect of grapefruit juice on the pharmacokinetics of amlodipine in healthy volunteers. Eur J Clin Pharmacol. 1996;51:189–193. doi: 10.1007/s002280050183. 10.1007/s002280050183. [DOI] [PubMed] [Google Scholar]
- 26.Lundahl J, Regardh CG, Edgar B, Johnson G. Relatioship between time of intake of grapefruit juice and its effect on pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur J Clin Pharmacol. 1995;49:61–67. doi: 10.1007/BF00192360. [DOI] [PubMed] [Google Scholar]
- 27.Bailey DG, Arnold JMO, Bend JR, Tran LT, Spence JD. Grapefruit juice–felodipine interaction: reproducibility and characterization with the extended release drug formulation. Br J Clin Pharmacol. 1995;40:135–140. [PMC free article] [PubMed] [Google Scholar]
- 28.Tokuma Y, Fujiwara T, Noguchi H. Determination of (+) and (-) -nilvadipine in human plasma using chiral stationary-phase liquid chromatography and gas chromatography-mass spectrometry, and a preliminary pharmacokinetic study in humans. J Pharm Sci. 1987;76:310–313. doi: 10.1002/jps.2600760410. [DOI] [PubMed] [Google Scholar]