Abstract
Aims
To test the hypothesis that the α2-adrenergic agonist, dexmedetomidine, dilates the pupil and does not alter the pupillary light reflex of anaesthetized patients.
Methods
Eight volunteers were administered general anaesthesia with propofol, nitrous oxide and alfentanil. One hour and 25 min after induction of anaesthesia, a 45 min infusion of dexmedetomidine was begun, targeting a plasma concentration of 0.6 ng ml−1. Pupil size, pupillary light reflex amplitude, light reflex recovery time, and reflex dilation were measured before and during dexmedetomidine infusion.
Results
Dexmedetomidine produced no change in pupil size and light reflex recovery time, increased the light reflex from 0.30 ± 0.14 to 0.37 ± 0.12 mm and significantly reduced pupillary reflex dilation by 72 ± 62%.
Conclusions
These pupillary effects of dexmedetomidine in humans are difficult to reconcile with the findings obtained in cats and rats that have demonstrated a direct inhibitory effect of α2-adrenergic agonists on the pupilloconstrictor nucleus. The increase in the magnitude of the light reflex in response to dexmedetomidine does not necessarily involve an anxiolytic mechanism.
Keywords: anaesthetics, intravenous: dexmedetomidine, pupil: size, reflex dilation, light reflex
Introduction
Clonidine constricts the human pupil and enhances the light reflex [1, 2], but produces mydriasis in cats and rats. The mechanisms of these effects are not known, but animal studies have provided some insight into how noradrenergic pathways alter pupillary reflexes. Koss has demonstrated that noradrenaline inhibits the pupilloconstrictor nucleus of cats via an α2-adrenergic mechanism [3], and the source of this inhibition is thought to be located in the locus coeruleus [4]. Clonidine also activates inhibitory receptors on cell bodies within the locus coeruleus [5], thus reducing the tonic inhibitory tone of these cells on the pupilloconstrictor neurones. The predominate effect of these opposing actions on pupil size, in conscious or anaesthetized cats and rats, is the pupilloconstrictor nucleus, as indicated by mydriasis following clonidine administration and reversal of this effect by the α2-adrenergic antagonist, yohimbine [3].
The relevance of this information to humans is unknown, but of interest, because of the value of pupillary reflexes in diverse clinical and experimental settings [6–10]. Szabadi et al. have proposed a mechanism of action of α2-adrenoceptor agonists on the human pupil that is the same as that demonstrated in cats and rats, with one exception—that, in humans, the autoreceptor inhibitory effects within the locus coeruleus are greater than the pupilloconstrictor inhibitory effects, whereas the reverse is true in cats and rats [4]. Exploring the anxiolytic properties of the α2-adrenoceptor agonists, these same investigators have found that anxiety depresses the pupillary light reflex while clonidine and diazepam [11] prevent this response, implying that some drugs eliminate emotional effects on the light reflex.
To clarify the effect of α2-adrenoceptor agonists on the human pupil, we measured the effect of the α2-adrenoceptor agonist dexmedetomidine on pupil size and light reactivity during opioid-supplemented general anaesthesia. Dexmedetomidine is an intravenously administered, highly specific α2-adrenoceptor agonist with α2 to α1 receptor selectivity approximately 10 times greater than that of clonidine [12]. Because the locus coeruleus neurones are quiescent during opioid-supplemented general anaesthesia [13–16], we hypothesized that this anaesthetic model would provide a good environment for investigating dexmedetomidine's pupillary effects. We further hypothesized that because of this anaesthetic-induced depressant effect on the locus coeruleus, we would observe miosis from the anaesthetic [17] and then mydriasis following administration of dexmedetomidine through its inhibitory action on the pupilloconstrictor nucleus. Finally, because anaesthesia would eliminate the effects of mental and emotional stimuli, we anticipated that dexmedetomidine would have no direct effect on the magnitude of the light reflex.
Methods
With approval from the Committee on Human Research at the University of California, San Francisco and written informed consent, we studied three male and five female fasting, healthy volunteers. Morphometric characteristics included age 32 ± 8 years, weight 72 ± 11 kg, height 174 ± 12 cm. We excluded any subjects with body weight >130% normal and those with a history of alcohol or drug abuse, or a history of cardiac, pulmonary, hepatic or renal disease.
On the study day, a catheter was inserted into an arm vein and 10 ml kg−1 of lactated Ringer's solution was administered before the induction of anaesthesia. Anaesthesia was induced with alfentanil (30 µg kg−1) and propofol (3 mg kg−1). After tracheal intubation without muscle relaxants, a deep surgical level of general anaesthesia was maintained with 70% nitrous oxide in oxygen, i.v. propofol (100 µg kg−1 min−1) and alfentanil (0.5 µg kg−1 min−1). A radial artery cannula was placed in the contralateral arm to permit arterial blood pressure measurements. Ventilation was controlled to maintain end-tidal CO2 between 35 and 40 mmHg. Forced air warming was used to maintain oesophageal temperature at 36–37 °C.
Approximately 30 min after induction of anaesthesia, the muscle relaxant rocuronium was administered as part of a separate study [18] measuring the effects of dexmedetomidine on the degree of muscular relaxation obtained.
Eighty-five min after induction of general anaesthesia, dexmedetomidine was administered using an infusion pump (Harvard Apparatus 22) controlled by a computer software program based upon pharmacodynamic data for intravenous dexmedetomidine [19] (Steven Shafer, Stanpump, Stanford, CA). The target plasma concentration was 0.6 ng ml−1, which is known to produce profound sedation in awake subjects [20]. The accuracy of the infusion was determined by collecting arterial blood samples for plasma dexmedetomidine analysis before and at 15, 30, and 45 min after the infusion was begun. After the last sampling, rocuronium-induced paralysis was reversed with neostigmine and glycopyrolate, and the propfol and alfentanil infusions were discontinued. Volunteers were allowed to awaken from anaesthesia, then were discharged home approximately 3 h later.
Blood samples were iced immediately after being drawn, and the plasma was separated by centrifugation. Plasma was stored at −70 °C until analysis using gas chromatography-mass spectrometry. This combined method has a lower limit of detection of 20 pg mol−1 and a coefficient of variation of 5.7% in the relevant concentration range.
Pupillary measurements
Pupillary measurements were obtained using a portable infrared pupillometer (Pupilscan®, Fairville Medical Optics, Inc., Amersham UK), for which the technique has been described previously [17]. Briefly, the pupil is stimulated by a 0.5 s flash of visible light (130 candelas/m2) and a concurrent 5 s infrared scan measures the pupillary response. We measured pupil diameter and light reflex amplitude using this technique every 2 min starting 30 min before and continuing every 2 min until the end of dexmedetomidine infusion. All measurements were taken from the right eye; the left eye was covered with an opaque bandage. To exclude light from the right eye during measurement, a hollow rubber cup was placed over the eye during each scan. Ten minutes before and 30 min after the beginning of dexmedetomidine infusion, a 3 s electrical tetanus (100 Hz, 60 mAmp) was delivered to the medial surface of the left forearm via subcutaneous steel needles. This stimulus was delivered by a Digi Stim 2 stimulator (Neuro Technology, Houston TX); a laptop computer triggered the pupillary scan and the stimulator simultaneously. The scans were later analysed for reflex pupillary dilation to the nearest 0.05 mm, which is the resolution of the instrument. The light reflex was analysed for pupil diameter and light reflex amplitude for each volunteer using the average of 5 measurements from the 10 min interval prior to each tetanic stimulus. Because the 75% recovery time of the light reflex has been used as a measure of sympathetic function [21], we also used the same averaged scan to analyse the effect of dexmedetomidine on this parameter.
Data analysis
The effects of dexmedetomidine on blood pressure, heart rate, pupil size, 75% recovery times, pupillary light reflex amplitude, and reflex dilation were determined using two-tailed paired t-tests. Effect size and 95% confidence intervals were calculated for each parameter. Applied to eight subjects, the power of the paired t-test (P < 0.05) to detect a difference of 0.15 mm in pupil size and 0.1 mm in light reflex amplitude was 0.95 and 0.84, respectively. Data are reported as means±s.d. and P < 0.05 was considered significant.
Results
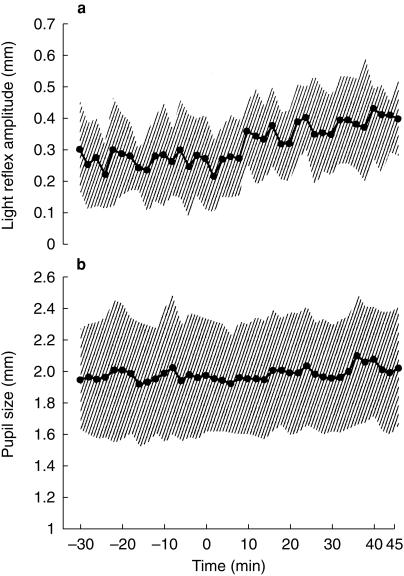
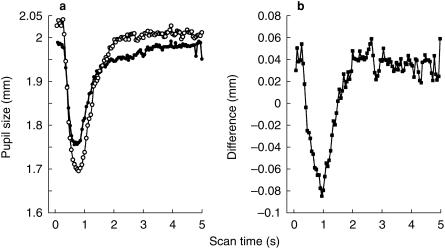
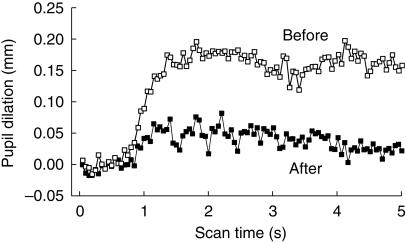
Relative to preinfusion control values, dexmedetomidine did not affect pupil size (Figure 1, Table 1). That is, pupil size did not change in four volunteers and was minimally altered in four—two in whom the pupil dilated 0.1 mm and two in whom the pupil constricted 0.1 mm over the 45 min observation period. Dexmedetomidine increased the pupillary light reflex by 37 ± 36% compared with preinfusion values (P < 0.05, Figure 1 and Table 1). The alteration in the light reflex configuration was observed only in the early phase of the reflex (Figure 2); the redilation (75% recovery) phase was not affected (Table 1, Figure 2). Dexmedetomidne reduced pupillary reflex dilation in response to tetanic stimulation by 72 ± 62% (P < 0.05, Figure 3). Latency was not measured, but the averaged scans from all eight volunteers suggest that dexmedetomidine delayed the onset of reflex dilation (Figure 3).
Figure 1.
Pupil size (a) and light reflex amplitude (b) for all volunteers for the duration of the study. Dexmedetomidine infusion to target a plasma concentration of 0.6 ng ml−1 was started at time 0. Shaded areas show the standard deviations. Statistical analysis shown in Table 1.
Table 1.
Effects of dexmedetomidine on human pupillary reflexes
| Light reflex (mm) | Reflex dilation (mm) | Pupil size (mm) | 75% recovery (s) | |
|---|---|---|---|---|
| Before dexmedetomidine | 0.30 ± 0.14 | 0.13 ± 0.13 | 2.0 ± 0.36 | 1.4 ± 0.40 |
| After dexmedetomidine | 0.37 ± 0.12* | 0.03 ± 0.09* | 2.0 ± 0.32 | 1.5 ± 0.20 |
| Effect (95% CI) | 0.07 (0.03,0.11) | −0.10 (−0.17,0.04) | 0.03 (−0.08,0.03) | 0.14 (−0.46,0.17) |
Data are mean±s.d.
P < 0.05.
Figure 2.
(a) Averaged scans for all volunteers demonstrating the light reflex before (•) and after (○) dexmedetomidine. (b) The difference between the scans before and after dexmedetomidine. This difference represents the added light reflex brought about by dexmedetomidine. The increase in the light reflex is limited to the first 2 s and does not involve the recovery portion of the reflex. The light stimulus was presented during the first 0.5 s of each scan.
Figure 3.
Reduction of pupillary reflex dilation by dexmedetomidine. Reflex dilation is shown before (top) and after (bottom) dexmedetomidine. The noxious stimulus was delivered for the first 3 s of each scan. To eliminate the effect of the light stimulus on the dilation produced by the noxious stimulus, the records were obtained by subtracting the scan during the noxious stimulus from the scan immediately preceding that scan. Scans are the averaged scans following the noxious stimulus for all eight volunteers. Statistical analysis shown in Table 1.
Plasma dexmedetomidine concentration was 1.05 ± 0.16 ng ml−1 at 15 min, 0.97 ± 0.14 at 30 min, and 0.94 ± 0.14 ng ml−1 at 45 min after the start of the infusion. Systolic blood pressure increased from 97 ± 9 mmHg prior to dexmedetomidine infusion to 112 ± 11 mmHg (P < 0.05) 30 min after beginning of infusion, while heart rate decreased from 60 ± 12 beats min−1 to 57 ± 13 beats min−1 (P < 0.05).
Discussion
One method of analysing central pathways in humans is to use receptor specific drugs as pharmacologic tools to demonstrate or refute the presence of certain neural pathways in the human brain that have been shown, by more invasive procedures, to exist in other mammals. Thus, Koss and others recorded directly from the ciliary nerves of anaesthetized cats and reported that the α2-adrenergic antagonist yohimbine blocked the inhibitory influence of sciatic nerve stimulation on these nerves [3]. Pharmacologic manipulations of the noradrenaline stores by reserpine and α-methyl-p-tyrosine tyrosine led these authors to conclude that a noradrenaline pathway originates within the lower brain stem and produces postsynaptic inhibition of the pupilloconstrictor nucleus via an α2-adrenergic receptor.
The above stated theory, however, cannot explain our findings in anaesthetized humans. This study demonstrates that the α2-adrenergic agonist, dexmedetomidine, did not change pupil size, enhanced the light reflex, and reduced pupillary reflex dilation. Because each of these parameters may be controlled by different mechanisms in the brain these are discussed separately.
We observed no change in pupil size after dexmedetomidine. If the basic circuitry of the cat applies to humans and is different only in the relative effects of α2-agonists on the pupilloconstrictor neurones and locus coeruleus autoreceptors, we would have expected to observe pupillary dilation after dexmedetomidine. Previous studies have shown that the locus coeruleus is quiescent during deep levels of anaesthesia supplemented with opioids such as we were using [6–10]. If this nucleus did inhibit the pupilloconstrictor nucleus in the awake state, its depression during opioid-supplemented general anaesthesia would produce miosis, just as we observed. However, because dexmedetomidine could not lower the tonic firing of these cells below zero, the predominate effect of the drug would then necessarily be exerted on the inhibitory receptors located on the pupilloconstrictor nucleus. This would dilate the pupil. The alternative explanation that a small basal tonic discharge of locus coeruleus neurones would be suppressed by dexmedetomidine and this effect would be exactly offset by postsynaptic inhibition at the pupilloconstrictor nucleus seems highly unlikely. Therefore, because we observed no change in pupil size, we cannot accept the cat model to explain our data from humans.
Clonidine constricts the pupils of awake subjects and previous studies (unpublished) in our laboratory have shown that dexmedetomidine produces a dose related pupillary constriction in awake volunteers. Presumably general anaesthesia removes the inhibitions that are exerted upon the pupilloconstrictor nucleus and it is partially by removing these inhibitory mechanisms that leads to pupillary constriction in the awake state after administration of either opioids or α2-adrenoceptor agonists. A possible additional factor in producing miosis in the awake state is the reduction in peripheral sympathetic tone of the iris musculature [22]. We have not observed any additional constriction of the pupil after administration of either opioids [17] or α2-adrenoceptor agonists [23] during general anaesthesia, provided subjects are not stimulated by noxious stimuli. Sharpe has reported that clonidine injected near the pupilloconstrictor nucleus of dogs produces miosis [24]. It is therefore likely that the locus of drug effect that produces miosis in awake humans is also in or near the pupilloconstrictor nucleus.
We found that the pupil would dilate following a noxious stimulus and a reduction in this dilation in the presence of dexmedetomidine. The cat model might explain this effect by postulating reduced activation of the locus coeruleus neurones that inhibit the pupilloconstrictor nucleus [4] via the α2-adrenoreceptor. Although this mechanism appears to explain reflex dilation in the cat and rat, no such evidence exists in humans. Other theories may apply to dexmedetomidine's effect in humans. α2-adrenergic receptors are located within the dorsal horn of the spinal cord [25] and agonists at these receptor sites are analgesics [26]. The observed reduction in pupillary reflex dilation might simply be due to this spinal effect of reducing nociceptive transmission to the midbrain sites. Alternatively, dexmedetomidine might suppress release of transmitters other than noradrenaline at the pupilloconstrictor nucleus. Specifically, it has been suggested that dopamine may play a role in pupillary reflex dilation in humans [27] and dexmedetomidine is known to inhibit release of dopamine as well as noradrenaline from central presynaptic terminals [28, 29]. Unfortunately, the results of our present study are inadequate to address these theories.
Dexmedetomidine increased the amplitude of the light reflex in our anaesthetized subjects. All other known anaesthetic adjuvants either depress or produce no change [17] in the light reflex. The increase was limited to the early components of the light reflex (Figure 2), indicating a central effect, as opposed to an effect on the peripheral sympathetic innervation, which alters the recovery portion of the light reflex [20]. We did not anticipate this result but it is consistent with studies performed on awake subjects following clonidine [1] and also with those performed on awake cats [30]. Because this alteration of the pupillary light reflex was brought about without any change in pupil size, these results support the contention by Sharpe that independent mechanisms account for the effects of α2-adrenoceptor agonists and opioids on pupil size and on the light reflex [30].
In anaesthetized cats, hypothalamic stimulation produces an attenuation of the light reflex amplitude without changing the sympathectomized pupil size [31]. Based upon this evidence and the fact that clonidine enhances the light reflex, it has been suggested that α2-adrenoceptor agonists suppress a hypothalamic centre that has a tonic inhibitory effect on the light reflex pathway. Conceivably, a similar mechanism could explain our results. Depresssion of the hypothalamic autonomic reflex centres by α2 adrenoceptor agonists such as clonidine during general anaesthesia usually results in mild hypotension and bradycardia [32, 33] but the haemodynamic effects of intravenous dexmedetomidine in humans are confounded by activation of peripheral α2 vasoconstrictor effects [18, 34] that in our anaesthetized volunteers resulted in an elevation of blood pressure.
Anxiety is known to decrease the amplitude of the human light reflex [1]. The depression of the light reflex is thought to be due to the previously discussed inhibitory mechanism exerted from the hypothalamus upon the light reflex pathway. Despite our subjects being neither anxious nor engaged in strenuous mental activity, the light reflex was enhanced to the same degree as that reported by others when administering clonidine to anxious subjects threatened by electric shock (painful stimulus) [1]. Accordingly, the α2-adrenoceptor agonists appear to increase the light reflex independently of any anxiolytic effect, a conclusion suggested by other investigators [1].
The use of general anaesthesia for the study of pupillary pharmacology has both advantages and disadvantages. General anaesthesia eliminates the confounding variables of sedation, nausea, vomiting, and central excitation that are often confounding factors in pupillary studies on awake subjects. General anaesthesia also functionally eliminates the peripheral sympathetic pathway innervating the dilator muscle of the iris [35, 36]. Other investigators have observed the 75% recovery time to be prolonged following clonidine administration to awake subjects [37], but this was not observed in the present study, presumably because the peripheral sympathetic nervous system that contributes to the shape of the light reflex is not active during general anaesthesia. Pupillary reflexes during general anaesthesia are thought to occur exclusively through the parasympathetic system originating in the pupilloconstrictor nucleus and innervating the constrictor muscle of the iris.
The anaesthetic also introduced other drugs such as alfentanil, propofol, and rocuronium into the study. However, these drugs were given by bolus injections followed by continuous infusions and therefore stable levels were considered to be present [38] before dexmedetomidine was administered. Alfentanil has no effect on the light reflex in human volunteers but does diminish pupillary reflex dilation [17]. Consequently, the dilations we observed were small compared with those measured during nonopioid based anaesthetics [39]. However, they were still large enough to detect the additional depressant effect of dexmedetomidine. Our finding of reflex dilation in the presence of these drugs demonstrates that the pupil was not in a state of ‘fixed miosis’, thereby allowing us to detect any mydriasis due to dexmedetomidine, if present. Because of the long duration of action of intravenous dexmedetomidine [40], we did not attempt to observe the decay of the drug effects that we observed on the pupil.
The clinical implications of this study relate to the use of dexmedetomidine in the critical care setting. A sedative that produced mydriasis in the presence of other agents such as opioids and propofol would confound the neurological evaluation of unstable patients. Data on the pupillary effects of α2-adrenoceptor agonists obtained from cats are already being applied in the critical care environment. One case report has attributed non reactive dilated pupils in the perioperative period to noradrenaline induced inhibition of the ‘Edinger Westfal nucleus via an α2 effect’ [41]. Our data were gathered from volunteers receiving commonly used agents, which, as previously discussed, were expected to reveal the mydriatic effect of α2-adrenoceptor agonists. Instead, dexmedetomidine produced no change in pupil size and increased the light reflex. These pharmacologic effects would be advantageous for monitoring the integrity of the midbrain, and the 2nd and 3rd cranial nerves, in critically ill patients.
In conclusion, we studied the effect of dexmedetomidine on human pupillary reflexes during general anaesthesia. Our findings that dexmedetomidine did not alter pupil size, enhanced the light reflex and reduced reflex dilation cannot be explained by an inhibitory α2 adrenergic pathway from the locus coeruleus to the pupilloconstrictor nucleus as is thought to exist in cats. One theory to explain our data is to postulate an action of dexmedetomidine on receptors close to or in the pupilloconstrictor nucleus that produce miosis and also reduce reflex dilation of the pupil. Dexmedetomidine-induced enhancement of the light reflex may be due to a separate mechanism involving depression of an inhibitory pathway originating within the hypothalamus.
Acknowledgments
This study was supported by Fairville Medical Optics, Inc. (Amersham, England).
References
- 1.Bitsios P, Szabadi E, Bradshaw CM. The effects of clonidine on the fear-inhibited light reflex. J Psychpharm. 1998;12:27–33. doi: 10.1177/026988119801200204. [DOI] [PubMed] [Google Scholar]
- 2.Clifford JM, Day MD, Orwin JM. Reversal of clonidine induced miosis by the alpha 2-adrenoceptor antagonist RX781094. Br J Clin Pharmacol. 1982;14:99–101. doi: 10.1111/j.1365-2125.1982.tb04941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koss MC. Pupillary dilation as an index of central nervous system alpha 2 adrenoceptor activation. J Pharmacol Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 4.Szabadi E, Bradshaw CM. Autonomic pharmacology of alpha 2 adrenoceptors. J Psychopharmacol. 1996;10(Suppl):6–18. [Google Scholar]
- 5.Aghajanian GK, VanderMaelen CP. Alpha 2 adrenoceptor mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- 6.Steiner T, Mendoza G, De Georgia M, Schellinger P, Holle R, Hacke W. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997;28:711–715. doi: 10.1161/01.str.28.4.711. [DOI] [PubMed] [Google Scholar]
- 7.Kardon R. Pupillary light reflex. Curr Opin Ophthalmol. 1995;6:20–26. doi: 10.1097/00055735-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Meyer S, Gibb T, Jurkovich GJ. Evaluation and significance of the pupillary light reflex in trauma patients. Ann Emerg Med. 1993;22:1052–1057. doi: 10.1016/s0196-0644(05)82750-7. [DOI] [PubMed] [Google Scholar]
- 9.Belani KG, Sessler DI, Larson MD, et al. The Pupillary Light Reflex. Effects Anesthetics Hyperthermia Anesthesiology. 1993;79:23–27. doi: 10.1097/00000542-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Larson MD, Muhiudeen I. Pupillometric analysis of the ‘absent light reflex’. Arch Neurol. 1995;52:369–372. doi: 10.1001/archneur.1995.00540280051018. [DOI] [PubMed] [Google Scholar]
- 11.Bitsios P, Szabadi E, Bradshaw CM. Sensitivity of the fear inhibited light reflex to diazepam. Psychopharmacology. 1998;135:93–98. doi: 10.1007/s002130050489. 10.1007/s002130050489. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of selectivity, specificity, and potency of medetomidine as an alpha 2 adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 13.Valentino RJ, Wehby RG. Morphine effects on locus coeruleus neurons are dependent on the state of arousal and availability of external stimuli – studies in anesthetized and unanesthetized rats. J Pharmacol Exp Ther. 1988;244:1178–1186. [PubMed] [Google Scholar]
- 14.Astier B, VanBockstaele EJ, Aston-Jones G, Pieribone VA. Anatomical evidence for multiple pathways leading from the rostral ventrolateral medulla to the locus coeruleus in rat. Neurosci Lett. 1990;118:141–146. doi: 10.1016/0304-3940(90)90612-d. [DOI] [PubMed] [Google Scholar]
- 15.Cedarbaum JM, Aghajanian GK. Activation of locus coeruleus neurons by peripheral stimuli: Modulation by a collateral inhibitory mechanism. Life Sci. 1978;23:1383–1392. doi: 10.1016/0024-3205(78)90398-3. [DOI] [PubMed] [Google Scholar]
- 16.Bird SJ, Kuhar MJ. Iontophoretic application of opiates to the locus coeruleus. Brain Res. 1977;122:523–533. doi: 10.1016/0006-8993(77)90462-0. [DOI] [PubMed] [Google Scholar]
- 17.Larson MD, Kurz A, Sessler DI, Dechert M, Bjorksten AR, Tayefeh F. Alfentanil blocks reflex pupillary dilation in response to noxious stimulation but does not diminish the light reflex. Anesthesiology. 1997;87:849–855. doi: 10.1097/00000542-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Talke PO, Caldwell JE, Richardson CA, Kirkegaard–nielsen H, Stafford M. The effects of dexmedetomidine on neuromuscular blockade in human volunteers. Anesth Analg. 1999;88:633–639. doi: 10.1097/00000539-199903000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Talke PO, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Anal. 1997:1136–1142. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Smith SA. Pupillary function in autonomic failure. In: Bannister R, editor. Autonomic Failure. 2. Oxford: Oxford University Press; pp. 393–412. [Google Scholar]
- 22.Phillips MA, Szabadi E, Bradshaw CM. Comparison of the effects of clonidine and yohimbine on pupillary diameter at different illumination levels. Br J Clin Pharacol. 2000;50:65–68. doi: 10.1046/j.1365-2125.2000.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel M, Larson MD, Eger EI, Noorani M, Wieskopf RB. Fentanyl, clonidine, and repeated increases in desflurane concentration, but not nitrous oxide or esmolol, block the transient mydriasis caused by rapid increases in desflurane concentration. Anesth Analg. 1995;81:372–378. doi: 10.1097/00000539-199508000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Sharpe LG, Pickworth WB. Opposite pupillary size effects in the cat and dog after microinjections of morphine, normorphine, and clonidine in the Edinger-Westfal nucleus. Brain Res Bull. 1985;15:329–333. doi: 10.1016/0361-9230(85)90159-5. [DOI] [PubMed] [Google Scholar]
- 25.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 26.Eisenach JC, DeKock M, Klimsch W. Alpha 2 adrenergic agonists for regional anesthesia: a clinical review of clonidine 1984–95. Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Larson MD, Berry PR. 23rd Pupil Colloquium. Nottingham, England: 1999. Does dopamine mediate pupillary reflex dilatation in anaesthetized humans? August, (Abstract) [Google Scholar]
- 28.Anden NE, Grabawska-Anden M, Strombom U. Different alpha adrenoceptors in the central nervous system mediating biochemical and functional effects of clonidine and receptor blocking agents. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:43–52. doi: 10.1007/BF00506488. [DOI] [PubMed] [Google Scholar]
- 29.Scheinin H, Virtanen R, MacDonald E, Lammintausta R, Scheinin M. Medetomidine—a novel alpha 2 adrenoceptor agonist: a review of its pharmacodynamic effects. Prog Neuro Psychopharm Biol Pschchiat. 1989;13:635–651. doi: 10.1016/0278-5846(89)90051-1. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe LG. Separate neural mechanisms mediate sufentanil-induced pupillary responses in the cat. J Pharmacol Exp Ther. 1991;256:845–849. [PubMed] [Google Scholar]
- 31.Lowenstein O, Loewenfeld IE. In: The Pupil, the Eye. Davson H, editor. New York: Academic Press; 1969. p. 299. [Google Scholar]
- 32.Talke PO, Li J, Jain U, Leung J, Drasner K, Hollenberg M, Mangano DT. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. The Study Perioperative Ischemia Research Group Anesthesiology. 1995;82:620–633. doi: 10.1097/00000542-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Chen DG, Dai XZ, Zimmerman BG, Bache RS. Postsynaptic alpha 1 and alpha 2 adrenergic mechanisms in coronary vasoconstriction. J Cardiovasc Pharmacol. 1988;11:61–67. doi: 10.1097/00005344-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Loewenfeld IE. Reflex dilation of the pupil. Docum Ophthalmol. 1958;12:185–448. doi: 10.1007/BF00913471. [DOI] [PubMed] [Google Scholar]
- 36.Larson MD, Tayefeh F, Sessler DI, Daniel M, Noorani M. Sympathetic nervous system does not mediate reflex pupillary dilation during desflurane anesthesia. Anesthesiology. 1996;85:748–754. doi: 10.1097/00000542-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Morley MJ, Bradshaw CM, Szabadi E. Effects of clonidine and yohimbine on the pupillary light reflex and carbachol-evoked sweating in healthy volunteers. Br J Clin Pharmacol. 1991;31:99–101. doi: 10.1111/j.1365-2125.1991.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youngs EJ, Shafer SL. Chapter 2. Basic Pharmacokinetic and Pharmacodynamic Principles. In: White PF, editor. Intravenous Anesthesia. Baltimore: Williams & Wilkins; 1997. pp. 10–26. [Google Scholar]
- 39.Larson MD, Sessler DI, Washington DE, Merrifield BR, Hynson JA, McGuire J. Pupillary response to noxious stimulation during isoflurane and propofol anesthesia. Anesth Analg. 1993;76:1072–1078. doi: 10.1213/00000539-199305000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–820. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Hegi TR, Schupbach RW, Masche U, Schmid ER. Fully dilated, non reactive pupils during cardiac anesthesia. Anesth Analg. 1999;89:265. doi: 10.1097/00000539-199907000-00073. [DOI] [PubMed] [Google Scholar]