Abstract
Aims
To determine the extent of drug use in children and the types of drugs that children use.
Methods
Cross-sectional study and cohort study, using computerized pharmacy dispensing records for all children aged 0–16 years in the northern part of The Netherlands in 1998. The main outcome measures were proportion of children that used drugs (per sex and age group), mean number of drugs per child, 10 most widely used drug groups and cumulative proportions of drugs users (per drug group) during the first 2 years of life.
Results
Drug use was the highest among infants, decreased till adolescence and increased from there. Overall, approximately 60% of all children used at least one drug in 1998. At younger ages, boys used more drugs than girls and at older ages girls used more drugs than boys. Systemic antibiotics were used by 21% of the children and were by far the most widely used drugs. Other frequently used drugs were analgesics (10%), corticosteroids for dermatologic use (9%), anthistamines (8%) and antiasthmatics (7%). Approximately 10% of the children had used at least one drug at the age of 1 month and at the age of 2 years this proportion was 81%.
Conclusions
The majority of children was exposed to one or more drugs and this exposure started at very young age. This shows the importance of good guidelines for drug use in children and emphasizes the necessity of research of pharmacokinetic and pharmacodynamic properties in children to obtain safety, efficacy and quality evidence of these drugs.
Keywords: children, drug use, drug utilization study, paediatrics, pharmacoepidemiology, pharmacy data
Introduction
Knowledge of prescription patterns is an important tool in rational drug therapy. Rational drug therapy is important for all drug users, but it is of paramount importance for children [1]. Many drugs have been investigated in adults only, but are often used by children as well [2–4]. However, children differ from adults regarding pharmacokinetics and pharmcodynamics [5]. Moreover, as children are still developing physically and mentally, they may be particularly vulnerable to possible harmful effects of drugs. It is therefore important to establish which drugs children use and to ascertain that these are not harmful. Although this subject is increasingly attracting attention, information of drug use in children is still scarce [6, 7]. With some exceptions [4, 8–10], most drug utilization studies were performed before the early nineties [11–17]. Often a limited number of drugs (frequently antibiotics) or a specific age group (frequently infants) was studied. Furthermore, a great number of studies was based on interviews with parents or based on small samples of children. Taken together, these previous studies show that drug use in children is very common. In an epidemiological evaluation of drug use in children, Bonati found numbers of prescribed drugs per child ranging from 0.7 to 3.0 [6]. The present study was undertaken to obtain a complete survey of the total drug use of children of all ages, based on computerized pharmacy records. These records offer very precise and objective information of all drugs prescribed to children. The specific aims were to determine the extent of drug use in children and the types of drugs that children use. Moreover, drug utilization in infants was studied specifically.
Methods
Population
The study was performed with pharmacy dispensing data from the InterAction database, which is part of the collaboration between community pharmacists in the northern part of the Netherlands and the University of Groningen. This database comprises all prescriptions of approximately 120 000 people since 1994, regardless of reimbursement status. Not only prescriptions by general practitioners are included. In the Netherlands, drugs for outpatients are supplied by community pharmacies, and therefore these prescriptions are included in the database as well. Drugs used during hospital stay (inpatients), vaccines, and over-the-counter medication (OTC) are not included. Amongst other things, an anonymous registration number, date of birth, gender, GP, date of drug delivery, quantity dispensed, prescribed daily dosage and ATC-code are registered. In The Netherlands it is common that people are registered with one pharmacy, and obtain all their medication from that pharmacy, so a complete medication history is registered and available in the database. Even without purchasing drugs, people can register with a pharmacy and a number of health insurance companies obliges people to do so. The total population that is covered by the database cannot be estimated by general population statistics because the districts used by the general population statistics do not entirely correspond with the adherence areas of the participating pharmacies. Analysis of a subarea for which general population statistics were available, showed that approximately 95% of all children (0–16 years) are registered in the database (ranging from 91% to 103%, depending on age). Therefore, this study used the number of children that were registered in the database as an estimate of the total number of children.
Design
The study consisted of three parts. In all parts pharmacy dispensing data were used as an estimate for actual drug use [18]. In the first part we examined the extent of drug use in children. For that, all children younger than 17 years in 1998 were selected from the database (n = 25 020) and identified by registration number. Prescriptions were divided into subgroups according to the Anatomical Therapeutical Chemical (ATC) classification system (2nd level), except for ATC subgroups N02 (analgesics) and M01A (NSAID's) which were combined [19]. Prescriptions for vitamin K, which is given to all children that receive breast-feeding, were excluded. Children were classified into the following age groups: 0–1 years (infants), 2–5 years (preschool children), 6–11 years (schoolchildren) and 12–16 years (adolescents). Age was determined at date of purchase of the prescriptions. The number of different ATC subgroups prescribed per child was counted. To minimize the possible distortion of chronic treatment, ATC subgroups were counted only once per child. The average of the number of children registered in the database on 1 January 1998 and 31 December 1998 (including children without prescriptions in 1998) was used as the estimate of the total number of children in 1998 and was calculated per age group and per sex. Using this average, the prevalence rate was defined as the number children/100 children/year that used drugs from one or more different ATC subgroups and was calculated per age group and per sex separately. 95% confidence intervals were estimated using SPSS 9.0. The children that had used drugs were considered in more detail: the average number of different ATC subgroups per drug using child was calculated per age group.
In the second part of the study we examined the kind of medication that children used. Therefore, all prescriptions from 1998 for children younger than 17 years at date of purchase were identified (n = 373 925). These prescriptions were classified by ATC subgroup (2nd level) and counted. Again, ATC subgroups were counted only once per child, resulting in the number of children that had used any drug of the particular ATC subgroup in 1998. Of the 10 most widely used ATC subgroups, the number and proportion of drug using children were calculated. These most widely used ATC subgroups were examined in more detail. For that analysis, the same procedure was followed using ATC-codes of the 4th and 5th level.
In the third part of the study we examined the drug use of infants in more detail. A cohort was defined as all children born between 1/1/94 and 31/12/96 that were registered in the database (n = 4511). The cumulative numbers and proportions of drug using children (% of children in cohort) of the 10 most widely used ATC subgroups (2nd level) were determined at age of 1, 3, 6, 12, 18 and 24 months.
Results
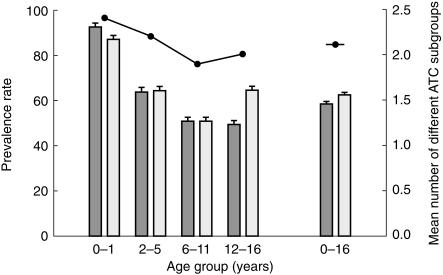
Figure 1 shows the prevalence rates (number of children/100 children/year that used at least one drug) in 1998 per age group and per sex. Overall (0–16 years), 60% of the children used one or more drug. For both boys and girls, the greatest annual prevalence of drug users was found in the 0–1 years age group (92 and 87%, respectively) and decreased with age until the start of adolescence (both 51%). From there the prevalence rate of drug use in boys decreased somewhat further (49%) whereas the prevalence rate of drug use in girls showed an increase (64%). When ATC main group G (genito urinary system and sex hormones) was excluded, prevalence rates were 49% in adolescent boys and 56% in adolescent girls. On average, the prevalence of drug users increased slightly from the start of adolescence.
Figure 1.
Prevalence rate, defined as the number of children/100 children/year (n = 25 020) that used at least one drug in 1998 and the mean number of different ATC subgroups that these children used per year. Error bars represent 95% confidence-intervals. y boys; □ girls; • number of different ATC subgroups.
The change in the mean number of different ATC subgroups was similar to that in the prevalence of drug users: a decrease with age until adolescence and an increase thereafter. Overall (0–16 years), these children used 2.2 different kind of drugs per year.
Table 1 shows the ATC subgroups that were most widely used by children in 1998. Of the 25 020 children registered in the database, 15 001 children used one or more drugs in 1998. Systemic antibiotics were by far the most widely used drugs: 21% of all children used drugs from this subgroup. The use of drugs from the remaining nine subgroups was considerably lower, ranging from 10% (analgesics and anti-inflammatory products) to 5% (cough and cold preparations). Broad-spectrum penicillins were used by 14% of all children and narrow-spectrum penicillin's by 3%. Macrolides (4%) and sulphonamides/trimethoprim (2%) were also frequently used. Paracetamol was by far the most widely used analgesic: 7% of all children used paracetamol, whereas NSAIDs were used by only 2% of all children. The ATC subgroup of antihistamines appeared to be a heterogeneous group which can be divided into drugs that are mainly used for cough or asthma (deptropine and promethazine) and drugs that are used for allergy (loratadine, cetirizine and terfenadine). Selective β2-adrenoceptor-agonists (by inhalation) and glucocorticoids (by inhalation) were the most widely used antiasthmatics (both used by 5% of the children). The use of the anticholinerigic ipratropium (by inhalation) was considerably lower (used by less than 1% of all children).
Table 1.
The 10 most widely used ATC subgroups, defined as the 10 highest numbers and proportions (%) of children (n = 25 020) that used at least one drug from that ATC subgroup.
| Drug(group) | ATC code | Number of children | (%1) |
|---|---|---|---|
| Systemic antibiotics | J01 | 5162 | (21) |
| Broad-spectrum penicillins | J01CA | 3417 | (14) |
| Macrolides | J01F | 1000 | (4) |
| Narrow-spectrum penicillins | J01CE + J01CF | 762 | (3) |
| Sulphonamides and trimethoprim | J01E | 569 | (2) |
| Other | 485 | (2) | |
| Analgesics+anti-inflammatory products | N02/M01A | 2555 | (10) |
| Paracetamol | N02BE01 | 1819 | (7) |
| NSAIDs | M01A | 571 | (2) |
| Other | 241 | (1) | |
| Corticosteroids, dermatological preparations | D07 | 2158 | (9) |
| Antihistamines for systemic use | R06 | 2085 | (8) |
| Deptropine | R06AX16 | 687 | (3) |
| Promethazine | R06AD02 | 511 | (2) |
| Loratadine | R06AX13 | 481 | (2) |
| Cetirizine | R06AE07 | 233 | (1) |
| Terfenadine | R06AX12 | 99 | (0) |
| Other | 253 | (1) | |
| Anti-asthmatics | R03 | 1854 | (7) |
| Selective β2-adrenoceptor agonist2 | R03AC | 1306 | (5) |
| Glucocorticoids2 | R03BA | 1241 | (5) |
| Ipratropium2 | R03BB01 | 133 | (0) |
| Other | 194 | (1) | |
| Nasal preparations | R01 | 1577 | (6) |
| Emollients and protectives | D02 | 1471 | (6) |
| Antifungals for dermatological use | D01 | 1333 | (5) |
| Ophthalmologics | S01 | 1231 | (5) |
| Cough and cold preparations | R05 | 1157 | (5) |
| Any drug | 15001 | (60) |
Proportions do not add up to 100% because children can be counted in more than one group.
by inhalation.
Table 2 shows the cumulative numbers and proportions (%) of drug using children of 2 years and under of the total cohort of 4511 children, determined at the age of 1, 3, 6, 12, 18 and 24 months. At the age of 1 month, already 10% of the children had used one or more drugs. In more detail, 2% of the children had received an ophthalmologic preparation (98% anti-infectives), 1% had received an antibacterial for systemic use, 1% had received an antifungal for dermatological use and 1% had used a nasal preparation (100% decongestants). By the age of 2 years, 46% of the children had used an antibacterial for systemic use, 33% an analgesic or anti-inflammatory drug (almost exclusively paracetamol) and 31% had used an antihistamine for systemic use. Overall, 65% of the 1 year-old children and 81% of the 2 year-old children had used at least one drug.
Table 2.
Cumulative numbers and proportions (%) of drug using children of the total cohort of 4511 children during the first 2 years of life, by ATC subgroup.
| 1 month | 3 months | 6 months | 12 months | 18 months | 24 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATC sub group | n | % | n | % | n | % | n | % | n | % | n | % | |
| J01 | Systemic antibiotics | 47 | 1 | 142 | 3 | 396 | 9 | 1224 | 27 | 1718 | 38 | 2085 | 46 |
| N02/M01A | Analgesics + anti-inflammatory products | 11 | 0 | 81 | 2 | 398 | 9 | 1067 | 24 | 1334 | 30 | 1476 | 33 |
| D07 | Corticosteroids, dermatological preparations | 9 | 0 | 56 | 1 | 211 | 5 | 417 | 9 | 571 | 13 | 708 | 16 |
| R06 | Antihistamines for systemic use | 8 | 0 | 97 | 2 | 399 | 9 | 996 | 22 | 1249 | 28 | 1398 | 31 |
| R03 | Anti-asthmatics | 1 | 0 | 17 | 0 | 59 | 1 | 205 | 5 | 301 | 7 | 388 | 9 |
| R01 | Nasal preparations | 44 | 1 | 132 | 3 | 268 | 6 | 612 | 14 | 807 | 18 | 935 | 21 |
| D02 | Emollients and protectives | 36 | 1 | 165 | 4 | 352 | 8 | 603 | 13 | 788 | 18 | 920 | 20 |
| D01 | Antifungals for dermatological use | 44 | 1 | 151 | 3 | 246 | 6 | 432 | 10 | 587 | 13 | 692 | 15 |
| S01 | Ophthalmologics | 86 | 2 | 163 | 4 | 272 | 6 | 485 | 11 | 671 | 15 | 835 | 19 |
| R05 | Cough and cold preparations | 12 | 0 | 104 | 2 | 281 | 6 | 603 | 13 | 742 | 16 | 838 | 19 |
| Any drug | 471 | 10 | 1175 | 26 | 1945 | 43 | 2931 | 65 | 3338 | 74 | 3651 | 81 | |
Discussion
This study shows that the InterAction database is a valuable source for information of drug utilization. The large size of the database enhances its reliability. Additionally, as data were recorded prospectively, information bias was reduced. Furthermore, follow-up is possible because people usually obtain all their drugs from one pharmacy. There are some weaknesses however. The database is not able to detect loss to follow up (by moving, for instance). This means that children who are lost to follow up, are still part of the total population. This might result in underestimations of drug use. On the other hand, the use of the database population as an estimate for the total number of children resulted in a small underestimation of the total number of children and therefore resulted in a small overestimation of drug use. Although it was possible to make corrections for this, we did not do so, because we wanted to edit the data as little as possible. Drug use during hospital stay and OTC-medication are not registered. As hospitals only supply drugs to inpatients and children are rarely hospitalized, it is not likely that this has influenced the outcome of the study. However, the failure to register OTC-medication is likely to have resulted in an underestimation of the use of drugs that are available without prescription as well. Regarding the 10 most widely prescribed drugs, this will be the case for analgesics, nasal preparations, emollients/protectives and cough/cold preparations. In the Netherlands, the other drugs listed in Table 1 are only available on prescription and the use of these drugs is therefore fully registered by the database.
The findings that drug use (both the prevalence of drug users as the mean number of different ATC subgroups per drug using child) in children was the highest among infants, decreased until adolescence and increased thereafter, are comparable with other studies [8, 20, 21]. Furthermore, the differences between drug use of boys and girls are also described in other studies [8, 17]. This pattern is in keeping with the number of contacts that boys and girls have with GPs at different ages [22]. The prominence of girls after adolescence (49% of the adolescent boys and 64% of the adolescent girls used at least one drug) could not entirely be attributed to drugs from ATC main group G (which includes oral contraceptives and gynaecological preparations). Exclusion of this drug group from the analysis only halved the difference in prevalence rates between boys and girls (boys still 49%, girls 56%). In addition, several studies show that the prominence of girls after adolescence continues into adult life [20, 21].
Systemic antibiotics were by far the most widely used drugs in children. This has been described in other studies as well [1, 6, 8, 12, 17]. Overall, 21% of the Dutch children used at least one antibacterial for systemic use, whereas a similar study among Danish children in 1997 found a percentage of 29% [10]. Although comparisons with other studies are hampered by major methodological differences, the use of systemic antibiotics in children in the Netherlands is low [6, 8, 11, 15]. This might reflect a conservative attitude of Dutch doctors towards prescribing of drugs in general, and antibiotics in particular. Furthermore, comparisons with other countries show that the relative share of broad-spectrum penicillins in the group for systemic antibiotics (approximately four times higher than narrow-spectrum penicillins) was high in the Netherlands [6, 8, 11, 15]. Since prescription of antibiotics with the smallest possible spectrum is recommended, our finding suggests that this advice is not always followed in the Netherlands. The pattern of use for analgesic and anti-inflammatory drugs is characteristic: the majority of children used paracetamol. In the Netherlands, this drug is generally considered as the first choice analgesic/antipyretic [23]. The finding that antihistamines were the fourth most widely prescribed ATC subgroup is distorted somewhat by the widespread use of deptropine and promethazine. These drugs are mainly used for asthmatic conditions and cough. The use of antiallergics, such as loratadine, cetirizine and terfenadine was much lower. In this case, the ATC classification does not entirely correspond with the use of these drugs in daily practice. From a therapeutic point of view, the use of antihistamines was overestimated and the use of antiasthmatics and cough medication was underestimated using the ATC classification. The widespread use of deptropine (used by 3% of the children), which is mainly used for its anticholinergic properties, is surprising, since it was removed from the national guidelines in 1997, due to adverse effects [24]. This illustrates that it is difficult to change prescribing habits. The use of the pharmacological alternative for deptropine, ipratropium (used by less than 1% of the children), was still very low. The other drugs from the antiasthmatic group were used to a larger extent: selective β2-adrenoceptor agonists and glucocorticoids by inhalation were both used by 5% of the children (1306 and 1241 children in total, respectively). As guidelines recommend the use glucocorticoids by inhalation in combination with β2-adrenoreceptor agonists [24], this would mean that there were very few children that used β2-adrenoreceptor agonists alone. Thus, the great majority of children that received asthma medication, received maintanance treatment.
It is remarkable that 10% of the children had used at least one drug by the age of 1 month. As noted before, almost all these are commonly used for infections. This might be due to a high susceptibility for infections and the severe course of infections at younger ages. A relatively great concern for an infant's health might play an important role as well. As 65% of the 1 year-old children and 81% of the 2 year-old children had used at least one drug, it can be said that the exposure to drugs started at very young age. Rasmussen & Smedby reported similar findings [13]. The most widely used drugs during infancy were systemic antibiotics (used by 46% of the 2 year-old children), analgesics (33%) and antihistamines (31%). This pattern is similar to the overall (0–16 years) pattern of the most widely used drugs. Only antiasthmatics were less widely used during infancy. This might have arisen since asthma is often not yet fully expressed in this age group. It might also be partially due to the frequent use of deptropine (ATC subgroup of antihistamines) as an antiasthmatic in infants instead of recommended antiasthmatics.
We conclude that the majority of children was exposed to one or more drugs and that this exposure started at very young age. This shows the importance of guidelines for drug use in children and emphasizes the necessity for research of pharmacokinetic and pharmacodynamic properties in children to ensure efficacy and safety. This is especially the case for systemic antibiotics, as these are by far the most widely prescribed drugs in children.
References
- 1.Sanz EJ. Drug use in non-hospitalized children. Pharmaceutisch Weekblad Scientific Edition. 1992;14:1–8. doi: 10.1007/BF01989218. [DOI] [PubMed] [Google Scholar]
- 2.Impicciatore P, Choonara I. Status of new medicines approved by the European Medicines Evaluation Agency regarding paediatric use. Br J Clin Pharmacol. 1999;48:15–18. doi: 10.1046/j.1365-2125.1999.00981.x. 10.1046/j.1365-2125.1999.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilton LV, Pearce G, Mann RD. The use of newly marketed drugs in children and adolescents prescribed in general practice. Pharmacol Drug Safety. 1999;8:S37–S45. doi: 10.1002/(sici)1099-1557(199904)8:1+<s37::aid-pds400>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. Br Med J. 2000;320:79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walson PD, Getschman S, Koren G. Principles of drug prescribing in infants and children. A practical guide. Drugs. 1993;46:281–288. doi: 10.2165/00003495-199346020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Bonati M. Epidemiologic evaluation of drug use in children. J Clin Pharmacol. 1994;34:300–305. doi: 10.1002/j.1552-4604.1994.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanz EJ. Drug prescribing for children in general practice. Acta Paediatr. 1998;87:489–490. doi: 10.1080/08035259850158155. [DOI] [PubMed] [Google Scholar]
- 8.Thrane N, Sørensen HT. A 1-year population-based study of drug prescriptions for Danish children. Acta Paediatr. 1999;88:1131–1136. doi: 10.1080/08035259950168216. [DOI] [PubMed] [Google Scholar]
- 9.Maison P, Guillemot D, Vauzelle-Kervroëdan F, et al. Trends in aspirin, paracetamol and non-steroidal anti-inflammatory drug use in children between. and 1992 in France. Eur J Clin Pharmcol. 1981;1998:659–664. doi: 10.1007/s002280050530. [DOI] [PubMed] [Google Scholar]
- 10.Thrane N, Steffensen FH, Mortensen JT, Schønheyder HC, Sørensen HT. A population-based study of antibiotic prescriptions for Danish children. Pediatr Infect Dis. 1999;18:333–337. doi: 10.1097/00006454-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Sanz EJ, Boada J. Drug utilization in pediatric outpatients in Tenerife (Canary Islands) Eur J Clin Pharmacol. 1988;34:495–499. doi: 10.1007/BF01046708. [DOI] [PubMed] [Google Scholar]
- 12.Sanz EJ, Bergman U, Dahlström M. Pediatric drug prescribing. A comparison of Tenerife (Canary Islands, Spain) and Sweden. Eur J Clin Pharmacol. 1989;37:65–68. doi: 10.1007/BF00609427. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen F, Smedby B. Life table methods aplied to the use of medical care and of prescription drugs in early childhood. J Epidemiol Community Health. 1989;43:140–146. doi: 10.1136/jech.43.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collet JP, Bossard N, Floret D, Gillet J, Honegger D, Boissel JP. and the Epicrèche research group. Drug prescription in young children: results of a survey in France. Eur J Clin Pharmacol. 1991;41:489–491. doi: 10.1007/BF00626376. [DOI] [PubMed] [Google Scholar]
- 15.Straand J, Roksstad K, Heggedal U. Drug prescribing for children in general practice. A report from the Møre & Romsdal prescription study. Acta Paediatr. 1998;87:218–224. doi: 10.1080/08035259850157705. [DOI] [PubMed] [Google Scholar]
- 16.Rylance GW, Woods CG, Cullen RE, Rylance ME. Use of drugs by children. Br Med J. 1988;297:445–447. doi: 10.1136/bmj.297.6646.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wessling A, Söderman P, Boëthius G. Monitoring of drug prescriptions for children in the county of Jämtland and in Sweden as a whole in 1977–1987. Acta Paediatr Scand. 1991;80:944–952. doi: 10.1111/j.1651-2227.1991.tb11757.x. [DOI] [PubMed] [Google Scholar]
- 18.Lau HS, De Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assesment. J Clin Epidemiol. 1997;50:619–625. doi: 10.1016/s0895-4356(97)00040-1. 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 19.Who Collaborating Centre for Drug Statistic Methodology. Guidelines for ATC classification and DDD assignment. Oslo, WHO.
- 20.Eggen AE. Patterns of medicine use in a general population (0–80 years). The influence of age, gender, diseases and place of residence on drug use in Norway. Pharmacol Drug Safety. 1997;6:179–187. doi: 10.1002/(SICI)1099-1557(199705)6:3<179::AID-PDS258>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Carmen Del Rio M, Prada C, Alvarez FJ. The use of medication by the Spanish population. Pharmacol Drug Safety. 1997;6:41–48. doi: 10.1002/(SICI)1099-1557(199701)6:1<41::AID-PDS250>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Van Suijlekom-Smit LWA, Bruijnzeels MA, Van der Wouden JC, Van der Velden J, Visser HKA, Dokter HJ. For what health problems in children is the GP consulted, and how often? Ned Tijdschr Geneeskd. 1995;139:1684–1689. (English summary) [PubMed] [Google Scholar]
- 23.Rosmalen CFH, Thomas S, Van der Laan JR, Van Lennep MJ, Vink R. Farmacotherapie voor de huisarts. Achtergronden. Houten: Bohn Stafleu Van Loghum BV.
- 24.Dirksen WJ, Geijer RMM, De Haan M, De Koning G, Flikweert S, Kolnaar BGM. NHG-standaard astma bij kinderen (eerste herziening) Huisarts Wet. 1998;41:130–143. [Google Scholar]