Abstract
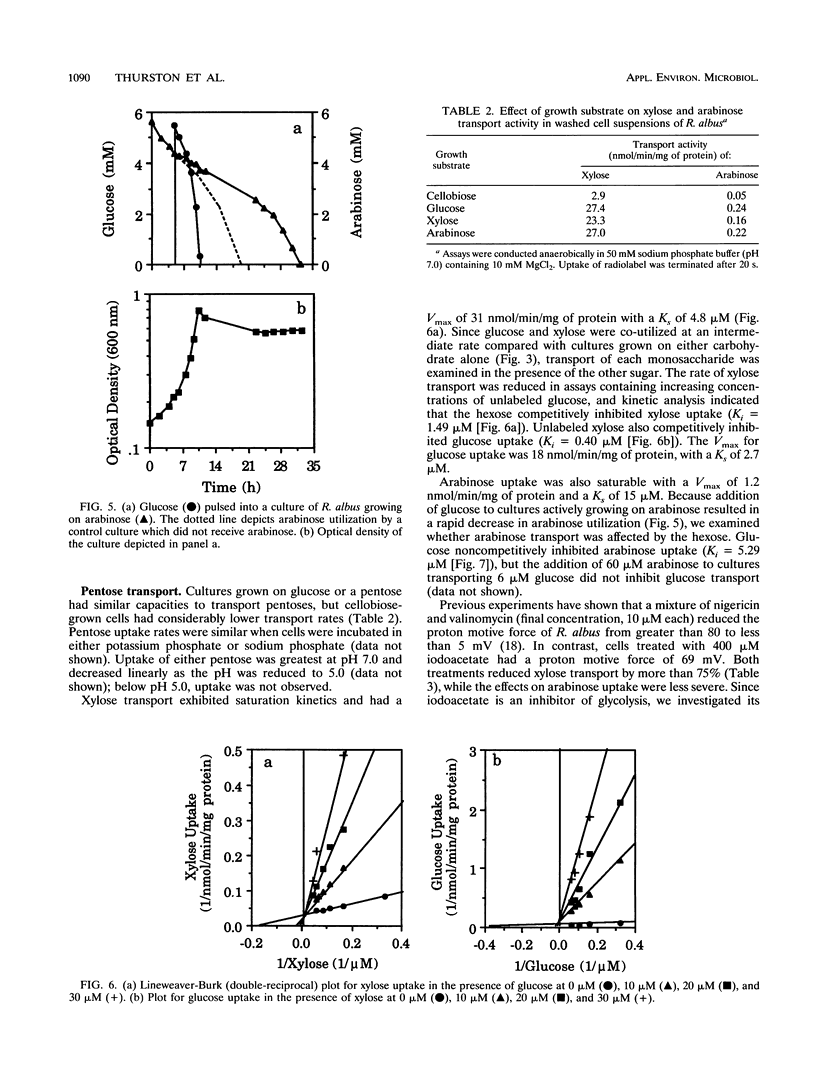
Ruminococcus albus is an important fibrolytic ruminal bacteria which degrades hemicellulose and ferments the resulting pentose sugars. However, little information is available on the utilization of pentoses by this organism or the effect of hexose sugars on pentose metabolism. Enzymatic studies indicated that R. albus metabolized pentoses via the pentose phosphate pathway and possessed constitutive transketolase activity. Cellobiose was preferred over xylose and arabinose, and it appeared that the disaccharide decreased pentose metabolism by repression of transport activity and catabolic enzymes (isomerases and kinases). Glucose and xylose were co-utilized, and transport studies suggested that there was a common transport system for both sugars. In contrast, glucose was preferred over arabinose and the hexose noncompetitively inhibited the transport of arabinose. Since R. albus lacks a glucose phosphotransferase system, the inhibition of arabinose uptake could not be explained by previously described models of inducer exclusion involving such a system. Because accumulation of radiolabeled xylose, arabinose, and glucose proceeded in the absence of a proton motive force and since transport was correlated with the intracellular ATP concentration, it appeared that monosaccharide uptake was driven by ATP hydrolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Hungate R. E. alpha-L-arabinofuranosidase from Ruminococcus albus 8: purification and possible role in hydrolysis of alfalfa cell wall. Appl Environ Microbiol. 1984 May;47(5):1135–1140. doi: 10.1128/aem.47.5.1135-1140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Stack R. J., Hungate R. E. Muralytic Activities of Ruminococcus albus 8. Appl Environ Microbiol. 1984 May;47(5):1141–1145. doi: 10.1128/aem.47.5.1141-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundin A., Richardsson A., Thore A. Continous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976 Oct;75(2):611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Matte A., Forsberg C. W., Verrinder Gibbins A. M. Enzymes associated with metabolism of xylose and other pentoses by Prevotella (Bacteroides) ruminicola strains, Selenomonas ruminantium D, and Fibrobacter succinogenes S85. Can J Microbiol. 1992 May;38(5):370–376. doi: 10.1139/m92-063. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanna D. K., Sanderson K. E. Uptake and catabolism of D-xylose in Salmonella typhimurium LT2. J Bacteriol. 1979 Jul;139(1):64–70. doi: 10.1128/jb.139.1.64-70.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. J., Dawson K. A. Xylose and arabinose utilization by the rumen bacterium Butyrivibrio fibrisolvens. FEMS Microbiol Lett. 1993 Nov 1;113(3):291–296. doi: 10.1111/j.1574-6968.1993.tb06529.x. [DOI] [PubMed] [Google Scholar]
- Strobel H. J. Evidence for catabolite inhibition in regulation of pentose utilization and transport in the ruminal bacterium Selenomonas ruminantium. Appl Environ Microbiol. 1993 Jan;59(1):40–46. doi: 10.1128/aem.59.1.40-46.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. J. Pentose utilization and transport by the ruminal bacterium Prevotella ruminicola. Arch Microbiol. 1993;159(5):465–471. doi: 10.1007/BF00288595. [DOI] [PubMed] [Google Scholar]
- Thurston B., Dawson K. A., Strobel H. J. Cellobiose versus glucose utilization by the ruminal bacterium Ruminococcus albus. Appl Environ Microbiol. 1993 Aug;59(8):2631–2637. doi: 10.1128/aem.59.8.2631-2637.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



