Abstract
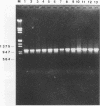
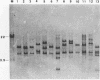
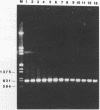
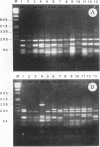
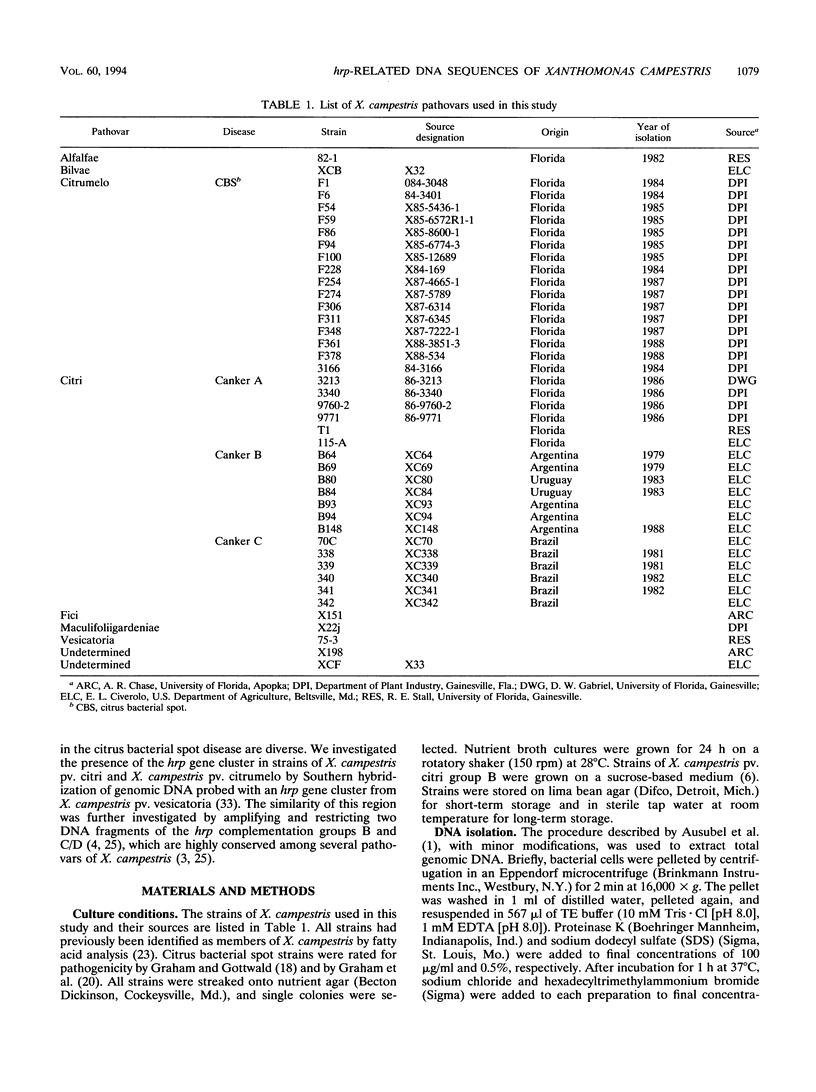
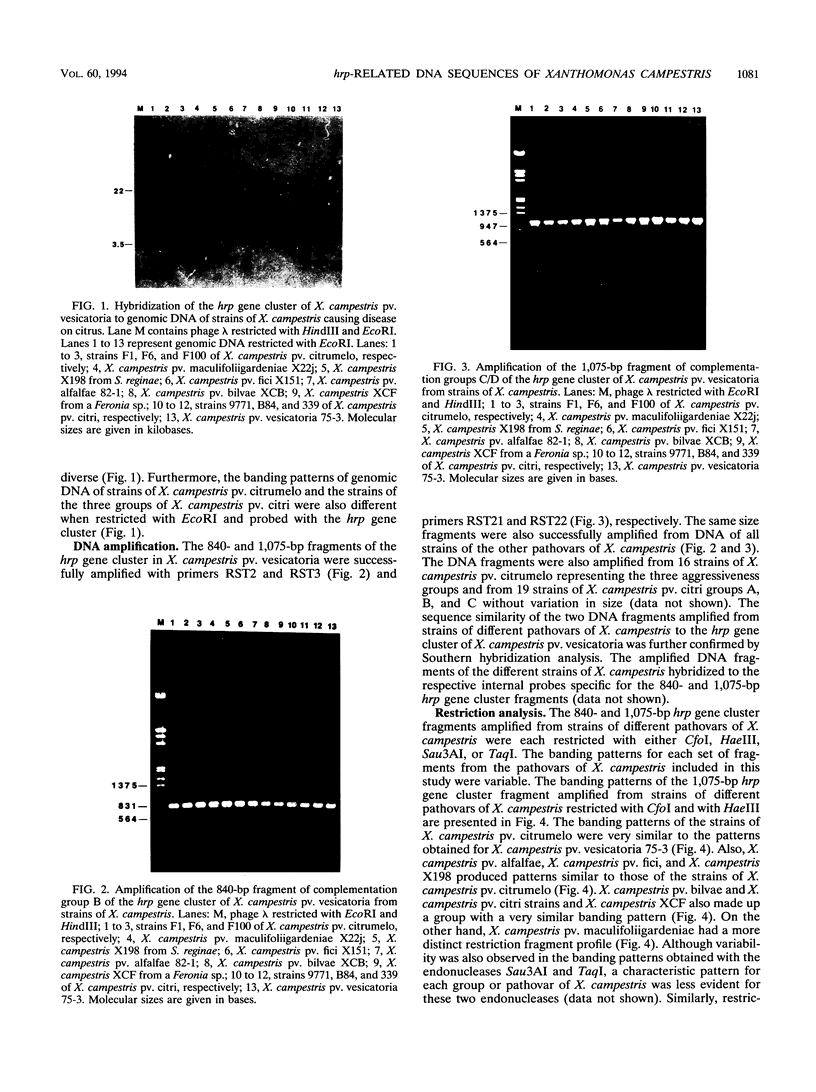
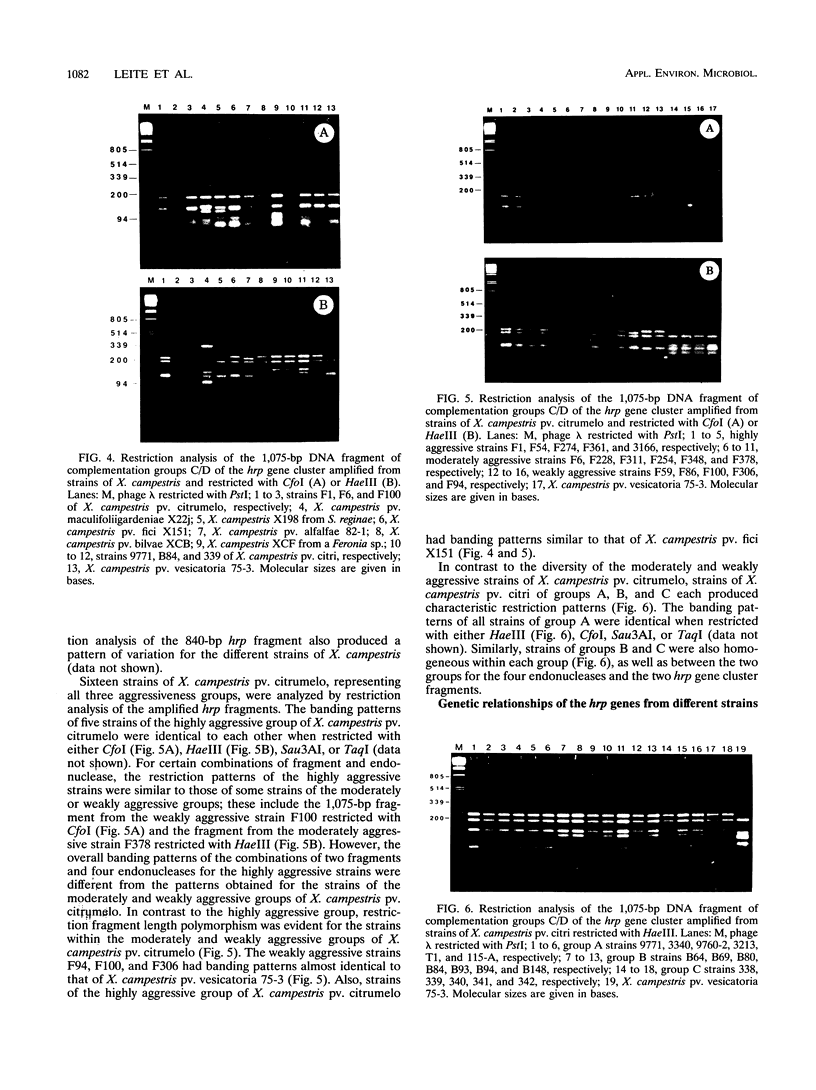
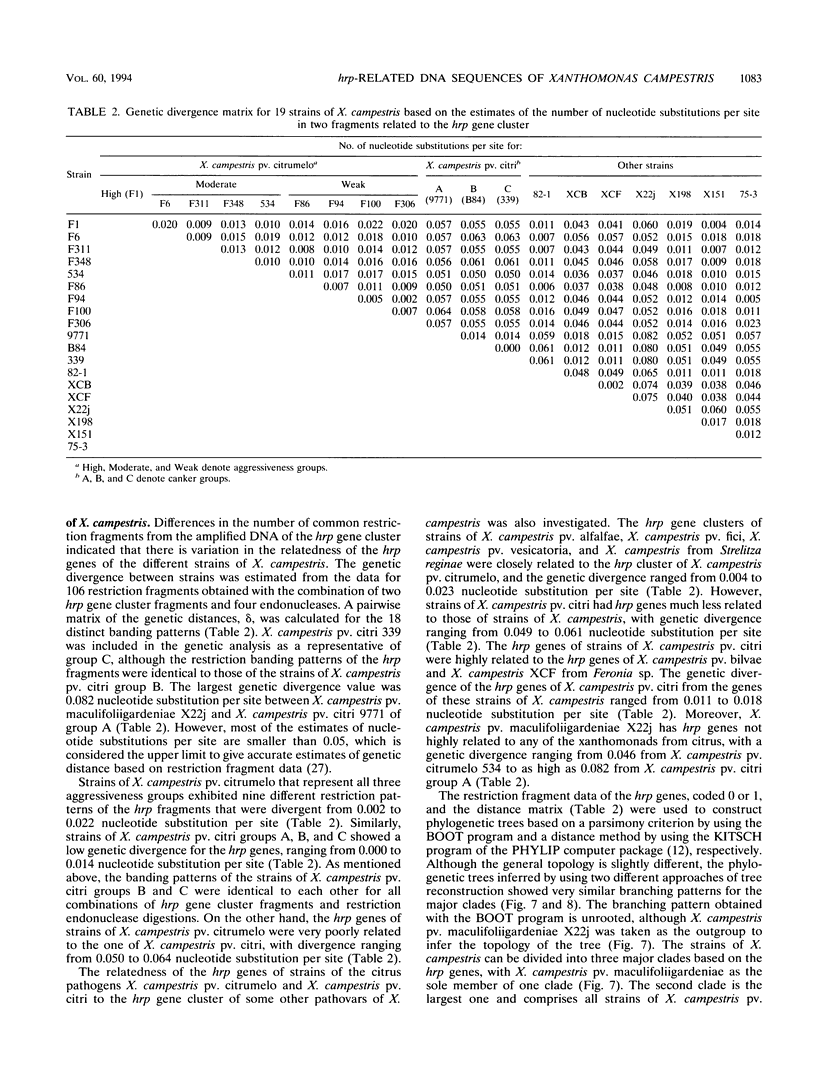
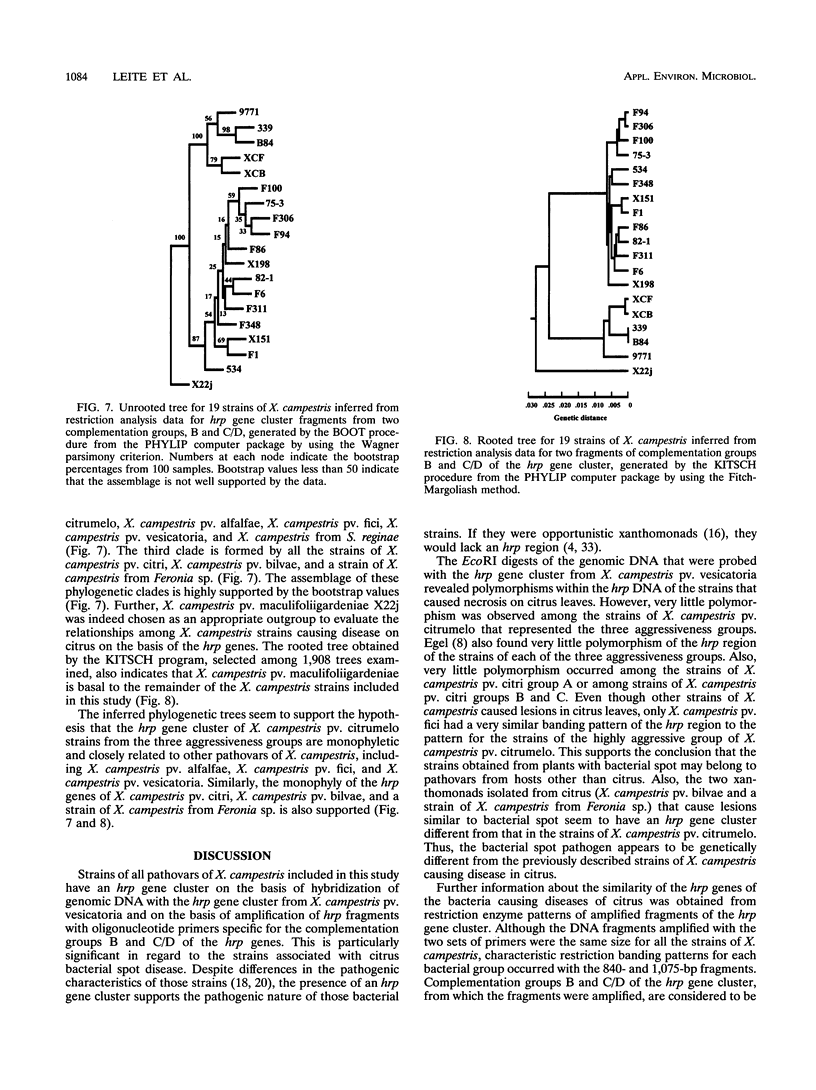
The hrp gene cluster of strains of Xanthomonas campestris that cause diseases of citrus was examined by Southern hybridization of genomic DNA and by restriction endonuclease analysis of enzymatically amplified DNA fragments of the hrp gene cluster. The hrp genes were present in all strains of the pathovars of X. campestris tested in this study, including strains of the three aggressiveness groups of the citrus bacterial spot pathogen, X. campestris pv. citrumelo. X. campestris pv. citri strains in groups A, B, and C, which cause citrus canker A, B, and C, respectively, each produced characteristic restriction banding patterns of amplified hrp fragments. The restriction banding patterns of all strains within each group were identical. In contrast, restriction fragment length polymorphism was evident among strains of the moderately and weakly aggressive groups of X. campestris pv. citrumelo. X. campestris pv. citrumelo strains in the highly aggressive group had a homogeneous restriction banding pattern. The characteristic banding patterns obtained for each bacterial group indicate that X. campestris strains causing disease in citrus can be reliably differentiated and identified by restriction analysis of amplified DNA fragments of the hrp gene cluster. In addition, the phylogenetic analysis based on the restriction banding patterns of amplified fragments suggests a polyphyletic relationship of the hrp genes among the strains of X. campestris that cause disease in citrus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel D. S., Graham J. H., Stall R. E. Genomic Relatedness of Xanthomonas campestris Strains Causing Diseases of Citrus. Appl Environ Microbiol. 1991 Sep;57(9):2724–2730. doi: 10.1128/aem.57.9.2724-2730.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Leite R. P., Jr, Minsavage G. V., Bonas U., Stall R. E. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1994 Apr;60(4):1068–1077. doi: 10.1128/aem.60.4.1068-1077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P. B., Peet R. C., Panopoulos N. J. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986 Nov;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. R., Smillie T. J., Rogers R. D. Platelet activating factor antagonist design: structure of methyl trans-5-(3,4-dimethoxyphenyl)-2,3,4,5-tetrahydro-2-oxo-4- furancarboxylate. Acta Crystallogr C. 1989 Feb 15;45(Pt 2):297–300. doi: 10.1107/s0108270188011151. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]