Abstract
Aims
We evaluated the inhibitory effect of haloperidol and its metabolites on CYP2D6 activity in order to better understand the potential role of these metabolites in drug interactions involving haloperidol.
Methods
The inhibitory effects of haloperidol and five of its metabolites on dextrorphan formation from dextromethorphan, a marker probe of CYP2D6 activity, were measured in human liver microsomal preparations. Apparent kinetic parameters for enzyme inhibition were determined by nonlinear regression analysis of the data.
Results
Racemic reduced haloperidol and its metabolite, RHPTP competitively inhibited dextromethorphan O-demethylation with estimated Ki values (0.24 µm and 0.09 µm, respectively) that were substantially lower than that of haloperidol (0.89 µm). The inhibitory effect of S(–)-reduced haloperidol was more potent than the R(+)-enantiomer, with estimated Ki values of 0.11 µm and 1.1 µm, respectively. The pyridinium metabolite of haloperidol, HPP+ inhibited the enzyme activity noncompetitively with a Ki value of 0.79 µm. The N-dealkylated metabolites of haloperidol (FBPA and CPHP) had a diminished inhibitory potency. While FBPA showed no notable inhibitory effect on dextrorphan formation, CPHP showed moderate competitive inhibition with a Ki value of 20.9 µm.
Conclusions
The principal metabolites of haloperidol inhibit CYP2D6, suggesting that they might contribute to the inhibitory effects of the drug. Reduced haloperidol seems to inhibit CYP2D6 activity in an enantioselective manner with the physiologically occurring S(–) enantiomer being more potent.
Keywords: CYP2D6, enantiomer, haloperidol, inhibition, interaction, metabolites, reduced haloperidol
Introduction
Haloperidol is widely used for the treatment of schizophrenia and other psychiatric disorders. It is not unusual for haloperidol to be coadministered with other drugs including other antipsychotics, antidepressants, and benzodiazepines [1]. Many authors have reported that antipsychotics including haloperidol increase the plasma concentrations of coadministered drugs that are known substrates of CYP2D6 [2–4]. Haloperidol has strong inhibitory effects on CYP2D6 catalysed dextromethorphan O-demethylation in vitro, but extrapolation of these data to clinical inhibition in vivo has raised the possibility that reported in vivo interactions of haloperidol with CYP2D6 substrates [2, 4, 5] might result from the high accumulation of haloperidol in liver tissue and/or a contribution of haloperidol metabolites. In a study with haloperidol in vitro, preincubation increased the inhibition of CYP2D6, suggesting a possible contribution of haloperidol metabolites to this inhibitory action of the drug on this enzyme and/or mechanism-based inhibition by haloperidol or some of its metabolites. Most antipsychotics are extensively metabolized to many active or inactive metabolites [8]. For example, chlorpromazine has been postulated to have 168 theoretical metabolites in humans [9].
Haloperidol undergoes oxidative N-dealkylation, oxidation to several pyridinium metabolites, glucuronidation, and carbonyl reduction via CYP3A4, CYP2D6 and a carbonyl reductase (Figure 1) [10–13]. The oxidation of reduced haloperidol to haloperidol appears to be mediated by CYP3A4 and CYP2D6 [14–16]. Some of these metabolites have significant pharmacological effects (e.g. high affinity binding to haloperidol-sensitive sigma receptor binding sites in the brain, increase of prolactin secretion and homovanillic acid concentration, and inhibition of apomorphine induced stereotypy in rats by reduced haloperidol) [17–20]. Toxic effects of haloperidol metabolites have also been reported (e.g. MPP+-like neurotoxic effects of haloperidol pyridinium metabolites) [21–23]. However, no information exists about the contribution of haloperidol metabolites to drug interactions involving CYP2D6.
Figure 1.
Metabolic pathways of haloperidol. Values in parentheses indicate the reference number. Solid arrows indicate the known metabolic pathways and dotted arrows metabolic pathways for which the enzymes involved have not been reported yet. HPTP and RHPTP are found in vitro, but not detected in in vivo studies [44]. Abbreviations are: FBPA: p-fluorobenzoyl propionic acid, CPHP: 4-chlorophenyl-4-hydroxypiperidine, FBHP: 4-(p-fluorophenyl)-4-hydroxybutyric acid, HAL-GLU: haloperidol glucuronide, S(–)RHAL: S(–) reduced haloperidol enantiomer, RHAL-GLU: reduced haloperidol glucuronide, RHAL-SUL: reduced haloperidol sulphate, HPTP: 4-(4-(chlorophenyl)-1-[4-(fluorophenyl)-4-oxybutyl]-1,2,3 [6, -tetrahydropyridine, HPP+: 4-(4-(chlorophenyl)-1-[4-(fluorophenyl)-4-oxybutyl]-pyridinium, RHPTP:: 4-(4-(chlorophenyl)-1-[4-(fluorophenyl)-4-hydroxybutyl]-1,2,3,6-tetrahydropyridine, RHPP + : 4-(4-(chlorophenyl)-1–4-(fluorophenyl)-4-hydroxybutyl-pyridinium: CR, carbonyl reductase.
Reduced haloperidol has been found to be a major metabolite of haloperidol in humans, but most pharmacokinetic and pharmacodynamic studies on reduced haloperidol have been performed using synthetic racemates. Unlike its parent molecule haloperidol, reduced haloperidol exists as two enantiomeric forms with an asymmetric chiral centre (Figure 1) [24]. Only the S(–)-enantiomer of reduced haloperidol is formed from haloperidol by carbonyl reductase in human tissue [25]. In the present study, we compared the inhibitory effect of S(–)-and R(+)-reduced haloperidol enantiomers and of the other principal metabolites of haloperidol on CYP2D6 catalysed dextromethorphan O-demethylation in vitro using human liver microsomal preparations.
Methods
Haloperidol, dextromethorphan hydrobromide, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, β-NADP and EDTA were purchased from Sigma Chemical Co. (St Louis, MO, USA). Racemic reduced haloperidol, 3-(p-fluorobenzoyl)propionic acid (FBPA) and 4- (p-chlorophenyl)-4-hydroxypiperidine (CPHP) were obtained from Janssen Pharmaceutica N.V. (Beerse, Belgium). Levallorphan was purchased from U.S.P.C. (Rockville, MD, USA). The haloperidol pyridinium species, 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4- oxobutyl]pyridinium ion (HPP+) and the reduced 1,2,3,6-tetrahydropyridine derivative (RHPTP) were generous gifts from Dr Neal Castagnoli, Jr. (Department of Chemistry, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA). All other chemicals and reagents used were of the highest commercially available quality.
Reduced haloperidol enantiomers were separated according to the method of Eyles & Pond [25]. Briefly, the reduced haloperidol racemate (rac-reduced haloperidol) was dissolved in methanol, and then separated using a Chiralcel OJ cellulose chiral column (J.T. Baker Inc., Phillipsburg, NJ, USA). The mobile phase, composed of hexane: isopropanol (95 : 5, v/v), was delivered at a flow rate of 1.4 ml min−1. The S(–)- and R(+)-enantiomers of reduced haloperidol were detected using a Waters 996 Photodiode Array Detector set at a wavelength of 245 nm and eluted at 22 and 27 min, respectively. The separated enantiomers were collected in glass tubes and concentrated in a Speedvac SC110 model RH40–12 (Savant Instruments Inc., Farmingdale, NY, USA). The complete separation of each enantiomer was confirmed through repeated injection of the concentrated harvest. The final concentrations of each enantiomer collected were measured from a standard curve obtained from rac-RHAL with a Spectronic® Genesys™ 5 u.v. spectrophotometer (Milton Roy, Rochester, NY, USA) set at a wavelength of 245 nm.
Human liver tissues designated HL-7, HL-10, and HL-13 were obtained under the auspices of the Washington Regional Transplant Consortium (Washington, DC) and human liver microsomes were prepared as described previously [26]. Protein concentrations were measured by the Bradford method of Pollard et al. [27].
The activity of CYP2D6 was determined from the rate of O-demethylation of dextromethorphan to dextrorphan [28] and was expressed as the quantity of the latter formed per unit of protein concentration and time. Inhibition experiments were performed as described previously [29]. Briefly, dextromethorphan (10–75 µm) without and with haloperidol or one of its metabolites (0.5–50 µm) were prewarmed at 37°C for 5 min in 0.1 m phosphate buffer (pH 7.4) and an NADPH regenerating system (1.3 mm β-NADP, 3.3 mm glucose-6-phosphate, 3.3 mm MgCl2 and 1 U ml−1 glucose-6-phosphate dehydrogenase). The reaction was started by adding human liver microsomes (final concentration 0.25 mg ml−1) and was terminated, after 30 min incubation, by adding 20 µl of 60% perchloric acid and 40 µl of 16 µm levallorphan, as internal standard, on ice. After centrifugation at 16 000×g in a microfuge for 5 min, 50 µl of supernatant was injected into the h.p.l.c. system. Dextrorphan, dextromethorphan and levallorphan were separated on a Dupont Zorbax SB-phenyl column (150×4.6 mm). The mobile phase of acetonitrile: methanol: 10 mm potassium phosphate buffer (23 : 20 : 57, v/v) with 0.114% of triethylamine, pH 4.0, was delivered at 0.5 ml min−1. Chromatograms were generated by a Spectrovision FD-300 Dual Monochromator Fluorescence detector (Groton Technology Inc., Concord, MA) set at an excitation wavelength of 200 nm and emission wavelength of 304 nm. Under these conditions, dextrorphan, levallorphan, and dextromethorphan were eluted at 5.2, 7.6, and 10.2 min, respectively. The standard curve for dextrorphan was linear between 40 and 830 ng ml−1 and the interday coefficient of variation of the assay was between 1.4% and 11.6%.
The apparent kinetic parameters for dextrorphan formation (Vmax, Km) and enzyme inhibition by haloperidol and its metabolites (IC50, Ki) were determined by nonlinear least square regression analysis using WinNonlin Version 1.5 (Scientific Consulting, Inc., Apex, NC). Different models of enzyme inhibition, i.e. pure and partial competitive inhibition, noncompetitive inhibition, mixed type inhibition, and uncompetitive inhibition [30], were fitted to the data. The best model of inhibition was determined by the following criteria: visual inspection of Lineweaver-Burk, Dixon and secondary plots of the Lineweaver-Burk plots (the ordinate intercept of Lineweaver-Burk plots against inhibitor concentration); the size of the sum of squares of the residuals, AIC (Akiake Index Criteria) and SC (Schwartz Criteria) values, the standard error and 95% confidence interval of the parameter estimates, and the random distribution of the residuals from nonlinear regression analysis. The simplest model was chosen if more than one model met the above criteria. All incubations in all livers were carried out in duplicate and the estimated kinetic parameters and values for the extent of inhibition are represented as mean (±s.d.) data from the three microsomal preparations. The estimated Vmax and Km values of the three microsomal preparations used ranged from 0.19 to 0.46 nmol min−1 mg−1 protein and from 12.0 to 21.3 µm, respectively.
Results
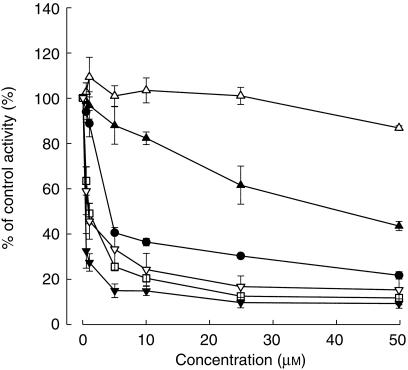
Rac-reduced haloperidol and haloperidol pyridinium metabolites (HPP+, RHPTP) strongly inhibited dextromethorphan O-demethylation (Figure 2). They decreased the dextrorphan formation rate to 24.0%, 18.5%, and 12.5% of control activity at 10 µm, respectively, which was comparable with that of parent haloperidol, which reduced CYP2D6 activity to 36.3% of control at the same concentration. When 10 µm dextromethorphan was used, the estimated mean IC50 values of rac-reduced haloperidol, HPP+ and RHPTP were 0.89±0.3, 0.34±0.07, and 1.34±0.37 µm, respectively, which were lower than that of haloperidol (5.70±0.91 µm). The N-dealkylated metabolite CPHP showed moderate inhibition with a mean IC50 of 40.4±1.7 µm, while the other haloperidol metabolite generated by the same reaction, FBPA showed no remarkable inhibition of dextromethorphan O-demethylation.
Figure 2.
Effect of haloperidol (•) and its metabolites (FBPA; ▴ CPHP; □ HPP+ ▾ RHPTP; rac-RHAL) on CYP2D6 catalysed dextromethorphan O-demethylation in human liver microsomes. Human liver microsomes were incubated with 25 µm dextromethorphan and haloperidol or its metabolites (0–50 µm) for 30 min. Each data point is the mean ± s.d. of the percent of control activity measured by the dextrorphan formation rate from three different liver microsomal preparations.
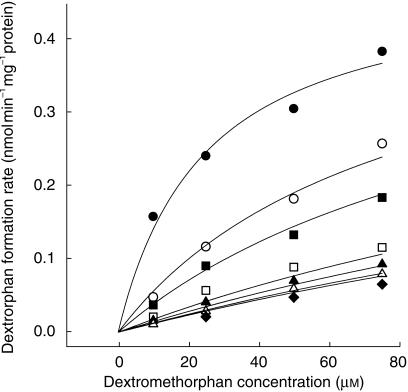
The data obtained from all the haloperidol metabolites, except HPP+, were best described by a competitive inhibition model (Table 1). Except for FBPA and CPHP, Dixon plots showed nonlinearity, suggesting saturation of inhibition at high concentrations of haloperidol and its metabolites (Figure 3). The inhibition data obtained from these metabolites were best described by a partial inhibition model (Figure 4, Table 1). RHPTP and rac-reduced haloperidol showed the lowest Ki values (0.09 and 0.24 µm, respectively) estimated from this model. The Ki values of RHPTP and rac-reduced haloperidol were substantially lower than that of haloperidol (0.89 µm) and CPHP (20.9 µm). Noncompetitive inhibition described the effect of HPP+ on CYP2D6 activity, and the mean estimated Ki was 0.79 µm. A 30-min preincubation of HPP+ brought about no additional increase in CYP2D6 inhibition (data not shown).
Table 1.
Inhibition of CYP2D6 catalysed dextromethorphan. O-demethylation in human liver microsomes by haloperidol and its metabolites.
| Haloperidol metabolites | Ki (µm) | Type of inhibition |
|---|---|---|
| Haloperidola | 0.89±0.15 | Partial competitive |
| CPHP | 20.9±1.6 | Competitive |
| FBPA | >500 | Competitive |
| rac-RHALa | 0.24±0.07 | Partial competitive |
| S(–) RHALa | 0.11±0.01 | Partial competitive |
| R(+) RHALa | 1.11±0.16 | Partial competitive |
| RHPTPa | 0.09±0.03 | Partial competitive |
| HPP+ | 0.79±0.11 | Noncompetitive |
Each value indicates mean ± s.d. of data from the three different livers.
Mean value of α, a factor by which Km changes when inhibitor occupies the enzyme: Haloperidol, 16.8; rac-RHAL, 35.7; S(–) RHAL, 33.3; R(+) RHAL, 22.2; RHPTP, 30.8.
Figure 3.
Dixon plots showing the inhibition of CYP2D6 catalysed dextromethorphan O-demethylation by haloperidol and its metabolites. Except for FBPA (b) and CPHP (c), Dixon plots of other haloperidol/metabolites showed nonlinear relationships. Human liver microsomes (HL-10) were incubated with 10 (•), 25 (♦), 50 (▴), and 75 µm (▾) dextromethorphan and haloperidol/metabolites (0–50 µm) for 30 min.
Figure 4.
Representative nonlinear regression fit of dextrorphan formation rate vs dextromethorphan (10–75 µm). Each symbol and solid line indicates the observed and predicted dextrorphan formation rate in the absence (•) and presence of reduced haloperidol 0.5 (○), 1 (▪), 5 (□), 10 (▴), 25 (▵) and 50 µm (♦), respectively. The inhibition of dextrorphan formation by reduced haloperidol was best described by a partial competitive inhibition model [30] with the following parameter estimates: Vmax 0.49 nmol min−1 mg−1 protein, Km 25.09 µm, Ki 0.19 µm and α 1 7.2.
The inhibitory effect of S(–)-reduced haloperidol was consistently more potent than that of the R(+)-enantiomer at all concentrations tested (Figure 5). S(–)-and R(+)-enantiomers decreased the dextrorphan formation rate to 40.2 and 81.8% of control activity at 0.5 µm, and 30.7 and 69.1% of control activity at 1 µm, respectively. The estimated IC50 values of the S(–)- and R(+)-enantiomers were 0.44±0.08 µm and 4.31±0.58 µm, respectively. The inhibitory effects of both enantiomers were best described by a partial competitive inhibition model, and the estimated Ki value of S(–)-reduced haloperidol was 10 fold lower than that of the R(+)-enantiomer (Table 1).
Figure 5.
Comparison of inhibition of CYP2D6 catalysed dextromethorphan O-demethylation by rac-reduced haloperidol (open columns) with S(–) (closed columns) and R(+) (shaded columns) reduced haloperidol enantiomers. Human liver microsomes were incubated with 25 µm dextromethorphan and rac-reduced haloperidol or S(–) or R(+)-reduced haloperidol enantiomer (0–50 µm) for 30 min. Each data point is the mean ± s.d. percent remaining activity after exposure to each inhibitor with three different liver microsomal preparations.
Discussion
These data clearly demonstrate that haloperidol metabolites are able to inhibit human liver cytochrome P450 2D6 in vitro and may contribute to clinical interactions of haloperidol with substrates of this CYP isoform. Although most haloperidol metabolites are formed by CYP3A4 in vitro [12, 13, 31], we focused on CYP2D6 in this study because the inhibitory effects of haloperidol on this enzyme have been shown to be much more potent than those on CYP3A [32]. This is not an unusual finding since several drugs have been reported to inhibit CYP isoforms that are not involved in their metabolism, e.g. halofantrine and quinidine [33, 34].
In the present study, rac-reduced haloperidol strongly inhibited dextromethorphan O-demethylation, which is consistent with the report of Tyndale et al. [6] in which reduced haloperidol strongly inhibited the metabolism of sparteine, a CYP2D6 substrate. Inhibition of CYP2D6 by reduced haloperidol appears to be more potent than that by haloperidol itself, since the estimated Ki value of the former was four fold less than that of the latter. Plasma and tissue concentrations of reduced haloperidol are nearly equal to or higher than those of haloperidol in psychiatric patients [7, 35, 36], suggesting that reduced haloperidol might contribute more to inhibition of CYP2D6 than its parent drug in patients who are taking haloperidol. Recently, it has been shown that only the S(–)-enantiomer of reduced haloperidol is formed in humans [25]. In the present study, S(–)-reduced haloperidol was eight fold more potent an inhibitor of CYP2D6 than the parent drug. It is possible that patients with high carbonyl reductase activity and high concentrations of S(–)-reduced haloperidol may be particularly vulnerable to CYP2D6–mediated drug interactions. It has been reported that the plasma concentration ratio of reduced haloperidol to haloperidol varies widely between patients taking single or multiple doses of haloperidol [37, 47]. Further in vivo studies are required to evaluate the contribution of reduced haloperidol to drug interactions with haloperidol.
In the present study, S(–)-reduced haloperidol was a more potent CYP2D6 inhibitor than the R(+)-enantiomer. The estimated Ki of the S(–)-enantiomer was 10 fold lower and the percent inhibition at low concentration was more than three fold greater than that of R(+)-reduced haloperidol, suggesting stereoselective inhibition of CYP2D6 activity by reduced haloperidol. This finding raises the question of whether stereoselectivity in the pharmacokinetics or pharmacodynamics of reduced haloperidol occurs. There are currently no data that allow us to evaluate this question. All previous pharmacological studies of reduced haloperidol have been carried out using the synthetic racemate [17–20, 36–38]. If we consider that S(–)-reduced haloperidol is the only form found in humans [25], pharmacological studies of reduced haloperidol should be performed with S(–)-enantiomer instead of a racemate.
Since the identification of a neurotoxic pyridinium metabolite (HPP+) of haloperidol by Subramanyam et al. [39], many reports have suggested a possible involvement of these metabolites in the extrapyramidal side-effects of haloperidol by analogy with neurotoxic MPP+ [21–23]. In this study, HPP+ strongly and noncompetitively inhibited CYP2D6 activity. The estimated Ki value for HPP+ was comparable with that of haloperidol. The inhibition of CYP2D6 by HPP+ seems not to be mechanism based, because preincubation of HPP+ with microsomal preparations had no additional inhibitory effect. The plasma concentration of HPP+ is related to the haloperidol daily dose and plasma drug concentration [40]. Igarashi et al. [41] have reported that the plasma HPP+ concentration measured in schizophrenic patients treated with 3–18 mg day−1 of haloperidol was 10–57 ng ml−1 which is comparable with the concentration of the parent drug [36, 42]. In the present study, the inhibition of RHPP+, a pyridinium metabolite of reduced haloperidol, was not evaluated because this compound was not available. However, this metabolite is also expected to have strong inhibition on CYP2D6 if we consider the inhibitory effect of reduced haloperidol and HPP+, precursors of RHPP+ formation [43] (see Figure 1). Furthermore, the plasma concentration of RHPP+ seems to be higher than those of parent drug and HPP+[40]. Together, these findings suggest that haloperidol pyridinium metabolites may be potent and important contributors to the in vivo inhibition of CYP2D6 activity.
Of the haloperidol metabolites tested, RHPTP was the most potent inhibitor with an estimated Ki of 0.09 µm. It appears that the tetrahydropyridine metabolites RHPTP and HPTP (4-(4-chlorophenyl)1–4-(4-fluorophenyl)-4-oxobutyl-1,2,3,6-tetrahydropyridine) are converted from reduced haloperidol and HPP+ by CYP3A4 and by carbonyl reductase in vitro, respectively [12, 43]. However, Avent et al. [44] found no tetrahydropyridine metabolites in human plasma and urine, suggesting that these are present only transiently at their site of formation and are not released into the circulation.
The N-dealkylation of haloperidol seems to result in the loss of much inhibitory effect of CYP2D6 activity. Although one of the metabolites resulting from N-dealkylation (CPHP) showed moderate inhibition with a Ki of 20.9 µm, the other metabolite FBPA had no detectable inhibitory effect on CYP2D6 activity. On the other hand, the reduction of the ketone group adjacent to the fluorophenyl ring and oxidation of the piperidinol ring of haloperidol increased inhibitory potency. These findings suggest that reduced haloperidol, and the tetrahydropyridine or pyridinium metabolites but not those resulting from N-dealkylation have some characteristics of a pharmacophore proposed by Strobl et al. [45] for inhibition of human liver CYP2D6.
In conclusion, the results obtained in the present study demonstrate that haloperidol metabolites have the ability to inhibit CYP2D6. The most potent include reduced haloperidol, and pyridinium and tetrahydropyridine metabolites. Their in vitro inhibitory potencies are higher than that of the parent drug, suggesting that these metabolites may contribute significantly to in vivo interactions between haloperidol and CYP2D6 substrates. Our data also suggest that the S(–) reduced haloperidol enantiomer inhibits CYP2D6 activity. Clinically, these findings imply that the interaction between haloperidol and CYP2D6 may be affected by clinical conditions in which the formation and/or the elimination of haloperidol metabolites are changed.
Acknowledgments
This study was supported in part by a Shannon Director's award (DAF) R55-GM56898, and by grants R01-GM56898–01 and T32–9M08386 from the National Institute of General Medical Sciences, Bethesda, MD. Dr Shin was supported by a Merck Sharp & Dohme International Fellowship Award in Clinical Pharmacology. We thank Dr Neal Castagnoli, Jr. (Department of Chemistry, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA) for the generous gift of HPP+ and RHPTP.
References
- 1.Rosholm JU, Hallas J, Gram LF. Concurrent use of more than one major psychotropic drug (polypsychopharmacy) in out-patients – a prescription database study. Br J Clin Pharmacol. 1994;37:533–538. doi: 10.1111/j.1365-2125.1994.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mihara K, Otani K, Ishida M, et al. Increase of plasma concentration of m-chlorophenylpiperazine, but not trazodon, with low-dose haloperidol. Ther Drug Monit. 1997;19:43–45. doi: 10.1097/00007691-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Maynard GL, Soni P. Thioridazine interferences with imipramine metabolism and measurement. Ther Drug Monit. 1996;18:729–731. doi: 10.1097/00007691-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC, Baldessarini RJ. Drug interactions with antipsychotic agents. J Clin Psychopharmacol. 1993;13:57–67. [PubMed] [Google Scholar]
- 5.Dinovo EC, Bost RO, Sunshine I, Gottschalk LA. Distribution of thioridazine and its metabolites in human tissues and fluids obtained postmortem. Clin Chem. 1978;24:1828–1830. [PubMed] [Google Scholar]
- 6.Tyndale RF, Kalow W, Inaba T. Oxidation of reduced haloperidol to haloperidol: involvement of human CYPIID6 (spartein/debrisoquine monooxygenase) Br J Clin Pharmacol. 1991;31:655–660. doi: 10.1111/j.1365-2125.1991.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korpi ER, Kleinman JE, Costakos DT, Linnoila M, Wyatt RJ. Reduced haloperidol in the post-mortem brains of haloperidol-treated patients. Psychiatric Res. 1984;11:259–269. doi: 10.1016/0165-1781(84)90074-x. [DOI] [PubMed] [Google Scholar]
- 8.Ereshefsky L. Pharmacokinetics and drug interactions: Update for new antipsychotics. J Clin Psychiatry. 1996;57(Suppl 11):12–25. [PubMed] [Google Scholar]
- 9.Forrest IS, Usdin E. Phenothiazines with aliphatic side chain. In: Usdin E, Forrest IS, editors. Pyschotherapeutic drugs. New York: Marcel Dekker, Inc.; 1977. pp. 699–753. [Google Scholar]
- 10.Tsang MW, Shader RI, Greenblatt DJ. Metabolism of haloperidol: Clinical implications and unanswered questions. J Clin Psychopharmacol. 1994;14:159–162. [PubMed] [Google Scholar]
- 11.Someya T, Shibasaki M, Noguchi T, Takahashi S, Inaba T. Haloperidol metabolism in psychiatric patients: Importance of glucuronidation and carbonyl reduction. J Clin Psychopharmacol. 1992;12:169–174. [PubMed] [Google Scholar]
- 12.Fang J, Baker GB, Silverstone PH, Coutts RT. Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol. 1997;17:227–233. doi: 10.1023/a:1026317929335. [DOI] [PubMed] [Google Scholar]
- 13.Pan LP, Wijnant P, De Vriendt C, Rosseel MT, Belpaire FM. Characterization of the cytochrome P450 isozymes in the in vitro N-dealkylation of haloperidol. Br J Clin Pharmacol. 1997;44:557–564. doi: 10.1046/j.1365-2125.1997.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo S, Odomi M. Involvement of human cytochrome P450 3A4 in reduced haloperidol oxidation. Eur J Clin Pharmacol. 1998;54:253–259. doi: 10.1007/s002280050455. 10.1007/s002280050455. [DOI] [PubMed] [Google Scholar]
- 15.Pan LP, De Vriendt C, Belpaire FM. In vitro characterization of the cytochrome P450 isoenzymes involved in the back oxidation and N-dealkylation of reduced haloperidol. Pharmacogenetics. 1998;8:383–389. doi: 10.1097/00008571-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Young D, Midha KK, Fossler MJ, et al. Effect of quinidine on the interconversion kinetics between haloperidol and reduced haloperidol in humans: implications for the involvement of cytochrome P450IID6. Eur J Clin Pharmacol. 1993;44:433–438. doi: 10.1007/BF00315539. [DOI] [PubMed] [Google Scholar]
- 17.Bowen WD, Moses EL, Tolentino PJ, Walker JM. Metabolites of haloperidol display preferential activity at sigma receptors compared to dopamine D-2 receptors. Eur J Pharmacol. 1990;177:111–118. doi: 10.1016/0014-2999(90)90260-d. [DOI] [PubMed] [Google Scholar]
- 18.Hays SE, Poland RE, Rubin RT. Prolactin releasing potencies of antipsychotic and related nonpsychotic compounds in female rats: Relation to clinical potencies. J Pharmacol Exp Ther. 1980;214:362–367. [PubMed] [Google Scholar]
- 19.Chang WH, Jaw SS, Wu HS, Tsai L, Yeh EK. Pharmacodynamics and pharmacokinetics of haloperidol and reduced haloperidol in guinea pigs. Pyschopharmacol. 1988;96:285–288. doi: 10.1007/BF00216051. [DOI] [PubMed] [Google Scholar]
- 20.Browning JL, Silverman PB, Harrington CA, Davis CM. Preliminary behavioral and pharmacological studies on the haloperidol metabolite reduced haloperidol. Soc Neuroscience Abstracts. 1982;8:470. [Google Scholar]
- 21.Igarashi K, Matsubara K, Kasuya F, Fukui M, Idzu T, Castagnoli N., Jr Effect of a pyridinium metabolites derived from haloperidol on the acitivities of striatal tyrosine hydroxylase in freely moving rats. Neurosci Lett. 1996;214:183–186. doi: 10.1016/0304-3940(96)12919-0. 10.1016/0304-3940(96)12919-0. [DOI] [PubMed] [Google Scholar]
- 22.Fang J, Zuo D, Yu PH. Comparison of cytotoxicity of a quaternary pyridinium metabolites of haloperidol (HPP+) with neurotoxin N-methyl-4-phenylpyridinium (MPP+) towards cultured dopaminergic neuroblastoma cells. Psychopharmacol. 1995;121:373–378. doi: 10.1007/BF02246077. [DOI] [PubMed] [Google Scholar]
- 23.Rollema H, Skolnik M, D'Engelbronner J, Igarashi K, Usuki E, Castagnoli N., Jr MPP+-like toxicity of pyridinium metabolites derived from haloperidol: In vivo microdialysis and in vitro mitochondrial studies. J Pharmacol Exp Ther. 1994;268:380–387. [PubMed] [Google Scholar]
- 24.Jaen JC, Caprathe BW, Priebe S, Wise LD. Synthesis of the enantiomers of reduced haloperidol. Pharm Res. 1991;8:1002–1005. doi: 10.1023/a:1015800923078. [DOI] [PubMed] [Google Scholar]
- 25.Eyles DW, Pond SM. Stereoselective reduction of haloperidol in human tissues. Biochem Pharmacol. 1992;44:867–871. doi: 10.1016/0006-2952(92)90117-2. [DOI] [PubMed] [Google Scholar]
- 26.Harris JW, Rahman A, Kim B-R, Guenggerich FP, Collins JM. Metabolism of taxol by human hepatic microsomes and liver slices: Participation of cytochrome P450 3A4 and unknown P450 enzyme. Cancer Res. 1994;54:4026–4035. [PubMed] [Google Scholar]
- 27.Pollard HB, Menard R, Brandt HA, Pazolzs CJ, Creutz CE, Ramu A. Application of Bradford's protein assay to adrenal gland subcellular fractions. Anal Biochem. 1978;86:761–763. doi: 10.1016/0003-2697(78)90805-9. [DOI] [PubMed] [Google Scholar]
- 28.Broly F, Libersa CL, Lhermitte M, Bechtel P, Dupuis B. Effect of quinidine on the dextromethorphan O-demethylase activity of microsomal fractions from human liver. Br J Clin Pharmacol. 1989;28:29–36. doi: 10.1111/j.1365-2125.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko J-W, Soukhova N, Thacker D, Chen P, Flockhart DA. Evaluation of omeprazole and lansoprazole as inhibitor of cytochrome P450 isoforms. Drug Metabol Dispos. 1997;25:853–862. [PubMed] [Google Scholar]
- 30.Segel IH. Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York: John Wiley & Sons, Inc.; 1975. [Google Scholar]
- 31.Igarashi K, Kasuya F, Fukui M, Usuki E, Castagnoli N., Jr Studies on the metabolism of haloperidol (HP): The role of CYP3A in the production of the neurotoxic pyridinium metabolite HPP+ found in rat brain following IP administration of HP. Life Sci. 1995;57:2439–2446. doi: 10.1016/0024-3205(95)02240-5. 10.1016/0024-3205(95)02240-5. [DOI] [PubMed] [Google Scholar]
- 32.Shin JG, Soukhova N, Desta Z, Flockhart DA. Inhibition of CYP2D6 by antipsychotic drugs. Clin Pharmacol Ther. 1998;2:188. [Google Scholar]
- 33.Halliday RC, Jones BC, Smith DA, Kitteringham NR, Park BK. An investigation of the interaction between halofantrine, CYP2D6 and CYP3A4: studies with human liver microsomes and heterologous enzyme expression systems. Br J Clin Pharmacol. 1995;40:369–378. doi: 10.1111/j.1365-2125.1995.tb04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otton SV, Crewe HK, Lennard MS, Tucker GT, Woods HF. Use of quinidine inhibition to define the role of the sparteine/debrisoquine cytochrome P450 in metoprolol oxidation by human liver microsomes. J Pharmcol Exp Ther. 1988;247:242–247. [PubMed] [Google Scholar]
- 35.Forsman A, Folsch G, Larsson MA. Metabolism of haloperidol. Curr Ther Res. 1978;24:567–568. [Google Scholar]
- 36.Shostak M, Perel JM, Stiller RL, Wyman W, Curran S. Plasma haloperidol and clinical response: a role for reduced haloperidol in antipsychotic acitivity. J Clin Psychopharmacol. 1987;7:394–400. [PubMed] [Google Scholar]
- 37.Midha KK, Chakraborty BS, Ganes DA, et al. Intersubject variation in the pharmacokinetics of haloperidol and reduced haloperidol. J Clin Psychopharmacol. 1989;9:98–104. doi: 10.1097/00004714-198904000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Jann MW, Chang W-H, Davis CM, et al. Haloperidol and reduced haloperidol plasma level in Chinese vs. non-Chinese psychiatric patients. Psychiatry Res. 1989;30:45–52. doi: 10.1016/0165-1781(89)90170-4. [DOI] [PubMed] [Google Scholar]
- 39.Subramanyam B, Pond SM, Eyles DW, Whiteford HA, Fouda HG, Castagnoli N., Jr Identification of potentially neurotoxic pyridinium metabolite in the urine of schizophrenic patients treated with haloperidol. Biochem Biophys Res Comm. 1991;181:573–578. doi: 10.1016/0006-291x(91)91228-5. [DOI] [PubMed] [Google Scholar]
- 40.Eyles DW, McLennan HR, Jones A, McGrath JJ, Stedman TJ, Pond SM. Quantitative analysis of one pyridinium metabolite of haloperidol and identification of another in schizophrenic patients. Clin Pharmacol Ther. 1994;56:512–520. doi: 10.1038/clpt.1994.172. [DOI] [PubMed] [Google Scholar]
- 41.Igarashi K, Kasuya F, Fukui M, Usuki E, Castagnoli N., Jr Simultaneous determination of haloperidol and its neurotoxic metabolite in plasma and brain tissue from schizophrenic patients treated with haloperidol using HPLC and solid-phase extraction. Jpn J Forensic Toxicol. 1995;13:31–38. [Google Scholar]
- 42.Ko GN, Korpi ER, Kirch DG. Haloperidol and reduced haloperidol concentrations in plasma and red blood cells from chronic schizophrenic patients. J Clin Psychopharmacol. 1989;9:186–190. [PubMed] [Google Scholar]
- 43.Eyles DW, McCrath JJ, Pond SM. Formation of pyridinium species of haloperidol in human liver and brain. Psychopharmacol. 1996;125:214–219. doi: 10.1007/BF02247331. [DOI] [PubMed] [Google Scholar]
- 44.Avent KM, Riker RR, Fraser GL, Van der Schyf CJ, Usuki E, Pond SM. Metabolism of haloperidol to pyridinium species in patients receiving high doses intravenously: Is HPTP an intermediate? Life Sci. 1997;61:2383–2390. doi: 10.1016/s0024-3205(97)00955-7. 10.1016/s0024-3205(97)00955-7. [DOI] [PubMed] [Google Scholar]
- 45.Strobl GR, Kruednener S, Stockigt J, Guengerich FP, Wolf T. Development of a pharmacophore for inhibition of human liver cytochrome P4502D6: molecular modeling and inhibition studies. J Med Chem. 1993;36:1136–1145. doi: 10.1021/jm00061a004. [DOI] [PubMed] [Google Scholar]
- 46.Inaba T, Kovasacs J. Haloperidol reductase in human and guinea pig livers. Drug Metab Dispos. 1989;17:330–333. [PubMed] [Google Scholar]
- 47.Someya T, Takahashi S, Shibasaki M, Inaba T, Cheung SW, Tang SW. Reduced haloperidol/haloperidol ratios in plasma: Polymorphism in Japanese psychiatric patients. Psychiatry Res. 1990;31:111–120. doi: 10.1016/0165-1781(90)90114-k. [DOI] [PubMed] [Google Scholar]