Abstract
Aims
To measure and compare the systemic bioavailability of fluticasone propionate aqueous nasal spray and a new nasal drop formulation, using a sensitive analytical method and high dose regimen.
Methods
Volunteers received four 800 µg doses of fluticasone propionate as a nasal spray or drops over 2 days, separated by an 8 h dose interval. On day 2, blood samples were collected for assay of fluticasone propionate plasma concentrations.
Results
The mean systemic exposure, for both formulations was 8.5 pg ml−1 h (drops) and 67.5 pg ml−1 h (spray). Mean absolute bioavailabilities were estimated to be 0.06% (drops) and 0.51% (spray), by reference to historical intravenous data.
Conclusions
Both formulations exhibited low systemic bioavailability, even at 12 times the normal daily dose. The bioavailability from the nasal drops was approximately eight times lower than from the nasal spray.
Keywords: bioavailability, fluticasone propionate aqueous nasal spray, fluticasone propionate nasal drops
Introduction
Fluticasone propionate (FP) is a potent corticosteroid with high topical activity in the nose. Prior to the present study, the intranasal bioavailability of FP had not been accurately measured, due to assay insensitivity and use of low doses. FP is available as an aqueous nasal spray (FPANS) for the treatment of rhinitis, and has recently been reformulated as an aqueous suspension in a single-dose, preservative-free nasal drop container for the treatment of nasal polyps. FPANS is established as a product with a low risk of systemic effects, and the present study investigated whether the drops formulation had the same profile. Nasal drops may produce an altered deposition pattern in the nose, or altered absorption characteristics relative to the established aqueous nasal spray. These factors may result in either an increase or a decrease in the systemic absorption of FP from the nose.
In the present study, four doses of 800 µg FP were given at 8 h intervals to rapidly produce high approximately steady-state plasma concentrations to define the plasma profiles. Daily doses exceeding 2400 µg are well tolerated when given as a nasal spray [1, 2]. A new assay was developed using the technique of liquid chromatography tandem mass spectrometry (LC-MS-MS), which is more sensitive than the radioimmunoassay used previously (50 pg ml−1) [1, 3]. The assay used in the present study provides a lower limit of quantification of 20 pg ml−1, sufficient to monitor systemic concentrations of inhaled FP at therapeutic doses [3]. The dose regimen used in the present study, in conjunction with a more sensitive assay, was designed to permit a pharmacokinetic comparison to be made between the two intranasal formulations.
Methods
FPANS was formulated as 100 µg micronized FP in 0.1 ml of 5% w/w dextrose solution containing 0.02% w/w benzalkonium chloride and 0.25% w/w phenylethylalcohol as preservatives. FP nasal drops were formulated as 400 µg in 0.4 ml of phosphate buffered saline. The entire contents were dripped into each nostril on each dosing occasion using the head down and forward position. This position required volunteers to be seated, leaning forward (approximate angle 45°) with the head bent towards the chest, to ensure that the nasal drops were delivered to the posterior region of the nasal cavity.
Twelve healthy volunteers (six male and six female; 18–50 years, body mass index 19–29 kg m−2) were enrolled into this randomised, open-label, two-way crossover study. The protocol was approved by the Ethics Committee, and all volunteers gave their informed consent. Volunteers received FP (800 µg) three times a day as FPANS or FP nasal drops over a 2 day period.
Four 800 µg doses of FP were administered, each separated by an 8 h dose interval (08.00 h, 16.00 h, midnight and 08.00 h the following day). After the last dose on day 2 (08.00 h), blood samples were collected over the following time-points for the assay of FP in plasma: (predose, 5, 10, 20, 30, 45 min, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h and 8 h postdose). Blood samples (5 ml) were drawn into lithium-heparin tubes, centrifuged within 30 min at 1500 g for 10 min, plasma separated and stored at −20° C until assay. Plasma samples were assayed for FP using solid phase extraction (on 96 well MicroLute II™ solid phase extraction blocks packed with 50 mg of Varian C18) and LC-MS-MS (using thermally and pneumatically assisted electrospray ionization and selected reaction monitoring). The method is accurate and precise with both intra- and interassay precision of less than 6% [3]. The method required 0.5 ml of plasma, and was validated over 20–1500 pg ml−1.
The FP plasma-concentration-time data were used to estimate the area under the plasma concentration-time curve (AUC), using WinNonlin version 1.1 and the linear trapezoidal method, maximum observed plasma concentration (Cmax) and time to maximum observed plasma concentration (tmax). For the calculation of FP plasma AUC, plasma concentration values below the detection limit were set equal to zero. Results were compared with previous data obtained from intravenously administered FP in a similar group of healthy subjects [4] to provide an estimate of the mean absolute systemic bioavailability of the two intranasal formulations.
Results
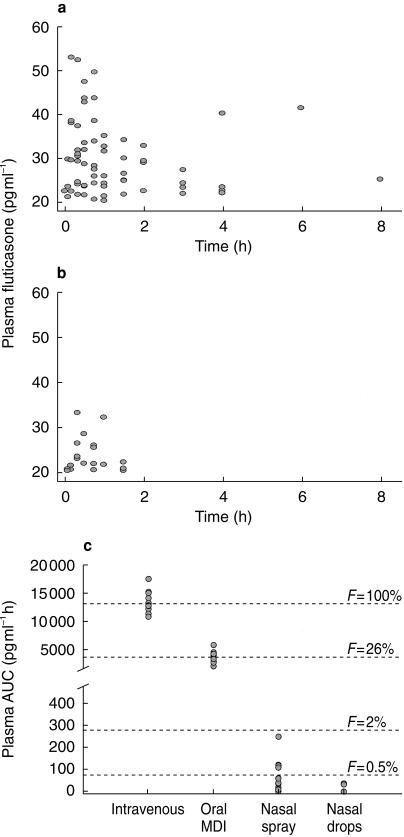
FP concentrations in plasma were undetectable in 2/12 of subjects on FPANS and 5/12 of subjects on nasal drops. The mean plasma AUC, Cmax, tmax for FPANS and FP nasal drops are shown in Table 1 and Figure 1.
Table 1.
Systemic exposure and bioavailability of fluticasone propionate administered as an aqueous nasal spray or nasal drop formulation in 12 healthy volunteers
| FP formulation | AUC (pg ml−1 h) | Cmax (pg ml−1) | tmax (h) |
|---|---|---|---|
| Nasal spray | 67.5 ± 20.7 | 31.4 ± 5.0 | 0.9 ± 0.4 |
| (800 µg tds) | (0–248.0) | (0–52.9) | (0.2–4.0) |
| Nasal drops | 8.5 ± 4.4 | 14.5 ± 3.8 | 0.36 ± 0.1 |
| (800 µg tds) | (0–36.6) | (0–33.2) | (0.08–0.75) |
| Median difference | 48.4 | 24.4 | 0.33 |
| 95% CI | (17.2, 107.8) | (1.5, 31.3) | (0.13, 1.76) |
| P | 0.005 | 0.026 | 0.25 |
Results are expressed as the mean±standard error (range). FP: fluticasone propionate, tds: three times daily.
Figure 1.
Individual plasma concentrations of fluticasone propionate in 12 healthy subjects under approximate steady-state conditions during an 8 h dosing interval following (a) 800 µg tds fluticasone propionate aqueous nasal spray or (b) 800 µg tds fluticasone propionate nasal drops. (c) Plasma AUC and absolute bioavailability (F) of intranasal fluticasone propionate relative to intravenous administration and in comparison with oral inhalation (normalized to 800 µg doses, n = 12).
There was an approximately eight fold lower relative bioavailability from the FP nasal drops compared with FPANS. The estimated absolute bioavailability from the two nasal formulations relative to intravenous administration (historical data) were 0.51% and 0.06% for FPANS and FP nasal drops, respectively (Figure 1).
Discussion
The present study showed that the systemic bioavailability from intranasally administered FP is very low, with the drops formulation (0.06%) producing an approximately eight-fold lower estimated bioavailability compared with FPANS (0.51%), estimated by reference to historical intravenous data (AUC(0,∞) following a 250 µg intravenous dose = 4159 pg ml−1 h [4]). The high dose rate (2400 µg day−1), coupled with a more sensitive assay, allowed reliable measurements of FP in plasma after intranasal dosing for the first time. FPANS and FP nasal drops produced approximately 50 and 400 times less systemic exposure, respectively, compared with FP administered by metered dose inhaler (MDI).
Other studies have shown that nasal drops are cleared more quickly from the nose than nasal spray [5, 6], and this supports our finding that nasal drops have a very low systemic bioavailability. In the present study, FP nasal drops administered in the head down and forward position, is intended to deliver the drug to the posterior region of the nose, to the ‘root’ of the polyps. Material deposited to this site is more likely to be cleared by the cilia and swallowed, reducing contact time with the mucosal surface. Both the FPANS and nasal drops are aqueous suspensions, and require solubilization before absorption. The dissolution rate of FP is slow; therefore the contact time and surface area probably limit the opportunity for absorption across the nasal mucosa. Following intranasal dosing of FP, the major portion of the dose is probably swallowed, and due to the negligible oral bioavailability of FP, does not result in significant systemic exposure.
In the present study we did not investigate the relationship between dose and systemic exposure to FP, but based the dose regimen on a previous study [1], and estimated the minimum FPANS dose rate required to produce a detectable concentration with the assay used. However, in other studies Cmax and AUC values for intranasally administered triamcinolone acetonide [7] and BDP [8] were shown to be dose-dependent. Compared with other intranasally administered corticosteroids FP appears to have low systemic bioavailability (FPANS (0.51%) and FP nasal drops (0.06%) compared with triamcinolone acetonide 44% [7, 9], beclomethasone dipropionate 44% [8] and budesonide 31% [10]). These higher values for triamcinolone acetonide, beclomethasone dipropionate and budesonide illustrate the importance of oral bioavailability in determining the systemic exposure to intranasal corticosteroids, and provide evidence that the nose may not be the major site of absorption. Consequently, this explains why only those agents with high oral bioavailability result in significant systemic exposure.
In conclusion, both FPANS and nasal drops exhibited a low systemic bioavailability. The bioavailability of the nasal drops was approximately eight times lower than FPANS.
Acknowledgments
Funding for the study (protocol number: FLTB1009) was provided by Glaxo Wellcome Research and Development. We thank Ruth Murray for writing and editing assistance during preparation of this manuscript. We also thank Tony McAllistair for his assistance.
References
- 1.McDowell JE, Mackie AE, Ventresca GP, Bye A. Pharmacokinetics and bioavailability of intra-nasal fluticasone in humans. Clin Drug Invest. 1997;1:103–105. [Google Scholar]
- 2.Harding SM. The human pharmacology of fluticasone propionate. Respir Med. 1990;84(Suppl A):25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- 3.Callejas SL, Biddlecombe RA, Jones AE, Joyce KB, Pereira AI, Pleasance S. Determination of the glucocorticoid fluticasone propionate in plasma by automated solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr B, Biomed Sci Applications. 1998;718:243–250. doi: 10.1016/s0378-4347(98)00374-0. [DOI] [PubMed] [Google Scholar]
- 4.Mackie AE, Ventresca GP, Fuller RW, Bye A. Pharmacokinetics of intravenous fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:539–542. doi: 10.1046/j.1365-2125.1996.36110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy JG, Lee SW, Wilson CG. Intra-nasal drug delivery by sprays and drops. J Pharm Pharmacol. 1985;37:294–297. doi: 10.1111/j.2042-7158.1985.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 6.Bryant ML, Brown P, Gurevich N, McDougall IR. Comparison of the clearance of radiolabelled nose drops and nasal spray as mucosally delivered vaccine. Nuclear Med Comm. 1999;20:171–174. doi: 10.1097/00006231-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hensel R, Mullen ME. Pharmacokinetic report: an open label pharmacokinetic study of RG5029Y (triamcinolone acetonide) aqueous nasal spray and Nasacort nasal inhaler. Rhône-Poulenc Rorer, Protocol no. RG 5029Y-101 (Data on file) 1993.
- 8.Daley-Yates P, Price AC, Pereira A, Richards DH. Absolute bioavailability of beclomethasone dipropionate administered via the inhaled, intra-nasal and oral routes in man. Allergy. 2000;55(Suppl 63):952. [Google Scholar]
- 9.Derendorf H. Pharmacokinetic and pharmacodynamic properties of inhaled corticosteroids in relation to efficacy and safety. Respir Med. 1997;91(Suppl A):22–28. doi: 10.1016/s0954-6111(97)90102-5. [DOI] [PubMed] [Google Scholar]
- 10.Thorsson L, Borga O, Edsbacker S. Systemic availability of budesonide after nasal administration of three different formulations; pressurized aerosol, aqueous spray pump and powder. Br J Clin Pharmacol. 1999;47:619–624. doi: 10.1046/j.1365-2125.1999.00956.x. 10.1046/j.1365-2125.1999.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]