Abstract
Aims
To investigate the steady-state pharmacokinetics of (R)- and (S)-methadone in a methadone maintenance population.
Methods
Eighteen patients recruited from a public methadone maintenance program underwent an interdosing interval pharmacokinetic study. Plasma and urine samples were collected and analysed for methadone and its major metabolite (EDDP) using stereoselective h.p.l.c. Methadone plasma protein binding was examined using ultrafiltration, and plasma α1-acid glycoprotein concentrations were quantified by radial immunoassay.
Results
(R)-methadone had a significantly (P < 0.05) greater unbound fraction (mean 173%) and total renal clearance (182%) compared with (S)-methadone, while maximum measured plasma concentrations (83%) and apparent partial clearance of methadone to EDDP (76%) were significantly (P < 0.001) lower. When protein binding was considered (R)-methadone plasma clearance of the unbound fraction (59%) and apparent partial intrinsic clearance to EDDP (44%) were significantly (P < 0.01) lower than for (S)-methadone, while (167%) was significantly (P < 0.001) greater. There were no significant (P > 0.2) differences between the methadone enantiomers for steady-state plasma clearance, trough plasma concentrations and unbound renal clearance. Patients excreted significantly (P < 0.0001) more (R)-methadone and (S)-EDDP than the corresponding enantiomers. Considerable interindividual variability was observed for the pharmacokinetic parameters, with coefficients of variation of up to 70%.
Conclusions
Steady-state pharmacokinetics of unbound methadone are stereoselective, and there is large interindividual variability consistent with CYP3A4 mediated metabolism to the major metabolite EDDP; the variability did not obscure a significant dose-plasma concentration relationship. Stereoselective differences in the pharmacokinetics of methadone may have important implications for pharmacokinetic-pharmacodynamic modelling but is unlikely to be important for therapeutic drug monitoring of methadone, in the setting of opioid dependence.
Keywords: 2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine, drug dependence, metabolism, methadone, pharmacokinetics, protein binding, stereoselectivity
Introduction
Since its formal introduction in 1965 [1], methadone has become the most widely used pharmacological agent for the treatment of opioid dependence [2]. Rac-methadone (6-dimethylamino-4,4-diphenyl-3-heptanone) is a chiral molecule of (R)- and (S)-enantiomeric forms in which (R)-methadone has a 10-fold higher affinity at µ and δ opioid receptors [3] and possesses up to 50 times the analgesic activity of (S)-methadone in human and animal models of antinociception [4]. (R)-methadone prevents the occurrence of opioid withdrawal symptoms, while (S)-methadone is ineffective [5, 6]. Despite this high eudismic ratio, methadone is used as the racemate in most countries.
Methadone is primarily eliminated from the body by metabolism with nine metabolites having been identified in human urine [7–9], although the data have been, in most cases, qualitative rather than quantitative. Mono N-demethylation, a primary metabolic pathway, results in the formation of a highly unstable compound which then undergoes spontaneous cyclization and dehydration to form 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP). Other identified metabolites appear to contribute quantitatively little to the overall metabolism of methadone [8, 10], although complete mass balance has not been achieved. After oral dosing, unchanged methadone and EDDP are found in similar amounts in human urine and account for up to 50% of the dose [8, 10, 11].
Few studies have examined the pharmacokinetics of the individual enantiomers after administration of rac-methadone. After a single dose for pain control, the two enantiomers differed significantly in their disposition [12], with (R)-methadone having a larger volume of distribution, longer terminal elimination half-life and higher total body clearance.
Examination of the disposition of the methadone enantiomers after chronic dosing of racemic methadone has been limited. Studies that have used a stereoselective assay have confined their investigations to enantiomeric ratios in plasma samples taken at single time points, for example at the end of an interdosing interval, and have reported wide interpatient variability [13–15]. Kreek and coworkers [16, 17] administered an equal amount of each stable isotope labelled methadone enantiomer, used mass-spectrometry to examine stereoselectivity in the pharmacokinetics of methadone in maintenance subjects, and reported similar results to those of Kristensen and coworkers [12].
Compared with the (S)-enantiomer, (R)-methadone has lower plasma protein binding, with α1-acid glycoprotein being the predominant binding protein [18, 19]. No study to date has examined the role of variability in protein binding in the pharmacokinetics of total (R)- and (S)-methadone. It is not known whether stereoselectivity in total body clearance is due to metabolism (intrinsic clearance) and/or protein binding.
The aim of the present study was to determine the steady-state pharmacokinetics of (R)- and (S)-methadone in a methadone maintenance population, and to examine factors which might contribute to their variability.
Methods
Patients
Ethical approval was obtained from the Royal Adelaide Hospital Research Ethics Committee. The patients had been enrolled in the South Australian Public Methadone Maintenance Program for at least 6 months (range 6 months to 10 years) and had not had a methadone dose change for at least 2 months. There were 11 males and 7 females; body weights ranged from 60 to 94 kg (mean± s.d.; 74 ± 10 kg); ages ranged from 21 to 45 years (35 ± 7 years). The once-daily methadone dose ranged from 7.5 to 130 mg day−1, which corresponded to 0.12–1.9 mg kg−1 (0.88 ± 0.50 mg kg−1). The patients were allowed to take benzodiazepines in therapeutic doses. The majority smoked cigarettes, 7 showed positive urinalysis for benzodiazepines, 10 for cannabinoids, 2 for opioids other than methadone, 1 for barbiturates, 2 for sympathomimetic amines and 4 consumed alcohol regularly in quantities less than 40 g day−1. Patients were excluded from the study if they were pregnant or had positive HIV serology.
Each patient was admitted to the inpatient facility of the maintenance program 1 h before their scheduled daily dose and remained in the unit for the subsequent 24 h. Methadone was administered as a syrup under supervision of the study personnel. An 18 gauge indwelling venous catheter (JelcoTM, Critikon Corp, Tampa, Fla, USA) was inserted into a forearm vein prior to the daily dose and kept patent with a Teflon stylet (JelcoTM). A 5 ml blood sample was collected before the dose and at the following times after dosing: 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 9 and 12 h, and at the end of the interdosing interval (23–24 h). Blood was centrifuged, and the plasma stored at −20°C until analysis. In 10 subjects (9 male, 1 female), a 24 h pooled urine sample was also obtained, volume and pH measured and an aliquot stored at −20°C until analysis. These patients have been described elsewhere [20], in which plasma rac-methadone concentration-effect relationships were investigated.
Chemicals
Rac-methadone as the hydrochloride salt, (R)- and (S)-methadone as the free bases, and (R)- and (S)-2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) as the perchlorate salts were obtained from the National Institute on Drug Abuse (Rockville, MD, USA). Racemic-EDDP as the hydroiodide salt was purchased from Alltech-Applied Science Labs (State College, PA, USA). 3-methoxymorphinan as the hydrobromide salt was a kind gift from Roche Products Pty Ltd (Sydney, NSW, Australia). Other materials were obtained from the following sources: bovine serum albumin (fraction V), and human α1-acid glycoprotein (purified from Cohn fraction VI) from Sigma Chemical Company (St Louis, MO, USA). H.p.l.c. grade acetonitrile, methanol and triethylamine were from BDH Laboratory Supplies (Poole, UK). All other reagents and chemicals were obtained from commercial sources and were of analytical grade quality.
Sample analysis
Quantification of (R)- and (S)-methadone in plasma and plasma ultrafiltrate
This method was based upon that of Norris & coworkers [21], with major modifications. The h.p.l.c. system comprised a LC-10AT pump (Shimadzu, Kyoto, Japan) at a flow rate of 1 ml min−1, a Sil-10A autoinjector (Shimadzu), a Cyclobond I 2000 RSP column (250 × 4.6 mm, Astec, Whippany, NJ, USA) with a Cyclobond I 2000 RSP precolumn (20 × 4.0 mm, Astec), and a SPD-M10A photo-diode array detector (Shimadzu) set at 210 nm. The system was controlled using Class-LC10 software (version 1, Shimadzu) running under Windows 3.11 (Microsoft Corporation, WA, USA) on a 486 DX IBM compatible computer. Optimal separation of the compounds of interest was achieved with a mobile phase of 9 : 11 : 80 (v/v) methanol:acetonitrile: 1% triethylamine (v/v) in water with the final pH adjusted to 6.0 with ortho-phosphodc acid. Retention times of (R)-methadone, (S)-methadone and 3-methoxymorphinan (internal standard) were 8.3, 9.6 and 21 min, respectively. Under these conditions all compounds of interest were baseline resolved. Enantioseparation of EDDP was not achieved, and the peak corresponding to EDDP was not resolved from (R)-methadone under these chromatography conditions. Recently Rudaz & Veuthey [22] reported similar inability to resolve EDDP from methadone with the Cyclobond I 2000 RSP column. However, EDDP is not back-extracted into 5 mm HCl. and did not interfere with the analysis of (R)- and (S)-methadone in this assay.
Patient samples were quantified with calibration curves consisting of eight standards over the concentration range 15–600 ng ml−1 of (R)- and (S)-methadone prepared in blank plasma. Plasma samples (1 ml) and internal standard (100 µl 5 µg ml−1 3-methoxymorphinan in water) were aliquoted into 10 ml tapered bottom plastic tubes, alkalinized (0.4 ml 0. 1 m Na2CO3 pH 10) and extracted with 6 ml of 30 : 70 (v/v) diethyl ether: hexane for 20 min on a rotary mixer. Samples were then centrifuged (2000 g, 10 min) and the organic phase transferred to a clean 10 ml tapered bottom plastic tube containing 0.25 ml 5 mm HCl and vortexed for 1 min. Samples were then centrifuged (2000 g, 10 min), the organic phase aspirated to waste and 100 µl of the acid phase was injected onto the chromatography system. The analysis of (R)- and (S)-methadone in plasma ultrafiltrate (see below) was performed as for plasma, with the following modifications: calibration curves consisted of eight standards over the concentration range 12.5–500 ng ml−1 of (R)- and (S)-methadone prepared in isotonic phosphate buffer (67 mm Na2HPO4/NaH2PO4 in 0.9% NaCl, final pH 7.4); only 0.35 ml plasma ultrafiltrate was assayed; and 50 µl of 5 µg ml-l 3-methoxymorphinan was used as the internal standard.
Interassay variability was monitored with quality control (QC) samples prepared in duplicate at three concentrations; low (LQC, 54 ng ml−1), medium (MQC, 90 ng ml−1) and high (HQC, 350 ng ml−1) of (R)- and (S)-methadone. Interassay accuracy and precision (mean ± %coefficient of variation (%CV); n = 14 assays on separate days) was 106 ± 7% (LQC), 103 ± 4% (MQC) and 100 ± 4% (HQC) for (R)-methadone, and 103 ± 7% (LQC), 105 ± 5% (MQC) and 103 ±6% (HQC) for (S)-methadone. Similarly, intra-assay accuracy and precision (n = 6 replicate samples) was 108 ± 8% (LQC), 106 ± 7% (MQC) and 107 ± 4% (HQC) for (R)-methadone, and 104 ± 7% (LQC), 106±6% (MQC) and 112 ± 5% (HQC) for (S)-methadone. The assay was both precise and accurate at the limit of quantification (15 ng ml−1), with interassay accuracy and precision (n = l 1 assays on separate days) being 101 ± 4% and 104 ± 4% for (R)- and (S)-methadone, respectively. Calculated from intra-assay replicate plasma QC samples, mean (n = 6 replicate samples) extraction efficiencies of (R)- and (S)-methadone were 101 ± 6% and 99 ± 7% (LQC); 101 ± 4% and 100 ± 3% (MQC); 101 ± 3% and 103 ± 2% (HQC), respectively, while extraction efficiency (n = 6 replicate samples) of the internal standard was 84 ± 1%. Similar assay validation and extraction efficiency results were obtained for the plasma ultrafiltrate assay.
Quantification of (R)-methadone, (S)-methadone, (R)-EDDP and (S)-EDDP in urine
Quantification of the enantiomers of methadone and EDDP was achieved using a previously validated stereoselective h.p.l.c. assay, which has been reported elsewhere [23]. Patient samples were quantified with calibration curves consisting of seven standards over the concentration range 0.13–12.5 µm of (R)- and (S)-methadone (39–3900 ng ml−1) and (R)- and (S)-EDDP (35–3500 ng ml−1) prepared in blank urine. Inter-assay variability was monitored with QC samples prepared in duplicate at three concentrations. Inter-assay inaccuracy (% bias) and precision (n = 4 assays on separate days) was < 10% for all compounds at all QC concentrations, with the exception of (S)-EDDP for the LQC precision, which was 13%. Similarly, intra-assay inaccuracy (% bias) and precision (n = 6, replicate samples) was < 12% for all compounds at all QC concentrations. The assay was both precise and accurate at the limit of quantification (39 ng ml−1 for (R)- and (S)-methadone and 35 ng ml−1 for (R)- and (S)-EDDP) with interassay inaccuracy (% bias) and precision (n = 4 assays on separate days) being < 6% for all compounds.
Quantification of rac-methadone in plasma
The method used for the analysis of rac-methadone in plasma has been previously reported [20]. Concentrations of rac-methadone obtained by this assay were used to compare the sum of (R)-and (S)-methadone in plasma obtained with the stereoselective assay. This was performed in order to provide a further validation of the stereoselective assays.
Determination of plasma (R)- and (S)-methadone unbound fraction
Determination of the unbound (nonprotein bound) (R)- and (S)-methadone concentration in plasma samples was performed using an ultrafiltration method modified from Wilkins and coworkers [24]. Initial experiments indicated very low unbound methadone concentrations in the patient samples. In order to quantify the unbound methadone concentrations it was necessary to increase the concentration of total (bound plus unbound) rac-methadone in plasma. This was achieved by the addition of 10–15 µl of a concentrated rac-methadone solution in isotonic phosphate buffer to a pooled 1.2 ml plasma sample obtained from each subjects' set of plasma samples. The volume of methadone solution was chosen to yield a rac-methadone concentration of 1500 ng ml−1, taking into consideration the original methadone concentration present in the sample. Preliminary investigations demonstrated that (R)- and (S)-methadone plasma protein binding (bound concentration vs bound/unbound concentration) was linear up to 5000 ng ml−1 of each enantiomer in the plasma of two healthy volunteers (results not shown). A 100 µl aliquot was taken, diluted with 900 µl of blank plasma and assayed for total (bound plus unbound) (R)- and (S)-methadone to accurately determine the concentration of total (bound plus unbound) (R)- and (S)-methadone. The remaining sample was warmed to 37°C in a shaking heated water bath, and a 1 ml aliquot was then transferred to an ultrafiltration device (MPS-1, Amicon, MA, USA) which had been prewarmed to 37°C. Prior to the addition of plasma, each device was passivated overnight with a solution of 60 g l−1 bovine serum albumin in isotonic phosphate buffer (pH 7.4), rinsed three times with isotonic phosphate buffer and dried. The passivation procedure was necessary as preliminary experiments indicated up to 30% nonspecific binding to the device. The filtration membrane (YMT 30000 molecular weight cut off, Amicon) did not demonstrate any nonspecific binding (< 1%). Samples were then processed according to the manufacturers' directions and the method of Wilkins and coworkers [24], using a Beckman J2–21 centrifuge with both the chamber and the fixed angle rotor (JA-20.1, Beckman) prewarmed to 37°C. Briefly, samples were centrifuged at 1500 g for a maximum of 30 min or until a maximum of 400 µl of filtrate had been collected. Concentrations of (R)- and (S)-methadone in the resulting plasma ultrafiltrate were quantified using h.p.l.c.
Quantification of plasma α1,-acid glycoprotein concentration
Plasma concentrations of α1-acid glycoprotein were determined using radial immunoassay plates (Behring Diagnostics, Marburg, Germany). Measurement of the diameter of the precipitated antibody was performed using a calibrated 10× magnification eyepiece. Two mutually perpendicular measurements were taken and the average value was used for calculations. Quality control samples were prepared at three concentrations (LQC: 30.5 mg dl−1, MQC: 61 mg dl−1, HQC: 122 mg dl−1) by diluting a commercially available reference sample(N/T Protein Control SL/H, Behring Diagnostics) in isotonic phosphate buffer. Inter-plate precision (n = 4 plates) was 2% (LQC), 4% (MQC) and 8% (HQC).
Pharmacokinetic and statistical analysis
(R)- and (S)-methadone unbound fraction (fu) was calculated as plasma ultrafiltrate concentration divided by total (protein bound and nonprotein bound) plasma concentration, and was expressed as a percentage. Area under the (R)- and (S)-methadone concentration-time curve during the dosing interval at steady-state () was determined by the linear trapezoidal method. Steady-state concentrations () were calculated by dividing by the dosing interval and normalized to a 70 mg rac-methadone (35 mg each enantiomer) dose. Time to reach (tmax) maximum measured steady-state plasma concentration (), minimum plasma concentration prestudy dose () and 24 h postdose () were obtained by direct observation of the data. , and were normalized to a 70 mg rac-methadone (35 mg each enantiomer) dose. Apparent plasma clearance at steady-state (CL/F) was calculated as dose/, renal clearance (CLR) as amount of methadone recovered in the 0–24 h urine sample divided by methadone , and apparent partial clearance of methadone to EDDP (CLMD→EDDP) as amount of EDDP recovered in the 0–24 h urine sample divided by methadone , taking into consideration molecular weight differences. Percent dose recovered (fe) was expressed as the percentage of the dose administered (assuming an equal amount of each enantiomer in the racemic dose) that was recovered in the 0–24 h urine sample, taking into consideration molecular weight differences in the case of EDDP. These pharmacokinetic parameters were also calculated with respect to the unbound enantiomers (, , , , , CLu/F, and CLMD→EDDPu), by either multiplying or dividing the corresponding parameters by fu. Peak to trough plasma concentration ratios for each patient were calculated by dividing by . Ratios of (R)- to (S)-methadone were calculated for and , and the resultant parameters corrected for plasma binding, by the division of (R)-methadone by (S)-methadone unbound fraction values. Additionally, ratios of total and unbound (R)- to (S)-methadone concentrations were calculated separately at the tmax of (R)-methadone (, ) and the tmax of (S)-methadone (, ) by the division of (R)-methadone by the corresponding (S)-methadone concentration at (R)-methadone tmax and the division of the (R)-methadone concentration at the (S)-methadone tmax by (S)-methadone , respectively. Ratios of plasma (R)- to (S)-methadone concentrations were also calculated at each time point.
As a validation procedure, ordinary least products linear regression analysis [25, 26] was used to compare the sum of plasma (R)- and (S)-methadone concentrations in the subjects' samples with the results from the racemic plasma assay using Excel (Excel v7.0a, Microsoft). Statistically significant differences between enantiomers were assessed using paired t-tests, while other comparisons were performed using un-paired t-tests and linear regression analysis was performed to yield Pearson's r values (GraphPad Prism v2.01, GraphPad Software, CA, USA). Mean ratios of (R)- to (S)-methadone concentrations calculated at each time point were compared with a theoretical value of 1 using a 2-tailed 1 sample t-test. Differences were considered significant at P < 0.05. All data are presented as mean±s.d.
Results
Ordinary least products linear regression analysis comparing the sum of (R)- and (S)-methadone plasma concentrations in patient samples (n = 185) with the results obtained with the racemic plasma assay yielded a strong and significant relationship (r2 = 0.98, P < 0.05) and the 95% confidence intervals of the slope (0.988; 1.052) and intercept (−18.4; 1.46) included 1 and 0, respectively.
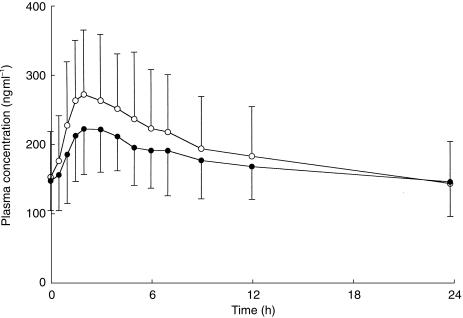
Figure 1 shows the interdosing interval mean plasma (R)- and (S)-methadone concentration-time profiles of the 18 subjects, normalized to a 70 mg rac-methadone dose.
Figure 1.
Mean plasma (R)-methadone (•) and (S)-methadone (○) concentration-time profiles for 18 methadone maintenance patients. Data are represented as concentration normalized to a 70 mg rac-methadone dose. Error bars indicate s.d.
Pre-dose () and 24 h postdose () plasma concentrations were not statistically significantly different (P value, mean difference; 95% CI) for (R)-methadone (P = 0.86, −1 ng ml−1; −11, 9 ng ml−1) or (S)-methadone (P = 0.31, 7 ng ml−1; −7, 21 ng ml−1). Similarly, (P = 0.55; Table 1) and (P = 0.95; Table 1) values were not statistically significantly different between the two enantiomers. In contrast (R)-methadone values were on average 83% those of (S)-methadone (P = 0.0002; Table 1). When corrected for protein binding, mean (R)-methadone ' and values were between 170% and 175% those of (S)-methadone (P < 0.0001; Table 2). The peak to trough plasma concentration ratio of (R)-methadone was a mean of 79% of that obtained for (S)-methadone (P = 0.0001; Table 1). (R)-methadone tmax (3.1 ± 1.9 h) values were significantly (P = 0.0072, 0.6 h; 0.2, 1.1 h) longer than for (S)-methadone (2.4 ± 1.6 h).
Table 1.
Disposition of total (bound plus unbound) (R)- and (S)-methadone following chronic dosing of between 7.5 mg and 130 mg rac-methadone once daily in 18 patients enrolled in a maintenance programme.
| Parameter1 | Mean | Range | %CV | P value2 (mean difference; 95% CI) | |
|---|---|---|---|---|---|
| (ng ml−l) | (R)-methadone | 147 | 42–223 | 30 | 0.55 |
| (S)-methadone | 153 | 31–261 | 43 | (−6; −24, 14) | |
| (ng ml−1) | (R)-methadone | 148 | 40–243 | 34 | 0.78 |
| (S)-methadone | 146 | 31–242 | 42 | (2; −15, 20) | |
| (ng ml−1) | (R)-methadone | 251 | 120–362 | 27 | 0.0002 |
| (S)-methadone | 303 | 116–447 | 32 | (−52; −74, −29) | |
| (ng ml−1) | (R)-methadone | 168 | 67–237 | 29 | 0.0571 |
| (S)-methadone | 186 | 52–304 | 37 | (−18; −36, 1) | |
| (ng ml−1 h) | (R)-methadone | 3484 | 608–7263 | 53 | 0.21 |
| (S)-methadone | 3797 | 589–7229 | 55 | (−313; −814, 188) | |
| CL/F (ml min−1) | (R)-methadone | 161 | 103–363 | 42 | 0.86 |
| (S)-methadone | 159 | 79–465 | 60 | (2; −18, 21) | |
| CLR (ml min−1) | (R)-methadone | 27.4 | 11.9–46.6 | 37 | < 0.0001 |
| (S)-methadone | 15.1 | 7.0–26.5 | 39 | (12.4; 9.2, 15.5) | |
| CLMD→EDDP (ml min−1) | (R)-methadone | 21.9 | 3.6–37.3 | 48 | 0.0088 |
| (S)-methadone | 28.8 | 5.5–58.9 | 55 | (−6.9; −11.6, 2.2) | |
| P/T | (R)-methadone | 1.81 | 1.35–3.58 | 30 | < 0.0001 |
| (S)-methadone | 2.30 | 1.68–4.49 | 34 | (−0.49; −0.68, −0.29) |
=plasma concentration, normalized to a 70 mg rac-methadone dose, predose; =plasma concentration, normalized to a 70 mg rac-methadone dose, 24 h after dosing; =maximum measured plasma concentration, normalized to a 70 mg rac-methadone dose; =steady-state plasma concentration, normalized to a 70 mg rac-methadone dose; =area under the plasma concentration-time curve during the inter-dosing interval; CL/F = apparent plasma clearance at steady-state; CLR=renal clearance; CLMD→EDDP=apparent partial clearance of methadone to EDDP; P/T=peak to trough plasma concentration ratio.
Comparison of (R)-methadone vs (S)-methadone.
Table 2.
Disposition of unbound (R)- and (S)-methadone following chronic dosing of between 7.5 mg and 130 mg rac-methadone once daily in 18 patients enrolled in a maintenance programme.
| Parameter1 | Mean | Range | %C | P value2 (mean difference; 95% CI) | |
|---|---|---|---|---|---|
| fu (%) | (R)-methadone | 3.6 | 1.8–4.8 | 24 | < 0.0001 |
| (S)-methadone | 2.1 | 1.2–2.8 | 25 | (1.5; 1.3, 1.8) | |
| (ng ml−1) | (R)-methadone | 5.3 | 2.0–8.0 | 33 | < 0.0001 |
| (S)-methadone | 3.1 | 0.8–5.9 | 46 | (2.2; 1.6, 2.8) | |
| (ng ml−1) | (R)-methadone | 5.4 | 1.9–7.8 | 33 | < 0.0001 |
| (S)-methadone | 3.1 | 0.8–5.5 | 43 | (2.3; 1.7, 2.9) | |
| (ng ml−1) | (R)-methadone | 8.9 | 4.3–13.4 | 29 | < 0.0001 |
| (S)-methadone | 6.2 | 2.8–9.2 | 34 | (2.7; 2.0, 3.5) | |
| (ng ml−1) | (R)-methadone | 6.0 | 2.3–11.0 | 34 | < 0.0001 |
| (S)-methadone | 3.8 | 1.4–6.1 | 40 | (2.3; 1.6, 2.9) | |
| (ng ml−1 h) | (R)-methadone | 117 | 28–268 | 51 | 0.0003 |
| (S)-methadone | 70 | 16–126 | 46 | (47; 26, 68) | |
| CLu/F (ml min−1) | (R)-methadone | 4611 | 2214–10590 | 46 | 0.0001 |
| (S)-methadone | 7845 | 3994–17750 | 51 | (−3235; −4590, −1879) | |
| (ml min−1) | (R)-methadone | 847 | 256–1832 | 48 | 0.63 |
| (S)-methadone | 819 | 265–1444 | 44 | (28; −100, 155) | |
| CLMD→EDDPu (ml min−1) | (R)-methadone | 727 | 77–1890 | 70 | 0.0017 |
| (S)-methadone | 1656 | 207–3506 | 66 | (−930; −1406, −453) |
fu=unbound fraction; =unbound plasma concentration, normalized to a 70 mg rac-methadone dose, predose; =unbound plasma concentration, normalized to a 70 mg rac-methadone dose, 24 h after dosing; =maximum measured unbound plasma concentration, normalized to a 70 mg rac-methadone dose; =steady-state plasma concentration of unbound drug, normalized to a 70 mg rac-methadone dose; =area under the plasma unbound concentration–time curve during the interdosing interval; CLu/F =apparent plasma clearance at steady-state of the unbound drug; =renal clearance of the unbound drug; CLMD→EDDPu=apparent partial intrinsic clearance of methadone to EDDP.
Comparison of (R)-methadone vs (S)-methadone.
The ratio of plasma (R)- to (S)-methadone concentrations predose () was not significantly different (P = 0.58; Table 3) from that obtained at the end of the dosing interval (). Similarly, unbound plasma (R)- to (S)-methadone concentration ratios at predose () and at the end of the dosing interval () were not significantly different (P = 0.52; Table 3). In contrast, ratios of (R)- to (S)-methadone calculated separately at the tmax of (R)-methadone () and (S)-methadone () were significantly lower compared with (P < 0.0001 and P = 0.0002, respectively; Table 3). Similarly, ratios of unbound (R)- to (S)-methadone calculated separately at the tmax of (R)-methadone () and (S)-methadone () were significantly lower compared with (P = 0.0003 and P = 0.0008, respectively; Table 3). The ratios of (R)- to (S)-methadone concentrations were significantly lower than unity at 1 (P > 0. 0003, −0.16; −0.24, −0.09), 1.5 (P < 0.0001, −0.18; −0.24, −0.12), 2 (P = 0.0004: −0.16, −0.24, −0.08), 3 (P = 0.0053, −0.12; −0.20, −0.04) and 4 (P = 0.0139, −0.12; −0.22, −0.03) h postdose (Figure 2), but not at other times (0.94 ≥ P ≥ 0.14; Figure 2).
Table 3.
(R)/(S) enantiomeric ratio at peak and trough concentrations following chronic dosing of between 7.5 mg and 130 mg rac-methadone once daily in 18 patients enrolled in a maintenance programme.
| Parameter1 | Mean | Range | %CV | P value2 (mean difference; 95% CI) |
|---|---|---|---|---|
| 1.07 | 0.68–2.28 | 34 | 0.57 (−0.02; −0.10, 0.06) | |
| 1.10 | 0.70–1.97 | 27 | ||
| 0.88 | 0.68–1.40 | 19 | < 0.0001 (−0.21; −0.30, −0.13) | |
| 0.843 | 0.66–1.07 | 15 | 0.0002 (−0.26; −0.38, −0.15) | |
| 1.91 | 1.20–4.11 | 38 | 0.52 (−0.05; −0.20, 0.10) | |
| 1.96 | 1.24–3.54 | 33 | ||
| 1.55 | 1.09–2.52 | 26 | 0.0003 (−0.41; −0.60, −0.22) | |
| 1.454 | 1.09–2.04 | 20 | 0.0008 (−0.51; −0.77, −0.25) |
=ratio of (R)- to (S)-methadone plasma concentrations predose; =ratio of (R)- to (S)-methadone plasma concentrations 24 h after dosing; =ratio of (R)-methadone and the corresponding (S)-methadone concentration at (R)-methadone tmax; =ratio of (R)-methadone concentration at the (S)-methadone tmax and (S)-methadone ;=ratio of (R)- to (S)- methadone plasma concentrations of the unbound drug predose; =ratio of (R)- to (S)-methadone plasma concentrations of the unbound drug 24 h after dosing; =ratio of (R)-methadone and the corresponding (S)-methadone concentration of the unbound drug at (R)-methadone tmax; =ratio of (R)-methadone concentration of the unbound drug at the (S)-methadone tmax and (S)- methadone
Comparison within each group vs the parameter with a blank cell in the P value column
Statistically significant difference (P value, mean difference; 95% CI) compared with (P =0.043, 0.048; 0.002, 0.094)
statistically significant difference compared with (P = 0.047, 0.098; 0.002, 0.195).
Figure 3.
Relationship between dose and (a) (R)-methadone (•; r2 = 0.68, P < 0.0001) and (b) (S)-methadone(○r2 = 0.47, P = 0.002) Dashed lines represent 95% confidence intervals.
Figure 2.
Mean plasma methadone (R)/(S) concentration ratio vs time profiles for 18 methadone maintenance patients. * Statistically significantly different from unity. Error bars indicate s.d. The y-axis has been truncated to enlarge the scale.
There was a highly significant relationship between plasma and dose for (R)-methadone (r2 = 0.68, P < 0.0001; Figure 3a) and (S)-methadone (r2 = 0.47, P = 0.002; Figure 3b). The plasma of (R)-methadone was not statistically significantly different (P = 0.21; Table 1) from the value for (S)-methadone. (R)-methadone was an average of 90% that obtained for (S)-methadone (P = 0.0571; Table 1), but this was only of borderline statistical significance. However, when corrected for protein binding, plasma (P = 0.0003; Table 2) and (P < 0.0001; Table 2) of (R)-methadone were on average 167% and 158% of those obtained for (S)-methadone, respectively.
The plasma unbound fractions of (R)-methadone were 171% those of (S)-methadone (P < 0.0001; Table 2). There was no significant difference in unbound fractions between males and females for (R)-methadone (3.62 ± 0.98% vs 3.69 ± 0.65%, respectively; P = 0.88,−0.07%; −0.89, 1.02%) or (S)-methadone (2.15 ± 0.58% vs 2.04 ± 0.42%, respectively; P = 0.69, 0.11%; −0.69, 0.47%). There was a weak but significant relationship between plasma α1-acid glycoprotein concentration and ratio of bound/unbound methadone concentrations in the patient samples for (R)-methadone (r2= 0.31; P = 0.019; Figure 4a) and (S)-methadone (r2 = 0.30; P = 0.021; Figure 4b).
Figure 4.
Relationship between plasma α1-acid glycoprotein concentration and (a) plasma (R)-methadone (r2 = 0.31, P = 0.019) and (b) (S)-methadone (○; r2 = 0.30, P = 0.021) bound/unbound concentration ratio. Dashed lines represent 95% confidence intervals. The x-axis has been truncated to maintain a consistent scale.
The mean concentration of α1-acid glycoprotein in plasma samples from the patients was 115 ± 24 mg dl–1 (range: 73–155 mg dl−1). These concentrations were not significantly different (P = 0.68, 5 mg dl−1; -30, 20 mg dl−1) between males (117 ± 25 mg dl−1; range: 79–155 mg dl−1) and females (112 ± 23 mg dl−1; range: 73–150 mg dl−1).
The renal clearance of total (bound plus unbound) (R)-methadone was a mean of 182% that of (S)-methadone (P < 0.0001; Table 1). After correcting for protein binding, there was no significant difference for CLRu, between (R)-methadone and (S)-methadone (P = 0.63; Table 2). The excretion of unchanged (R)-methadone was a mean of 160% that of (S)-methadone (P < 0.0001; Table 4). Conversely, the excretion of (R)-EDDP was a mean of 68% of that obtained for (S)-EDDP (P < 0.0001; Table 4). These differences resulted in a significantly (P =0.0008. −1.36; −1.99, −0.73) lower urinary ratio of EDDP/methadone for the (R)-enantiomer (0.93 ± 0.50) compared with the (S)-enantiomer (2.29 ± 1.37). However, there was no significant difference in excretion for the sum of methadone and EDDP of the (R)-enantiomers (17.8 ± 4.2%) compared with the (S)-enantiomers (18.1 ± 5.0%; P = 0.75, −0.3%; −1.9, 1.5%), or between the sum of (R)- and (S)-methadone (15.6 ± 4.9%) and (R)- and (S)-EDDP (20.3 ± 8.2%; P = 0.17, −4.8%; −12.0, 2.5%). The mean total urinary recovery of (R)- and (S)-methadone and (R)- and (S)-EDDP was 35.9 ± 8.9% of the dose.
Table 4.
Urinary recovery during an interdosing interval of the enantiomers of methadone and EDDP from 10 methadone maintenance patients following chronic dosing of between 7.5mg and 130 mg rac-methadone once daily.
| Patient | Dose (mg kg−1) | (R)-methadone (%) | (S)-methadone (%) | % Dose recovered (R)-EDDP (%) | (S)-EDDP (%) | Total dose (%) |
|---|---|---|---|---|---|---|
| 9 | 1.91 | 8.6 | 5.3 | 9.8 | 14.2 | 37.9 |
| 10 | 0.33 | 6.1 | 3.7 | 10.7 | 16.6 | 37.1 |
| 11 | 0.82 | 10.6 | 7.8 | 8.2 | 11.5 | 38.1 |
| 12 | 0.30 | 10.7 | 6.5 | 8.9 | 12.9 | 39.0 |
| 13 | 0.86 | 13.3 | 6.7 | 10.8 | 15.2 | 46.0 |
| 14 | 0.77 | 12.0 | 7.2 | 3.8 | 5.3 | 28.3 |
| 15 | 0.12 | 5.8 | 3.3 | 1.9 | 2.9 | 13.9 |
| 16 | 1.41 | 7.3 | 4.3 | 11.0 | 16.6 | 39.2 |
| 17 | 0.93 | 13.7 | 10.1 | 6.5 | 8.6 | 38.9 |
| 18 | 0.68 | 8.0 | 4.6 | 10.6 | 17.4 | 40.6 |
| Mean | 0.88 | 9.6 | 6.01 | 8.2 | 12.12 | 35.9 |
| %CV | 57 | 30 | 35 | 39 | 41 | 25 |
Statistically significant difference (P value, mean difference; 95% CI) compared with (R)-methadone (P < 0.0001, −3.6; −4.6, −2.7)
statistically significant difference compared with (R)-EDDP (P = 0.0001, 3.9; 2.5, 5.3).
The apparent plasma clearance with respect to total (bound plus unbound) methadone was not significantly different for (R)-methadone and (S)-methadone (P = 0.86; Table 1). When corrected for protein binding, (R)-methadone CLu/F was on average 59% that of (S)- methadone (P = 0.0001; Table 2). The apparent partial clearance of (R)-methadone to (R)-EDDP was on average 76% of the clearance of (S)-methadone to (S)-EDDP (P = 0.0088; Table 1). When corrected for protein binding, apparent partial intrinsic clearance of (R)-methadone to (R)-EDDP was on average 44% of the clearance of (S)-methadone to (S)-EDDP (P = 0.0017; Table 2).
There was no statistically significant effect of gender on any of the pharmacokinetic parameters derived from plasma concentration-time profiles for either(R)-methadone (0.92 > P > 0.10) or (S)-methadone (0.83 > P > 0.10).
Discussion
To our knowledge, this is the first study to comprehensively describe the disposition of methadone in a large cohort of methadone maintenance patients, with respect to stereoselectivity. The study found substantial stereoselectivity of most pharmacokinetic parameters, and also intersubject variability, which could contribute to altered therapeutic efficacy. The significantly lower CLu/F for (R)-methadone, which describes the elimination of the unbound pharmacologically active methadone enantiomer, indicates that (R)-methadone has a lower intrinsic clearance when compared with (S)-methadone. Further, there was a significantly greater fraction of the dose excreted in the urine as (S)-EDDP and (R)-methadone, than the corresponding enantiomers, which resulted in a stereoselective difference in the urinary ratio of EDDP/methadone concentrations, suggesting significantly less (R)-methadone than (S)-methadone was metabolized to EDDP. Stereoselectivity in the renal clearance of methadone is unlikely to explain this phenomenon, as there was no difference in the renal clearance of unbound methadone. There was no significant difference between the enantiomers in the sum of methadone and EDDP, or between the sum of (R)- and (S)-methadone and (R)- and (S)-EDDP. These data show that important characteristics of drug metabolism would fail to be observed using nonchiral analytical techniques. These data are in contrast to the results of in vitro metabolism studies with methadone which found no stereoselectivity in the intrinsic clearance of methadone to EDDP [27]. Reasons for this lack of agreement include the possibility that methadone may be eliminated by other metabolic pathways which display stereoselectivity, and/or that there is stereoselectivity in the binding of methadone to proteins in the in vitro liver microsomal fraction, which may mask any stereoselectivity in the metabolism by the enzymes mediating the formation of EDDP. It is possible that there may be stereoselectivity in the renal clearance and/or in the elimination of EDDP via faeces, such that the combined urinary and faecal recovery of (R)- and (S)-EDDP are the same. In support of this, significant amounts of EDDP have been recovered in the faeces of methadone maintenance patients (6% [28] to 18% [29]), while methadone was found to account for less than 1% [28, 29]. N-demethylation and hydroxylation metabolites of EDDP have been reported [8, 10]. Although these metabolites appear to contribute very little to the metabolic profile of methadone, it is possible that there is stereoselectivity in the metabolism of EDDP to these other metabolites. We did not quantify EDDP in plasma or faeces, or other possible EDDP metabolites in urine, so are unable to examine these mechanisms further. We observed stereoselectivity in the apparent partial clearance to EDDP (CLMD→EDDP), and apparent partial intrinsic clearance to EDDP (CLMD→EDDP) with (R)-methadone values significantly lower than for (S)-methadone (44% and 76%, respectively). The reference to ‘apparent’ was used as calculations were based on urinary excretion data only and EDDP is eliminated to some extent in faeces [28, 29], and further metabolized to a limited, albeit poorly defined, extent [8, 10]. If one assumes that methadone is not eliminated by other metabolic pathways which display marked net stereoselectivity, and that there is no stereoselectivity in the microsomal protein binding of methadone, then comparison of these data with in vitro data [27] indicates that the net nonrenal elimination (faecal elimination and/or further metabolism) of (R)-EDDP is greater than that of (S)-EDDP.
Recent in vitro studies have shown CYP3A4 to be the major CYP isoform mediating EDDP formation from rac-methadone [30–32] and the individual enantiomers [27]. We observed a large degree of interindividual variability (4- to 5-fold) in , and CLu/F. These pharmacokinetic parameters are mainly determined by metabolic activity, and the interindividual variability observed in this study is consistent with reported in vitro[33–35] and in vivo[36–39] estimates of variability in hepatic CYP3A4 expression. Markedly higher interindividual variability was observed for (≈12-fold), CLMD→EDDP (≈ 10-fold) and CLMD→EDDPu (≈20-fold), however, these are influenced by additional factors which include in some cases dose, plasma binding, and nonrenal elimination of EDDP.
There was no difference in the steady-state plasma clearance (CL/F) between the two enantiomers. This observation is in contrast to Kristensen and coworkers [12], who reported that the mean plasma clearance of (R)-methadone (158 ml min−1) was significantly greater than for (S)-methadone (129 ml min−1), although the magnitude of the difference was small. In contrast, bioavailability was not found to be stereoselective [12]. Recent population pharmacokinetic modelling studies have shown that the pharmacokinetics of rac-methadone differ between single doses in healthy normal subjects and methadone maintenance patients after a single dose [40], and that there are time-dependent changes in the clearance and volume of distribution of rac-methadone in methadone maintenance patients while steady-state is being achieved [41]. Comparison with the results of Kristensen and coworkers [12] is difficult, as our study involved patients at steady-state, whereas Kristensen and coworkers studied acute methadone administration. The subject populations were also markedly different, as Kristensen and coworkers' patients were receiving opioids for chronic pain control, and our patients were in a methadone maintenance programme. Assuming a bioavailability of 90%, our results for methadone plasma clearance are in close agreement with those of Kristensen and coworkers [12], being 149 ml min−1 and 143 ml min−1 for (R)-and (S)-methadone, respectively.
The highly significant relationships between plasma and dose for both (R)-methadone and (S)-methadone indicate that the pharmacokinetics (extent of absorption, clearance) of each enantiomer are linear after administration of the racemate over a wide dosage range (7.5–130 mg day−1). This is the first time that this has been demonstrated for the individual methadone enantiomers, and confirms previous reports of the linearity of rac-methadone pharmacokinetics [42]. It also should be noted that this relationship was established in separate individuals taking a range of fixed doses, rather than within single individuals all given a range of dosages. This demonstrates that the extent of interindividual variation in metabolic activity does not prevent a dose-plasma concentration relationship, indeed approximately 68% and 47% of the large interindividual variability in the of (R)-methadone and (S)-methadone, respectively, is explained by variation in dose, while the remaining variability is mainly due to differences in clearance.
In contrast to the results for and CL/F, plasma and peak to trough plasma concentration ratios were significantly lower for total (bound plus unbound) (R)-methadone compared with (S)-methadone. Additionally, Figure 2 shows that the ratio of (R)- to (S)-methadone plasma concentrations is not stable over an interdosing interval. The ratio decreases as plasma concentrations increase, with values at 1–4 h postdose, significantly lower than unity, while ratios at other time points are not different to unity. Indeed, (R)/(S) ratios calculated predose () and postdose () were not significantly different, but were both significantly greater than ratios calculated separately at the tmax of (R)-methadone () and (S)-methadone (), with identical results obtained when protein binding was considered (Table 3).
These differences may be explained by volume of distribution differences. As (R)-methadone had a lower protein binding compared to (S)-methadone, it is likely to have a greater volume of distribution, as has been previously reported [12]. This would result in lower values for (R)-methadone in comparison with (S)-methadone, and hence a lower peak to trough concentration ratio. Recently, we have shown in methadone maintained patients that withdrawal severity can be related to the rate of change of plasma rac-methadone concentrations from the time of peak plasma concentration, and that this relationship has a very high Hill slope factor [20]. The stereoselective difference in the magnitude of fluctuation of plasma methadone concentrations reported here may have important implications for pharmacokinetic-pharmacodynamic modelling of methadone, as measurement of rac-methadone may not provide an accurate reflection of plasma concentrations of the active (R)-methadone enantiomer. In contrast, therapeutic drug monitoring for patient compliance is unlikely to require stereoselective measurement of plasma methadone concentrations given the close dose-plasma concentration relationship demonstrated for the individual methadone enantiomers found in this study, and for rac-methadone by others [41–43].
The plasma protein binding of methadone was found to be stereoselective, with unbound fractions of (R)-methadone (3.84 ± 0.86%) significantly greater than for (S)-methadone (2.11 ± 0.52%) in accord with previous findings [18, 44]. However, in contrast to previous studies [18, 19, 24, 44–46], we observed lower unbound fractions. A possible reason for this difference may be that the pH of plasma samples was not maintained at 7.4. Although the ultrafiltration device maintains a constant sample pH during the filtration process, there is evidence that the pH of plasma samples may increase during storage, and changes in plasma pH may alter unbound fractions of other drugs [47]. Despite this possibility, stereoselectivity in methadone plasma protein binding was observed. A weak relationship was found between plasma α1-acid glycoprotein concentration and the ratio of bound/unbound concentrations, confirming earlier reports in methadone maintenance patients [19] and other subject populations [18, 19, 48]. Although α1-acid glycoprotein has been reported to be the predominant binding protein, investigators have reported that albumin [19, 48] and lipoproteins [19] may also play a role. However, the individual role of each protein appears to be minor compared with that of α1-acid glycoprotein, as plasma albumin [18, 19], cholesterol [18], triglyceride [18] and total protein concentrations [18] have been shown not to contribute significantly to the binding of methadone.
The renal clearance of total (bound plus unbound) (R)- and (S)-methadone accounted for approximately 10–20% of CL/F, consistent with previous reports for rac-methadone [49–51]. The renal clearance of rac-methadone has previously been shown to be pH dependent [50, 51]. Examination for pH dependency of the renal clearance of the methadone enantiomers was not possible in the present study, due to a very narrow pH range amongst samples collected. Of the 10 samples, only two were below pH 6 and the remainder were within the range of 6.0–6.4 pH units. When corrected for protein binding, the renal clearance for each enantiomer (range: 256–1832 ml min−1) was between 2- and 15-fold greater than glomerular filtration rate, indicating extensive net tubular secretion. After correcting for protein binding there was no significant difference (P = 0.63) between the enantiomers, indicating that the net secretion and reabsorption of methadone is not a stereoselective process.
Plasma concentrations of methadone were quantified with both a chiral and nonchiral assay, allowing a thorough validation of the results. Ordinary least products linear regression analysis is sensitive to both fixed and proportional bias, unlike conventional linear-regression analysis, as it does not assume that one axis is error-free [25, 26]. Comparisons between the stereoselective and racemic assays of methadone in plasma yielded a very strong and highly significant correlation with the 95% confidence intervals of the slope including 1 (indicating no proportional bias), and the intercept including 0 (indicating no fixed bias). This analysis demonstrated excellent performance of the assays, indicating that it is unlikely that there was any interference from other licit and illicit drugs. Combined with the intra- and interassay precision and accuracy of quality control samples, these data indicate that the assays were selective, precise and accurate. A similar validation procedure was used for the analysis of (R)- and (S)-methadone and (R)- and (S)-EDDP in urine [23].
The data presented in this study highlight the importance of protein binding and stereochemical considerations when drawing conclusions on the pharmacokinetics and metabolism of compounds, and demonstrate that care should be taken when interpreting pharmacokinetic and metabolism data of chiral compounds based only upon results of nonchiral analytical techniques. In conclusion, we have shown that the pharmacokinetics of methadone are stereoselective, and that there is large interindividual variability, consistent with CYP3A4 mediated metabolism to the major metabolite EDDP. However this variability did not obscure a strong dose-plasma concentration relationship. Stereoselective differences in the pharmacokinetics of methadone may have important implications for pharmacokinetic-pharmacodynamic modelling but is unlikely to be important for therapeutic drug monitoring of compliance with methadone in the setting of opioid dependence.
Acknowledgments
The results were presented in part at the XIIIth International Congress of Pharmacology in 1998 (Munich, Germany). David Foster was supported by a Dawes Scholarship from the Royal Adelaide Hospital and a Dora Lush (Biomedical) Postgraduate Scholarship from the National Health and Medical Research Council of Australia. Kyle Dyer was supported by a National Drug Strategy Postgraduate Research Scholarship. The authors wish to thank the National Institute on Drug Abuse for supplying the drug compounds, and also the Royal Adelaide Hospital, the University of Adelaide and the National Health and Medical Research Council of Australia for financial support, and the staff at the Warinilia Clinic of the South Australian Methadone Maintenance Programme for their invaluable assistance in this project.
References
- 1.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction. JAMA. 1965;193:80–84. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 2.Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. Br Med J. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristensen K, Christensen CB, Christrup LL. The µ1, µ2, δ, κ opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:45–50. doi: 10.1016/0024-3205(94)00426-s. 10.1016/0024-3205(94)00406-i. [DOI] [PubMed] [Google Scholar]
- 4.Scott CC, Robbins EB, Chen KK. Pharmacologic comparison of the optical isomers of methadon. J Pharmacol Exp Ther. 1948;93:282–286. [PubMed] [Google Scholar]
- 5.Scherbaum N, Finkbeiner T, Leifert K, Gastpar M. The efficacy of l-methadone and racemic methadone in substitution treatment for opiate addicts-a double-blind comparison. Pharmacopsychiatry. 1996;29:212–215. doi: 10.1055/s-2007-979573. [DOI] [PubMed] [Google Scholar]
- 6.Isbell H, Eisenman AJ. The addiction liability of some of the drugs of the methadon series. Fed Proc. 1948;7:162. [PubMed] [Google Scholar]
- 7.Sullivan HR, Smits SE, Due SL, Booher RE, McMahon RE. Metabolism of d-methadone: Isolation and identification of analgesically active metabolises. Life Sci. 1972;11:1093–1104. [Google Scholar]
- 8.Sullivan HR, Due SL. Urinary metabolites of dl-methadone in maintenance subjects. J Med Chem. 1973;16:909–913. doi: 10.1021/jm00266a009. [DOI] [PubMed] [Google Scholar]
- 9.Pond SM, Kreek MJ, Tong TG, Raghunath J, Benowitz NL. Altered methadone pharmacokinetics in methadone-maintained pregnant women. J Pharmacol Exp Ther. 1985;233:1–6. [PubMed] [Google Scholar]
- 10.Änggård E, Gunne L-M, Homstrand J, McMahon RE, Sandberg CG, Sullivan HR. Disposition of methadone in methadone maintenance. Clin Pharmacol Ther. 1975;17:258–266. doi: 10.1002/cpt1975173258. [DOI] [PubMed] [Google Scholar]
- 11.Kreek MJ, Bencsath FA, Field FH. Effects of liver disease on urinary excretion of methadone and metabolites in maintenance patients: quantitation by direct probe chemical ionization mass spectrometry. Biomed Mass Spectrom. 1980;7:385–395. doi: 10.1002/bms.1200070906. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen K, Blemmer T, Angelo HR, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit. 1996;18:221–227. doi: 10.1097/00007691-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Pham-Huy C, Chikhichorfi N, Galons H, et al. Enantioselective high-performance liquid chromatography determination of methadone enantiomers and its major metabolite in human biological fluids using a new derivatized cyclodextrin-bonded phase. J Chromatogr B. 1997;700:155–163. doi: 10.1016/s0378-4347(97)00317-4. [DOI] [PubMed] [Google Scholar]
- 14.Beck O, Boreus LO, Lafolie P, Jacobsson G. Chiral analysis of methadone in plasma by high-performance liquid chromatography. J Chromatogr B. 1991;570:198–202. doi: 10.1016/0378-4347(91)80216-y. [DOI] [PubMed] [Google Scholar]
- 15.Eap CB, Finkbeiner T, Gastpar M, Scherbaum N, Powell K, Baumann P. Replacement of (R)-methadone by a double dose of (R,S) -methadone in addicts: interindividual variability of the (R)/(S) ratios and evidence of adaptive changes in methadone pharmacokinetics. Eur J Clin Pharmacol. 1996;50:385–389. doi: 10.1007/s002280050128. 10.1007/s002280050128. [DOI] [PubMed] [Google Scholar]
- 16.Kreek MJ, Hachey DL, Klein PD. Stereoselective disposition of methadone in man. Life Sci. 1979;24:925–932. doi: 10.1016/0024-3205(79)90343-6. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Hachey DL, Kreek MJ, lrving CS, Klein PD. Quantitation of methadone enantiomers in humans using stable isotope-labeled [2H3]-, [2H5]-, and [2H8]-Methadone. J Pharm Sci. 1982;71:40–43. doi: 10.1002/jps.2600710110. [DOI] [PubMed] [Google Scholar]
- 18.Eap CB, Cuendet C, Baumann P. Binding of d-methadone, 1-methadone, and dl-methadone to proteins in plasma of healthy volunteers: role of the variants of α1-acid glycoprotein. Clin Pharmacol Ther. 1990;47:338–346. doi: 10.1038/clpt.1990.37. [DOI] [PubMed] [Google Scholar]
- 19.Romach MK, Piafsky KM, Abel JG, Khouw V, Sellers EM. Methadone binding to orosomucoid (α1-acid glycoprotein): determinant of free fraction in plasma. Clin Pharmacol Ther. 1981;29:211–217. doi: 10.1038/clpt.1981.34. [DOI] [PubMed] [Google Scholar]
- 20.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999;65:685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 21.Norris RL, Ravenscroft PJ, Pond SM. Sensitive high-performance liquid chromatographic assay with ultraviolet detection of methadone enantiomers in plasma. J Chromatogr B. 1994;661:346–350. doi: 10.1016/0378-4347(94)00341-6. [DOI] [PubMed] [Google Scholar]
- 22.Rudaz S, Veuthey JL. Chiral stationary phases in HPLC for the stereoselective determination of methadone. Chirality. 1999;11:319–325. doi: 10.1002/(SICI)1520-636X(1999)11:4<319::AID-CHIR10>3.0.CO;2-Y. 10.1002/(sici)1520-636x(1999)11:4<319::aid-chir10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Foster DJR, Somogyi AA, Bochner F. Stereoselective quantification of methadone and its major oxidative metabolite, EDDP, in human urine using stereoselective high-performance liquid chromatography. J Chromatogr B. 2000;744:165–176. doi: 10.1016/s0378-4347(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins JN, Ashofteh A, Setoda D, Wheatley WS, Huigen H, Ling W. Ultrafiltration using the Amicon MPS-1 for assessing methadone plasma protein binding. Ther Drug Monit. 1997;19:83–87. doi: 10.1097/00007691-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Ludbrook J. Comparing methods of measurements. Clin Exp Pharmacol Physiol. 1997;24:193–203. doi: 10.1111/j.1440-1681.1997.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 26.Brace RA. Fitting straight lines to experimental data. Am J Physiol. 1977;233:R94–R99. doi: 10.1152/ajpregu.1977.233.3.R94. [DOI] [PubMed] [Google Scholar]
- 27.Foster DJR, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–412. doi: 10.1046/j.1365-2125.1999.00921.x. 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreek MJ, Bencsath FA, Fanizza A, Field FH. Effects of liver disease on fecal excretion of methadone and its unconjugated metabolites in maintenance patients. Quantitation by direct probe chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:544–549. doi: 10.1002/bms.1200101003. [DOI] [PubMed] [Google Scholar]
- 29.Verebely K, Volavka J, Mulé S, Resnick R. Methadone in man: pharmacokinetic and excretion studies in acute and chronic treatment. Clin Pharmacol Ther. 1975;18:180–190. doi: 10.1002/cpt1975182180. [DOI] [PubMed] [Google Scholar]
- 30.lribarne C, Berthou F, Baird S, et al. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–373. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 31.Iribarne C, Dreano Y, Bardou LG, Menez JF, Berthou F. Interaction of methadone with substrates of human hepatic cytochrome P450 3A4. Toxicology. 1997;117:13–23. doi: 10.1016/s0300-483x(96)03549-4. 10.1016/s0300-483x(96)03549-4. [DOI] [PubMed] [Google Scholar]
- 32.Moody DE, Alburges ME, Parker RJ, Collins JM, Strong JM. The involvement of cytochrome P450 3A4 in the N-demethylation of 1-α-acetylmethadol (laam), norlaam, and methadone. Drug Metab Dispos. 1997;25:1347–1353. [PubMed] [Google Scholar]
- 33.Forrester LM, Henderson CJ, Glancey MJ, et al. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281:359–368. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada T, Yamazaki H, Mimura M, lnui Y, Guengerich FP. lnterindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 35.Chauret N, Gauthier A, Martin J, Nicollgriffith DA. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab Dispos. 1997;25:1130–1136. [PubMed] [Google Scholar]
- 36.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro–in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–556. [PubMed] [Google Scholar]
- 37.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P4503A probe: II. Characterization of inter– and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther. 1994;271:557–566. [PubMed] [Google Scholar]
- 38.Lown KS, Thummel KE, Benedict PE, et al. The erythromycin breath test predicts the clearance of midazolam. Clin Pharmacol Ther. 1995;57:16–24. doi: 10.1016/0009-9236(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 39.Thummel KE, Shea DO, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 40.Wolff K, Rostami-Hodjegan A, Shires S, et al. The pharmacokinetics of methadone in healthy subjects and opiate users. Br J Clin Pharmacol. 1997;44:325–334. doi: 10.1046/j.1365-2125.1997.t01-1-00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rostami-Hodjegan A, Wolff K, Hay AW, Raistrick D, Calvert R, Tucker GT. Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Br J Clin Pharmacol. 1999;48:43–52. doi: 10.1046/j.1365-2125.1999.00974.x. 10.1046/j.1365-2125.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff K, Sanderson M, Hay AW, Raistrick D. Methadone concentrations in plasma and their relationship to drug dosage. Clin Chem. 1991;37:205–209. [PubMed] [Google Scholar]
- 43.Wolff K, Hay AW, Raistrick D, Calvert R. Steady-state pharmacokinetics of methadone in opioid addicts. Eur J Clin Pharmacol. 1993;44:189–194. doi: 10.1007/BF00315479. [DOI] [PubMed] [Google Scholar]
- 44.Eap CB, Cuendet C, Baumann P. Selectivity in the binding of psychotropic drugs to the variants of α1-acid glycoprotein. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:220–224. doi: 10.1007/BF00169251. [DOI] [PubMed] [Google Scholar]
- 45.Hervé F, Duché JC, d'athis P, Marché C, Barré J, Tillement JP. Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human α1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on α1-acid glycoprotein. Pharmacogenetics. 1996;6:403–415. doi: 10.1097/00008571-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Judis J. Binding of codeine, morphine, and methadone to human serum proteins. J Pharm Sci. 1977;66:802–806. doi: 10.1002/jps.2600660615. [DOI] [PubMed] [Google Scholar]
- 47.Ho Ngoc-Ta Trung A, Sirois G. Influence of plasma pH on quinidine uptake by erythrocytes: estimation of free drug fraction in plasma from blood/ and erythrocyte/plasma concentration ratios. Pharm Acta Helv. 1987;62:61–64. [PubMed] [Google Scholar]
- 48.Abramson FP. Methadone plasma protein binding: alterations in cancer and displacement from α1-acid glycoprotein. Clin Pharmacol Ther. 1982;32:652–658. doi: 10.1038/clpt.1982.217. [DOI] [PubMed] [Google Scholar]
- 49.Inturrisi CE, Colburn WA, Kaiko RF, Houde RW, Foley KM. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin Pharmacol Ther. 1987;41:392–401. doi: 10.1038/clpt.1987.47. [DOI] [PubMed] [Google Scholar]
- 50.Bellward GD, Warren PM, Howald W, Axelson JE, Abbott FS. Methadone maintenance: effect of urinary pH on renal clearance in chronic high and low doses. Clin Pharmacol Ther. 1977;22:92–99. doi: 10.1002/cpt197722192. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson MI, Widerlov E, Meresaar U, Änggård E. Effect of urinary pH on the disposition of methadone in man. Eur J Clin Pharmacol. 1982;22:337–342. doi: 10.1007/BF00548403. [DOI] [PubMed] [Google Scholar]