Abstract
Aims
The purpose of this study was to characterize the relationship between the degree of anticoagulation, assessed by APTT, and the plasma concentration of inogatran in healthy subjects and in patients with coronary artery disease.
Methods
Data from five phase I studies in 78 healthy males and two phase II multicentre studies in 948 patients of both sexes with unstable angina pectoris or non-Q-wave myocardial infarction were evaluated. A total of 3296 pairs of concentration-APTT samples were obtained before, during, and after intravenous infusions of inogatran. Mixed effects modelling was used for population pharmacodynamic analysis of the drug effect and for describing the variability in baseline APTT.
Results
The population mean baseline APTT was 29 s, but large variations between individuals (s.d. 3.6 s) were observed. The variability between studies (1.3 s) and centres (1.8 s) were of less importance, though statistically significant. APTT increased in a nonlinear manner with increasing inogatran concentration and the relationship was well described by a combined linear and Emax model. A significant part of the overall variability could be ascribed to the APTT reagent and equipment used at the different study centres. These method-dependent differences were compensated for by including the lower limit of the normal reference range as a covariate, affecting both baseline and Emax, in the model. For the typical healthy subject and patient, the method-corrected population mean parameters were: APTTbaseline 35 and 31 s, slope 8.0 and 5.8 s l µmol−1, Emax 36 and 34 s, and EC50 0.54 and 0.72 µmol l−1, respectively. The model predicted plasma concentration needed to double the APTT from the baseline value was 1.25 and 1.45 µmol l−1 in the healthy volunteer and patient, respectively.
Conclusions
The nonlinear relationship between APTT and inogatran concentration in plasma was well described by a combined linear and Emax model. Pooling of data was made possible by incorporating a centre-specific characteristic of the assay method in the model. Patients had lower baseline APTT and appeared to have less pronounced effect of inogatran than young healthy subjects.
Keywords: activated partial thromboplastin time, anticoagulation, APTT, inogatran, population pharmacodynamic modelling, thrombin inhibition
Introduction
Inogatran is a synthetic low-molecular-weight thrombin inhibitor, developed for the possible treatment and prophylaxis of arterial and venous thrombotic diseases. Thrombin is a key enzyme in the coagulation cascade, catalysing the formation of fibrin from fibrinogen [1]. In vitro studies have shown that the inhibition of thrombin by inogatran is reversible, competitive and selective [2]. For this class of drugs it is important that an optimal degree of anticoagulation is attained that gives the desired antithrombotic effect without increasing the risk for adverse effects, such as bleeding complications, to an unacceptable level. One common surrogate marker for the degree of anticoagulation is the activated partial thromboplastin time (APTT), which has long been used to monitor treatment with heparin [3] and more recently in the clinical evaluation of direct thrombin inhibitors, e.g. hirudin, hirulog and argatroban [4–10]. Therapeutic ranges for APTT have been established empirically for heparin in various indications [11], but difficulties exist due to lack of standardization of measurement methods between laboratories. After incubation of citrated plasma with a reagent containing phospholipids as a substitute for platelet membranes and a contact activator, calcium is added and the clotting time (APTT) is registered. Sources of variation in the APTT result include, e.g. type and source of reagents, including batch-to-batch variations, type of instrument used for clot detection, citrate concentration in the test tube, and type of test tube [12–18]. In addition, anticoagulants with differing mechanism of action produce different levels of antithrombotic effect and bleeding at the same level of APTT [3, 8, 19–22]. Antithrombotic effects have been demonstrated for inogatran at plasma concentrations of 0.3–3 µmol l−1 in experimental rat models [20, 22, 23]. The predicted therapeutic level is 1 µmol l−1, which is the concentration that causes a two-fold increase of APTT from the baseline when inogatran is added to human plasma [2].
The aim of this investigation was to characterize the relationship between plasma concentration of inogatran and APTT, measured in healthy volunteers and in patients with coronary artery disease, and to identify important factors influencing this relationship. Pharmacodynamic data from seven clinical trials involving a total of 1026 individuals were therefore evaluated using the population approach.
Methods
Clinical studies
Data from five pharmacokinetic studies in 78 healthy volunteers and two clinical multicentre studies in 948 patients with coronary artery disease were included in the analyses. The main design characteristics of the studies are given in Table 1. All study protocols were approved by the relevant ethics committees and the studies were performed according to GCP (Good Clinical Practice) standards. Written informed consent was obtained prior to enrolment. The pharmacokinetic and clinical results from these studies have previously been reported [24–26].
Table 1.
Main study characteristics of i.v. inogatran data included in the population pharmacodynamic analysis
| Study | Number of centres | Number of subjects | Study design | Dosing regime | Number of samples | Conc. range (µmol l–1) |
|---|---|---|---|---|---|---|
| A | 1 | 22 healthy | Open, dose-escalation 2–4 subjects/dose, 1–2 doses/subject | i.v. inf. (10′) | 183 | 0.10–7.06 |
| B | 1 | 16 healthy | 1 dose/subject | i.v. inf. (10′) | 155 | 0.02–2.41 |
| C | 1 | 16 healthy | Open, 2-way cross-over inogatran or placebo | i.v. inf. (4 h) | 477 | 0.02–2.38 |
| D | 1 | 12 healthy | Double-blind, 2-way cross-over inogatran+placebo or inogatran+aspirin 150 mg p.o. | i.v. inf. (10′) | 249 | 0.02–0.92 |
| E | 1 | 12 healthy | 1 dose/subject | i.v. inf. (10′) | 32 | 0.02–2.42 |
| F | 4 | 49 patients | Open, 4 parallel dose groups | i.v. inf.10′ + 3 h 50′ or 10′ + 71 h 50′ | 286 | 0.03–1.91 |
| G | 61 | 899 patients | Double-blind, 3 parallel dose groups | i.v. bolus+inf. 72 h | 1913 | 0.04–9.10 |
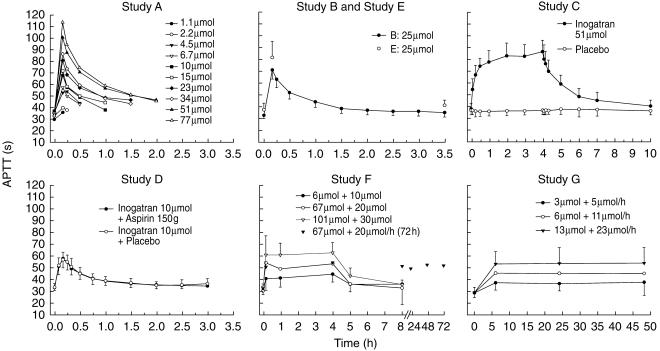
The amount of inogatran is given in Figure 1.
Healthy volunteers
Seventy-eight male volunteers, judged to be healthy by standard clinical and laboratory investigations, participated in five phase I pharmacokinetic studies (studies A-E) conducted at the AstraZeneca drug study unit (Göteborg, Sweden). Demographic information is summarized in Table 2. A total of 110 administrations of inogatran were given as i.v. infusions over 10 min (studies A-B, D-E) or 4 h (study C). In addition, placebo was given in study C. A total of 1096 pairs of blood samples (3–15 per subject) were drawn for subsequent determination of inogatran concentration and APTT and were all included in the analysis.
Table 2.
Subject characteristics. For continuous variables, the median (range) values are provided. For categorial variables the number (percentage) of subjects are given
| Healthy volunteers | Patients | |
|---|---|---|
| Gender | ||
| Male | 78 (100%) | 652 (69%) |
| Female | 0 | 296 (31%) |
| Age (years) | 28 (20–39) | 66 (32–81) |
| Weight (kg) | 76 (66–86) | 79 (50–121) |
| Smoking habits | ||
| Non-smoker | – | 414 (44%) |
| Previous smoker | – | 309 (33%) |
| Smoker | 2 (17%)1 | 223 (24%) |
| Baseline APTT (s) | 34 (28–49) | 29 (15–60) |
| Concomitant aspirin | 12 (15%) | 948 (100%) |
| Diagnosis | ||
| Unstable angina pectoris | 0 | 716 (76%) |
| Non Q-wave myocardial infarction | 0 | 232 (24%) |
| Hypertension | 0 | 333 (35%) |
| Diabetes | 0 | 153 (16%) |
| Cardiac failure | 0 | 124 (13%) |
| Clinical outcome | ||
| Death | 0 | 9 (1%) |
| Myocardial reinfarction | 0 | 41 (4%) |
| Refractory angina | 0 | 38 (4%) |
| Recurrect angina | 0 | 343 (36%) |
| None of the above | 78 (100%) | 517 (55%) |
Recorded in study E only
Patients
In a Swedish four-centre phase IIA study (study F) a total of 49 patients received inogatran as i.v. infusion during four hours (low, intermediate or high dose level) or three days (intermediate dose level). In a phase IIB study (study G) 904 patients from 61 Scandinavian centres obtained constant infusion with one of three inogatran dose levels for 3 days. In both studies, the patients should have a clinical diagnosis of unstable angina pectoris or non-Q-wave myocardial infarction, with an episode of chest pain of at least 10 min duration within the preceding 72 h, and should have been clinically stable in hospital for at least 8 h before inclusion. Patient characteristics are given in Table 2. In addition to the study drug, all patients received standard doses of aspirin throughout the studies. Standard treatments with nitroglycerin, β-adrenoceptor blockers and calcium antagonists were given at the discretion of the responsible physician. In 948 patients at least one plasma concentration vs APTT observation was recorded and the total number of data points were 2200 (≤ 4 per patient). All data points, except for one outlying value (APTT 90 s at 0.026 µmol l−1), were included in the pharmacodynamic modelling.
Study drug
The drug was administered as i.v. solutions containing a stable dihydrobromide salt of inogatran (base: MW 438.6, salt: MW 600.4). Dose information is given in Table 1.
Inogatran concentration in plasma
Venous blood samples were collected in 5 ml heparinized tubes. Plasma was recovered after centrifugation and stored at −20 °C until analysis. Plasma concentrations of inogatran were determined using reversed-phase liquid chromatography (LC) and positive electrospray ionization mass spectrometry. For quantification, an analogue to inogatran was used as internal standard. Inogatran was isolated from plasma (500 µl) by solid-phase extraction on octylsilica. LC separation was made on an ODS column using an acetonitrile/ammonium acetate/formic acid mobile phase. Inogatran and the internal standard were monitored by selected ion monitoring at m/z 439.2 and 453.2, respectively. The method was linear over the range 0.02–13 µmol l−1 (500 µl plasma sample), with a lower limit of quantification of 0.10 µmol l−1 in study A and 0.02 µmol l−1 in studies B-G. The coefficient of variation (n = 6) of daily plasma standards in study G, in which samples were analysed on 41 occasions, was 1.5–14.2% (mean 6.5%) at 0.02 µmol l−1 and 0.5–5.6% (mean 1.5%) at 6.8 µmol l−1.
Activated partial thromboplastin time
Blood samples were drawn in citrated tubes and plasma was separated. Activated partial thromboplastin time (APTT) was measured according to the standard method of the local laboratory. All samples from studies A-E were analysed by the same laboratory using the reagent PTT-Automate 10TM (Diagnostica Stago). The normal reference range of this laboratory was 30–42 s. In study F three additional laboratories were used with reference ranges of 23–34 s, 24–35 s, and 30–42 s, respectively. In study G, APTT was determined locally at 61 different centres. Lower reference values (RefLow) varied between 18 and 30 s (median 25 s) and upper reference values (RefUpp) were between 30 and 46 s (median 36 s) in these centres. The width of the reference intervals (RefWidth) varied between 6 and 20 s (median 12 s) and the midpoint (RefMid) between 26 and 38 s (median 31 s) for the different laboratories.
Pharmacodynamic modelling
Methodology and software
The population approach was applied for the pharmacodynamic modelling, using mixed effects models as implemented in the software package NONMEM (version V) [27]. The first-order (FO) method was used for estimation of mean and variability parameters. The postprocessor Xpose (version 2.0) [28] was used for model diagnostic purposes and for exploration of covariate relationships.
Baseline APTT
Prior to modelling of the drug effect, the observed baseline APTT values were characterized in order to quantify the variability between centres, between studies within centres, between individuals, and within individuals when no inogatran is present. A linear mixed effects model with four levels of variability was applied to all APTT measured immediately before drug administration for this purpose (studies A-G). In addition, the baseline values from all healthy volunteers and from patients (n = 138) at the three centres using corresponding APTT methodology (reference midpoint 36 s) were compared.
APTT after inogatran administration
To describe the effect of inogatran nonlinear mixed effects models were used. Different pharmacodynamic models were first fitted to absolute APTT values and inogatran plasma concentration data, pooled from the studies in healthy volunteers (studies A-E). The following structural models were compared (C = inogatran concentration):
Model 1: APTT = θ1+θ2 · log (C+1)
Model 2: APTT = θ1+θ2 · Cθ3
Model 3: APTT = θ1+θ2 · C/(θ3+C)
Model 4: APTT = θ1+θ2 · Cθ4/(+Cθ4)
Model 5: APTT = θ1+θ2 · C+θ3 C/(θ4+C)
The fixed effects (population mean) parameters (θ : s) and the random effects parameters (ω2 : s and σ2) were estimated, where ω2 is the variance of the individual deviations (η : s) from the population mean parameters (interindividual variability) and σ2 is the variance of the deviations from the model predicted APTT values (residual error, ε). Proportional and additive+proportional error models were tested. Interoccasion variability was modelled by the method proposed by Karlsson & Sheiner [29]. The models were compared according to goodness of fit plots and objective function values (OFV), which is approximately minus twice the logarithm of the maximum likelihood of the data [27]. The difference in objective function value (ΔOFV) between two hierarchical models is approximately χ2-distributed with n degrees of freedom, where n is the difference in the number of parameters between the two models. For example, a ΔOFV of 3.84 is significant at the 5% level, and 10.83 at the 0.1% level, for one degree of freedom.
The best model was selected for subsequent analysis of pooled patient data (studies F-G). The influence of covariates was then evaluated on these data, with the exception of concomitant aspirin medication that was studied in healthy volunteers only (study D). The covariates investigated in the patients were diagnosis, gender, age, weight, smoking habits, presence of hypertension, diabetes and cardiac failure, clinical outcome day 30, and centre-specific reference values for the APTT method (RefLow, RefUpp, RefMid and RefWidth), see Table 2 for details. Missing covariates were set to the study median (continuous covariate) or mode (categorial covariate).
The covariate analysis was performed in three steps.
Individual Bayesian estimates of the pharmacodynamic parameters were generated in NONMEM using the model without covariates. The individual parameter estimates were plotted against each covariate and potentially important covariates were identified using a generalized additive modelling procedure (GAM), proposed by Mandema et al. [30]. The GAM procedure was incorporated in Xpose and explores linear and nonlinear relationships [28].
All covariates selected by the GAM, and other covariates that were considered of interest, were then formally tested in a stepwise covariate analysis by NONMEM. Each covariate was included in the model, one at a time, assuming no covariance between the individual parameters (η : s). The covariate causing the largest reduction in OFV was kept in the population model. This procedure was then repeated until all statistically significant covariates have been included. The significance level during this model building phase was set to 0.05. Individual parameters (η : s) were then allowed to covary in the full model.
One covariate at a time was then deleted from the full model and the covariate causing the least increase in OFV was removed from the model, provided that the significance level was not less than 0.001. This procedure was repeated until the final model was reached, containing only those covariates that caused an improvement of the model at the significance level of 0.001.
Results
Baseline APTT
The overall population mean (s.e. mean) of the observed baseline APTT was 29.2 (0.01) s, but large differences were observed across individuals. In healthy volunteers the mean (95% C.I.) baseline value was 35.2 (34.3, 36.1) s, while in patients from matching study centres it was 31.1 (30.4, 31.7) s. The repeated observations after placebo infusion demonstrated that the APTT level was stable over time (Figure 1, study C). The major source of overall variability was attributed to interindividual differences (s.d. 3.6 s) and the variability between studies (within centre) contributed to less than one tenth (on the variance scale) of the total variability in APTT when no inogatran is present (Table 3). Intercentre variability was also significant, but less than the interindividual variability.
Figure 1.
Mean (± s.d. where applicable) APTT vs time curves. The doses are expressed for a 70 kg person when dosed per kg.
Table 3.
Variability estimates for baseline APTT
| Variability component | Standard deviation (σ) | Standard error (s.e. σ) | Significance level1 (P) |
|---|---|---|---|
| Intercentre | 1.8 s | 0.27 s | < 0.0005 |
| Interstudy | 1.3 s | 0.34 s | < 0.005 |
| Interindividual | 3.6 s | 0.16 s | < 0.0005 |
| Interoccasion+residual | 1.4 s | 0.01 s | N.A. |
Based on increase in OFV when the variability component was fixed to zero.
APTT after inogatran administration
For each study, the mean APTT values vs time after start of infusion are presented in Figure 1. The highest observed APTT was 129 s in the healthy subjects and 108 s in the patients, obtained after 10 min i.v. infusion of 83 µmol and a steady state infusion of 11.4 µmol h−1, respectively. The APTT was close to baseline 6 h after cessation of the infusion. The response was not affected by concomitant administration of aspirin (study D).
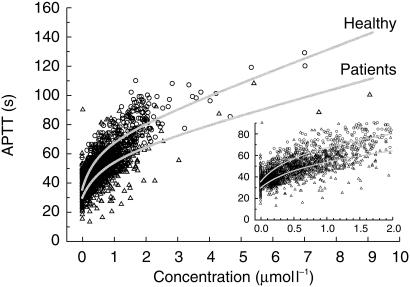
The prolongation of APTT increased in a nonlinear manner with increasing inogatran concentration (Figure 2). Individual plots of the increasing and decreasing phases of the APTT vs plasma concentration curves showed no signs of hysteresis. The pooled data from healthy volunteers was best described by the combined linear and Emax model (model 5) with residual error proportional to the predicted APTT. Interindividual variability components (η : s) were significant for APTTbaseline and Emax only, and these were highly correlated. This implies that a subject with a high baseline APTT is prone to have a higher degree of response to inogatran, compared to a subject with a low baseline value. All mean and variability parameter estimates are given in Table 4.
Figure 2.
Combined linear and Emax models (solid lines), fitted to pooled data from healthy volunteers (circles) and patients with coronary arterial disease (triangles). Models without covariates.
Table 4.
Parameter estimates (s.e. estimate) and objective function values for the pharmacodynamic modela
| Parameter | Healthy volunteers | Patients – no covariates | Patients – full model | Patients – final model |
|---|---|---|---|---|
| Structural model parameters (θ : s) | ||||
| APTTbaseline (θ1) | 35.1 (0.5) | 29.5 (0.1) | 29.2b (0.1) | 29.2b (0.1) |
| RefLow on APTTbaseline (θ5) | – | – | 0.36b (0.06) | 0.36b (0.06) |
| Age on APTTbaseline (θ6) | – | – | −0.039b (0.019) | – |
| SLOPE (θ2) | 8.0 (0.6) | 5.8 (1.4) | 5.6 (1.4) | 5.8 (1.4) |
| Emax (θ3) | 36 (2) | 31 (5) | 32c (5) | 31c (5) |
| RefLow on Emax (θ7) | – | – | 0.70c (0.23) | 0.67c (0.22) |
| Diagnosis on Emax (θ8) | – | – | −2.8c (1.2) | – |
| EC50 (θ4) | 0.54 (0.06) | 0.72 (0.12) | 0.74 (0.11) | 0.72 (0.12) |
| Interindividual variability parameters (ω : s) | ||||
| APTTbaseline (%) | 10 (2) | 12 (2) | 11 (2) | 11 (2) |
| Emax (%) | 20 (4) | 29 (8) | 28 (8) | 28 (8) |
| Correlation between APTTbaseline and Emax (r) | 1.00 (17%)d | 0.43 (39%)d | 0.37 (49%)d | 0.37 (49%)d |
| Interoccasion variability parameter (π) | ||||
| APTTbaseline (%) | 4.5 (1.1) | N.A. | N.A | N.A. |
| Residual error parameter (σ) | ||||
| Coefficient of variation (%) | 5.0 (1.0) | 8.9 (0.9) | 8.8 (0.9) | 8.8 (0.9) |
| Objective function value | 3371 | 9298 | 9222 | 9237 |
APTT=APTTbaseline+SLOPE C +Emax C/(EC50 + C), where C is the concentration in µmol l−1 and APTT is in s.
APTTbaseline=θ1 + θ5 (RefLow - 25)−θ6(Age-66), where 25 is the median of the lower limit of the reference range for the APTT method and 66 is the median age.
Emax=θ3 + θ7 (RefLow - 25) + θ8Diagnosis, where 0=Angina pectoris and 1 = Non-Q-wave myocardial infarction.
Relative s.e. of COV(APTTbaseline, Emax)
The combined linear and Emax model provided a good fit to the patient data too, although the structural parameters differed somewhat from those obtained in the healthy subjects. Interindividual variability parameters (ω:s) were similar for the two populations, but the residual variability (σ) was higher for the patient data compared to the data from healthy subjects. The forward inclusion of covariates resulted in a full model in which APTTbaseline was influenced by age and the APTT method, the lower reference value (RefLow) being the most explanatory covariate. Emax was also correlated to the RefLow as well as to the diagnosis. Age and diagnosis were, however, of borderline statistical significance (P = 0.004 and P = 0.010, respectively) according to the backward retention criterion and were therefore excluded from the final model, see Table 4.
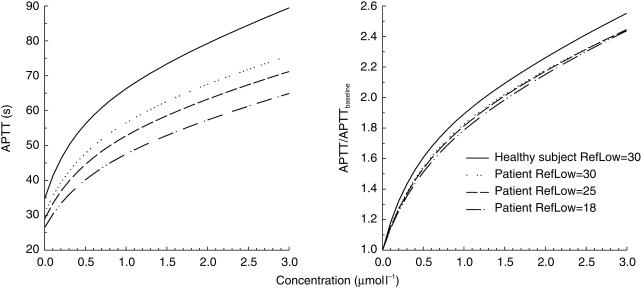
Patients thus have lower baseline APTT and seem to have less pronounced effect of inogatran than healthy subjects when the reference range of the APTT method is not taken into account (Figure 2, Table 4). The influence of the lower reference value of the assay method is illustrated in Figure 3 (left panel), where the model predicted APTT values for patients have been calculated at varying RefLow values. When comparing the curves at a RefLow of 30 s, the value for the phase I centre, a difference between patients and healthy volunteers is still indicated. The method-corrected model parameters were (mean (s.e. mean)): APTTbaseline 35 (0.5) and 31 (0.3) s, slope 8.0 (0.6) and 5.8 (1.4) s l µmol−1, Emax 36 (2.4) and 34 (5.1) s, and EC50 0.54 (0.06) and 0.72 (0.12) µmol l−1 for healthy subjects and patients, respectively. By taking the ratio between APTT and APTTbaseline the method-dependency is essentially eliminated in the expected therapeutic range and the difference between patients and healthy subjects is considerably less, see Figure 3 (right panel). For example, the concentration that is needed to increase the APTT to twice the baseline value in a typical patient is 1.55 µmol l−1 at a RefLow of 18 s and 1.45 µmol l−1 at a RefLow of 30 s. In comparison, the model predicts that the corresponding concentration is 1.25 µmol l−1 at RefLow 30 s in the typical healthy subject.
Figure 3.
Model predicted APTT (left panel) and APTT-ratio (right panel) vs inogatran plasma concentration in a typical patient and healthy subject at varying lower limits of the reference range (RefLow).
Discussion
The relationship between the degree of anticoagulation, APTT, the plasma concentration of the direct thrombin inhibitor inogatran was investigated in a large number of healthy volunteers and patients with coronary artery disease. The pharmacodynamic response after administration of heparins and other thrombin inhibitors has previously been described in the literature, mostly by linear models between APTT (or APTT-ratio) and drug levels (untransformed or after logarithmic transformations) [7, 17, 31–34]. Polynomial [35], exponential [36] and parabolic [37, 38] models have also been applied. We found that the nonlinear APTT response after inogatran was best described by a combined linear and Emax model, allowing predictions in the concentration range from zero (baseline APTT) to 9 µmol l−1. These ex vivo results are similar to previously reported in vitro data, which followed the same curvilinear shape with no maximum attained when inogatran was added to human plasma [2].
The analysis of the observed baseline APTT demonstrated that the variability between studies and study centres were small, compared with the individual variability, which justifies pooling of data from different studies and centres in the population pharmacodynamic modelling. The reason for the significant variability between studies performed at the same study centre on the same population (young healthy subjects) is not evident, but could be due to batch-to-batch variations not being fully compensated for. The variability between centres can be explained by methodological differences in the APTT method, as reflected by the diverse normal reference ranges reported. Information from the reference range was therefore included in the model of the drug effect. Of the characteristics tested, the lower reference value (RefLow) was the most significant covariate, influencing the pharmacodynamic parameters APTTbaseline and Emax. RefUpp and RefMid also had some influence on both parameters, while RefWidth did not affect any of them. It might have been expected that the RefWidth should reflect the sensitivity of the reagent used and therefore influence the degree of response to inogatran. However, since the criteria for defining the reference range varies between laboratories, the RefWidth will be influenced by several factors in addition to the assay sensitivity, e.g. the number of normal plasma samples and the variability between individuals selected for assessment of the APTT reference range. Nevertheless, this range contains some quantitative information about the sensitivity of the method, which can be accounted for by the lower reference value when predicting the APTT response. As long as the APTT method is not standardized, the normal reference range is a convenient characteristic since it can easily be obtained from accredited laboratories. Another means of compensating for the methodological differences could be to take the ratio between APTT and baseline APTT, where the latter is either the patient's pretreatment value or an average value from pooled normal plasma. This approach is sometimes used in clinical practice when monitoring heparin treatment. When modelling a pharmacodynamic response, however, it is generally most appropriate to include the baseline value as a parameter to be estimated in the model [39], since the baseline measurement is afflicted with random error. This study shows that most of the disparities in APTT-result obtained from different laboratories, for a typical inogatran patient, were eliminated by taking the APTT-ratio (APTT/APTTbaseline). Other studies have, however, shown that quite different ratios are obtained with varying reagents for heparin and hirudin [11, 13, 17, 40, 41]. More importantly, it has to our knowledge not been shown whether the APTT-ratio or the absolute APTT value is the clinically most relevant marker for anticoagulation, i.e. whether two patients with different absolute APTT, but the same ratio (APTT/APTTbaseline), are at comparable anticoagulation, or if the patient with the higher absolute APTT value is at higher risk, for example haemorrhage, everything else being equal. Which of the variables absolute APTT, absolute increase, or relative increase (APTT/APTTbaseline), is the best predictor for the clinical outcome after thrombin inhibition remains to be shown.
Differences in baseline APTT and response to inogatran between healthy volunteers and patients were observed. A formal test of a true difference could, however, not be performed due to imbalance in the dataset, where all data from healthy volunteers originated from one centre, whose reference interval was in the upper region. To be able to discern the patient factor from the other variability sources, by simultaneously fitting a mixed effects model to pooled healthy and patient data, five levels of random effects (study, centre, individual, occasion and residual variability) would ideally have been required. Such a model is not possible to implement within NONMEM. The results from the subanalysis of data from centres with matching reference values do, however, support the finding of different APTT values between the two study populations. Furthermore, it seems plausible that patients with acute thrombosis have lower APTT values than healthy subjects, since they are in a hypercoagulable state. Similar results have been reported where patients with thrombosis responded with lower APTT prolongation to heparin treatment, compared to when the drug was added in vitro to plasma from healthy subjects [14, 18, 42] and in a pure in vitro comparison between spiked plasma from patients and normal subjects the patient plasma was less sensitive to heparin [43]. Another contributing factor to the difference in APTT between patients and healthy subjects, observed in our study, could be that the patients were older, since a small age effect on the baseline value was indicated in the patient material (−2 s in the interval 32–81 years, P = 0.004). Gender, weight, smoking habits, hypertension, cardiac failure or diabetes had no influence on the baseline APTT or APTT response after inogatran, and no relationships between any of the pharmacodynamic parameters and the clinical outcome were seen.
The population approach is commonly applied to data collected from clinical trials in the late phases of drug development, typically large multicentre studies. Method-dependency can then be a complicating factor, which could introduce bias in the parameter estimates and create false covariate–parameter relationships, if overlooked. Clinical pharmacological observations from different sources using different methods can only be compared if method–effect interaction can be excluded. In a recent review of more than 200 clinical pharmacological publications on blood pressure, the authors found that the data were not reported with adequate methodological specification, although there are well-known method-dependencies in that field [44]. In the current study, methodological differences were accounted for by including a characteristic of the normal reference range as a covariate in the model, affecting both the baseline value and the degree of the response. This approach might be used in other areas as well when a method-interaction is present and data from several studies and/or study centres are to be combined in a population model.
In conclusion, the nonlinear relationship between APTT and inogatran concentration in plasma could be well described by a combined linear and Emax model. Pooling of data was made possible by incorporating a centre-specific characteristic of the assay method in the model. Patients had lower baseline APTT and appeared to have less pronounced effect of inogatran on APTT than healthy subjects. This difference could be due to the higher age of the patients or to them being in a hypercoagulable state.
Acknowledgments
In addition to the investigators and all other study personnel the authors wish to thank the following coworkers at AstraZeneca Mölndal: Christer Mattsson and Karin Wåhlander for sharing with us their expert knowledge of coagulation tests, and Claes Ekman and Lars Frison for valuable statistical discussions.
References
- 1.Mann KG. Prothrombin and thrombin. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 3. Philadelphia: JB Lippincott; 1994. pp. 71–79. [Google Scholar]
- 2.Teger-Nilsson A-C, Bylund R, Gustafsson D, Gyzander E, Eriksson U. In vitro effects of inogatran, a selective low molecular weight thrombin inhibitor. Thromb Res. 1997;85:133–145. doi: 10.1016/s0049-3848(96)00230-7. 10.1016/s0049-3848(96)00230-7. [DOI] [PubMed] [Google Scholar]
- 3.Kher A, Dieri RA, Hemker HC, Béguin S. Laboratory assessment of antithrombotic therapy: What tests and if so why? Haemostasis. 1997;27:211–218. doi: 10.1159/000217459. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM for the TIMI; A Investigators. Hirudin in acute myocardial infarction. Safety report from the thrombolysis and thrombin inhibition in myocardial infarction (TIMI) 9A trial. Circulation. 1994;90:1624–1630. doi: 10.1161/01.cir.90.4.1624. [DOI] [PubMed] [Google Scholar]
- 5.Clarke RJ, Mayo RN, Fitzgerald GA, Fitzgerald DJ. Combined administration of aspirin and a specific thrombin inhibitor in man. Circulation. 1991;83:1510–1518. doi: 10.1161/01.cir.83.5.1510. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KA. Clinical pharmacology and drug safety: Lessons from hirudin. Clin Pharmacol Ther. 1995;58:123–126. doi: 10.1016/0009-9236(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 7.Fox I, Dawson A, Loynds P, et al. Anticoagulant activity of HirulogTM, a direct thrombin inhibitor, in humans. Thromb Haemost. 1993;69:157–163. [PubMed] [Google Scholar]
- 8.Gusto IIa Investigators. Randomized trial of intravenous heparin versus recombinant hirudin for acute coronary syndromes. Circulation. 1994;90:1631–1637. doi: 10.1161/01.cir.90.4.1631. [DOI] [PubMed] [Google Scholar]
- 9.Gusto IIb Investigators. A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med. 1996;335:775–782. doi: 10.1056/NEJM199609123351103. [DOI] [PubMed] [Google Scholar]
- 10.Lee V. Initial experience with hirudin and streptokinase in acute myocardial infarction: Results of the thrombolysis in myocardial infarction (TIMI) 6 trial. Am J Cardiol. 1995;75:7–13. doi: 10.1016/s0002-9149(99)80517-7. 10.1016/s0002-9149(99)80517-7. [DOI] [PubMed] [Google Scholar]
- 11.Hirsh J. Heparin. New Engl J Med. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 12.Adcock DM, Kressin DC, Marlar RA. Effect of 3.2% vs 3.8% sodium citrate concentration on routine coagulation testing. Am J Clin Pathol. 1997;107:105–110. doi: 10.1093/ajcp/107.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Monreal M, Monreal L, Ruiz de Gopegui R, Espada Y, Angles AM, Monasterio J. Effects of two different doses of hirudin on APTT, determined with eight different reagents. Thromb Haemost. 1995;73:219–222. [PubMed] [Google Scholar]
- 14.Reed SV, Haddon ME, Denson KW. An attempt to standardize the APTT for heparin monitoring, using the P.T. ISI/INR system of calibration. Results of a 13 centre study. Thromb Res. 1994;74:515–522. doi: 10.1016/0049-3848(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 15.Shojania AM, Tetreault J, Turnbull G. The variations between heparin sensitivity of different lots of activated partial thromboplastin time reagent produced by the same manufacturer. Am J Clin Pathol. 1988;89:19–23. doi: 10.1093/ajcp/89.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Siegel JE, Bernard DW, Swami VK, Sazama K. Monitoring heparin therapy. APTT results from partial- vs full-draw tubes. Am J Clin Pathol. 1998;110:184–187. doi: 10.1093/ajcp/110.2.184. [DOI] [PubMed] [Google Scholar]
- 17.Tripodi A, Chantarangkul V, Arbini AA, Moia M, Mannucci PM. Effects of hirudin on activated partial thromboplastin time determined with ten different reagents. Thromb Haemost. 1993;70:286–288. [PubMed] [Google Scholar]
- 18.Van der Velde EA, Poller L. The APTT monitoring of heparin – the ISTH/ICSH Collaborative Study. Thromb Haemost. 1995;73:73–81. [PubMed] [Google Scholar]
- 19.Carteaux JP, Gast A, Tschopp TB, Roux S. Activated clotting time as an appropriate test to compare heparin and direct thrombin inhibitors such as hirudin or Ro 46–6240 in experimental arterial thrombosis. Circulation. 1995;91:1568–1574. doi: 10.1161/01.cir.91.5.1568. [DOI] [PubMed] [Google Scholar]
- 20.Elg M, Gustafsson D, Carlsson S. Antithrombotic effects and bleeding time of thrombin inhibitors and warfarin in the rat. Thromb Res. 1999;94:187–197. doi: 10.1016/s0049-3848(98)00213-8. 10.1016/s0049-3848(98)00213-8. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg JS, Nurmohamed MT, Gent M, et al. Use of Hirulog in the Prevention of venous thrombosis after major hip or knee surgery. Circulation. 1994;90:2385–2389. doi: 10.1161/01.cir.90.5.2385. [DOI] [PubMed] [Google Scholar]
- 22.Elg M, Gustafsson D, Deinum J. The importance of enzyme inhibition kinetics for the effect of thrombin inhibitors in a rat model of arterial thrombosis. Thromb Haemost. 1997;78:1286–1292. [PubMed] [Google Scholar]
- 23.Gustafsson D, Elg M, Lenfors M, Börjesson I, Teger-Nilsson A-C. Effects of inogatran, a new low-molecular-weight thrombin inhibitor, in rat models of venous and arterial thrombosis, thrombolysis and bleeding time. Blood Coagulation Fibrinolysis. 1996;7:69–79. doi: 10.1097/00001721-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Teger-Nilsson A-C, Eriksson U, Gustafsson D, Bylund R, Fager G, Held P. Phase I studies on inogatran, a new selective thrombin inhibitor. J Am Coll Cardiol, Special issue. Abstract from ACC Meeting, New Orleans, March, 1995. 1995:117A. [Google Scholar]
- 25.Andersen K, Dellborg M, Emanuelsson H, Grip L, Swedberg K. Thrombin inhibition with inogatran for unstable angina pectoris: evidence for reactivated ischemia after cessation of short-term treatment. Coronary Artery Disease. 1996;7:673–681. doi: 10.1097/00019501-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Thrombin Inhibition in Myocardial Ischemia (TRIM) study group. A low molecular weight, selective thrombin inhibitor, inogatran, vs heparin, in unstable coronary artery disease in 1209 patients. Eur Heart J. 1997;18:1416–1425. doi: 10.1093/oxfordjournals.eurheartj.a015467. [DOI] [PubMed] [Google Scholar]
- 27.NONMEM Users Guides. NONMEM Project Group. In: Beal SL, Sheiner LB, editors. San Francisco: University of California at San Francisco; 1992. [Google Scholar]
- 28.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based model building aid for population analysis with NONMEM. In: Aarons L, Balant LP, Danhof M, et al., editors. The population approach: measuring and managing variability in response concentration and dose. Brussels: European Commission; 1997. pp. 411–413. [Google Scholar]
- 29.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokin Biopharm. 1993;21:735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 30.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokin Biopharm. 1992;20:511–528. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 31.Baker BA, Adelman MD, Smith PA, Osborn JC. Inability of the activated partial thromboplastin time to predict heparin levels: time to reassess guidelines for heparin assays. Arch Intern Med. 1997;157:2475–2479. [PubMed] [Google Scholar]
- 32.Nurmohamed MT, Berckmans RJ, Morriën-Salomons WM, et al. Monitoring anticoagulant therapy by activated partial thromboplastin time: Hirudin assessment. Thromb Haemost. 1994;72:685–692. [PubMed] [Google Scholar]
- 33.Pötzsch B, Madlener K, Seelig C, Riess C, Greinacher A, Müller-Berghaus G. Monitoring of r-hirudin anticoagulation during cardiopulmonary bypass – assessment of the whole blood ecarin clotting time. Thromb Haemost. 1997;77:920–925. [PubMed] [Google Scholar]
- 34.Schenk JF, Glusa E, Radziwon P, Butti A, Markwardt F, Breddin HK. A recombinant hirudin (IK-HIR02) in healthy volunteers. I. Effects on coagulation parameters and bleeding time. Haemostasis. 1996;26:140–149. doi: 10.1159/000217199. [DOI] [PubMed] [Google Scholar]
- 35.Cirillo R, Lippi A, Subissi A, Agnelli G, Criscuoli M. Experimental pharmacology of hirunorm: a novel synthetic peptide thrombin inhibitor. Thromb Haemost. 1996;76:384–392. [PubMed] [Google Scholar]
- 36.Schoemaker RC, Cohen AF. Estimating impossible curves using NONMEM. Br J Clin Pharmacol. 1996;42:283–290. doi: 10.1046/j.1365-2125.1996.04231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksson H, Eriksson UG, Frison L, et al. Pharmacokinetics and pharmacodynamics of melagatran, a novel synthetic LMW thrombin inhibitor, in patients with acute DVT. Thromb Haemost. 1999;81:358–363. [PubMed] [Google Scholar]
- 38.Marbet GA, Verstraete M, Kienast J, et al. Clinical pharmacology of intravenously administered recombinant desulfatohirudin (CGP 39393) in healthy volunteers. J Cardiovasc Pharmacol. 1993;22:364–372. doi: 10.1097/00005344-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Colburn WA, Eldon MA. Models of drug action: Experimental design issues. In: Van Boxtel CJ, Holford NHG, Danhof M, editors. The in vivo study of drug action. Amsterdam: Elsevier; 1992. pp. 17–29. [Google Scholar]
- 40.Esslinger H-U, Haas S, Maurer R, Lassmann A, Dübbers K, Müller-Peltzer H. Pharmacodynamic and safety results of PEG-hirudin in healthy volunteers. Thromb Haemost. 1997;77:911–919. [PubMed] [Google Scholar]
- 41.Zanke B, Shojania AM. Comparison of two APTT methods of monitoring heparin therapy. APTT ratio and heparin response of pooled normal plasma. Am J Clin Pathol. 1990;93:684–689. doi: 10.1093/ajcp/93.5.684. [DOI] [PubMed] [Google Scholar]
- 42.Bain B, Forster T, Sleigh B. Heparin and the activated PTT. A difference between the in vitro and in vivo effects and implications for the therapeutic range. Am J Clin Pathol. 1980;74:515–522. doi: 10.1093/ajcp/74.5.668. [DOI] [PubMed] [Google Scholar]
- 43.Thomson JM. The control of heparin therapy by the activated partial thromboplastin time: sensitivity of various thromboplastins to heparin. In: Triplett DA, editor. Standardization of Coagulation Assays: an Overview. Skokie, IL: College of American Pathologists; 1982. pp. 195–206. [Google Scholar]
- 44.De Mey C, Enterling D. Issues of method-specificity and -dependency of blood pressure data: lack of reporting of methodological specifications. Eur J Clin Pharmacol. 1998;53:295–297. doi: 10.1007/s002280050381. 10.1007/s002280050381. [DOI] [PubMed] [Google Scholar]