Abstract
Aims
The population pharmacokinetics of 131I-mAbF19, a radiolabelled murine monoclonal antibody against fibroblast activation protein and a potential antitumour stroma agent, were investigated during two phase I studies in cancer patients.
Methods
131I-mAbF19 serum concentration-time data were obtained in 16 patients from two studies involving imaging and dosimetry in colorectal carcinoma and soft tissue sarcoma. Doses of 0.2, 1 and 2 mg antibody were administered as 60 min intravenous infusions. The data were analysed by nonlinear mixed effect modelling.
Results
The data were described by a two-compartment model. Population mean values were 109 ml h−1 for total serum clearance, 3.1 l for the volume of distribution of the central compartment, and 4.9 l for the volume of distribution at steady state. Mean terminal half-life was 38 h. Intersubject variability was high, but no patient covariates could be identified that further explained this variability. In particular, there was no influence of tumour type or mAbF19 dose.
Conclusions
The pharmacokinetics of antistromal mAbF19 were well defined in these two studies with different solid tumour types, and were comparable with those of other murine monoclonal antibodies that do not bind to normal tissue antigens or blood cells.
Keywords: cancer patients, fibroblast activation protein, mAbF19, monoclonal antibody, population pharmacokinetics
Introduction
mAbF19 is a murine monoclonal antibody directed against fibroblast activation protein (FAP), which is an inducible tumour stromal antigen of epithelial cancers and a classical antigen on a subset of soft tissue sarcomas [1, 2]. While being abundantly expressed in reactive stromal fibroblasts of > 90% of common epithelial cancers, the 95 kDa cell surface glycoprotein FAP shows a very limited distribution pattern in normal tissues and thus exhibits considerable potential for tumour targeting and therapy [2].
131I-radiolabeled mAbF19 has been investigated in two phase I studies in cancer patients. The first included 17 patients with hepatic metastases from colorectal carcinoma, and the highly selective expression pattern of FAP enabled imaging of lesions as small as 1 cm in diameter [3]. The second, hitherto unpublished study was a pilot investigation of dosimetry and localization in nine patients with soft tissue sarcoma. Only preliminary pharmacokinetic data for the colorectal carcinoma patients have been reported [3], and no prior publication has examined pharmacokinetics of an antibody that targets tumour stroma, in contrast to antigens or receptors expressed on tumour cells that might also be present in the circulation or in normal tissue. Therefore, an analysis is reported of pooled pharmacokinetic data from these two phase I studies of 131I-mAbF19.
Methods
Pharmacokinetic data were obtained from 7 out of 17 patients from the colorectal carcinoma study [3], and from the 9 patients from the soft tissue sarcoma study, which was a pilot investigation of dosimetry and localization of 131I-mAbF19 in histologically confirmed primary, recurrent or metastatic soft tissue sarcoma. Inclusion criteria, clinical procedures, informed consent and ethical review were very similar for both studies and have been documented [3]. Eligibility criteria relevant for pharmacokinetic analysis were: Karnofsky performance score (functional activity and well-being) > 70% [4], serum creatinine and bilirubin levels < 2 mg dl−1 and prothrombin time < 1.3 × control. Important exclusion criteria were: prior exposure to mouse immunoglobulin, serious cardiac disease, serious infection or other serious illness, and illness requiring the use of steroids or other anti-inflammatory agents. Baseline characteristics of the patients were (median and range): age 58 (27–74) years, weight 72 (36–107) kg, body surface area 1.83 (1.33–2.33) m2. Nine patients were men and seven were women.
Preparation, characterization and radio-iodination of mAbF19 were as previously described [3]. Doses were administered intravenously over 60 min. Pharmacokinetic data were available for the 0.2 mg (n = 4) and 2 mg (n = 3) doses in the colorectal carcinoma study. The dose of mAbF19 in the soft tissue sarcoma study was 1 mg for all patients. Serum samples were taken on 8–11 occasions between the end of infusion and study day 7. Serum radioactivity was counted in duplicate in a gamma counter with decay correction using appropriate standards of 131I. Coefficients of variation of serum concentrations derived from the counting procedure were within 1–2%.
Data analysis was by nonlinear mixed effects modelling (NONMEM, version V level 1.1) [5] combined with graphical analysis [6, 7]. A total of 143 serum concentrations was available from 16 patients. An intravenous two-compartment structural model was used (subroutines ADVAN3 and TRANS3), parameterized with total serum clearance (CL), central volume of distribution (V), volume of distribution at steady state (Vss), and intercompartmental clearance (Q). An exponential model was used for interindividual variabilities (η) of the pharmacokinetic parameters, and a proportional model for the residual (intraindividual) variability (ε). η and ε are modelled as random variables with zero mean and variances ω2 and σ2, respectively [5]. The first order conditional estimates method with interaction was used to obtain population mean and individual pharmacokinetic parameters. Other parameters such as half-life were calculated using standard formulas [8]. Goodness of fit was judged by the minimum value of the objective function, the standard errors of the pharmacokinetic parameters, the magnitude of their interindividual variabilities and of the residual variability, and by appropriate diagnostic graphs [5–7].
Results
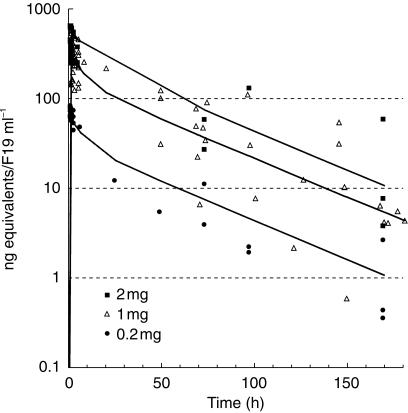
The combined 131I-mAbF19 serum concentration-time data from all patients, with the computed population mean curve at each dose level from the two-compartment model, are shown in Figure 1. Pharmacokinetic parameters and statistics from NONMEM analysis are listed in Table 1. The parameter standard errors (9–18% CV) and the residual variability (12.5% CV) indicated a satisfactory data fit. Plots of predicted vs observed serum concentrations exhibited a random distribution of the data points around the line of unity, and the weighted residual plots were essentially trendless. However, the interindividual variability was high, for example 54% CV for clearance. Mean values (± s.d.) of relevant secondary pharmacokinetic parameters were: initial half life 3.9 ± 1.9 h, terminal half-life 37.9 ± 12.6 h, proportion of area under the curve in the terminal phase 84 ± 15%. A graphical analysis (not shown) indicated that there were no evident correlations between pharmacokinetic variables and the following study/patient covariates: dose, tumour type, gender, age, body weight, or body surface area. Linear regression analysis (not shown) indicated that maximum serum concentrations increased proportionally with the dose, and that CL was independent of dose.
Figure 1.
Pooled serum 131I-mAbF19 concentration-time data from two studies in cancer patients, with population mean curves at the three dose levels.
Table 1.
Population pharmacokinetic parameters and statistical results, two-compartment model, following the administration of 131I-mAbF19 to cancer patients.
| Parameter | Population mean value | Standard error of estimate | Intersubject variability (CV) | Standard error of variability estimate |
|---|---|---|---|---|
| CL (ml h−1) | 109 | 14.6% | 54% | 31% |
| Q (ml h−1) | 142 | 18.2% | a | a |
| V (l) | 3.13 | 10.8% | 37% | 49% |
| Vss (l) | 4.87 | 9.2% | 31% | 45% |
| Residual variability | 12.5% | 31% |
not in final model.
Discussion
The results show that pharmacokinetic parameters of the murine therapeutic monoclonal antibody 131I-mAbF19 could be well defined by combining data from two Phase I studies in cancer patients, even though tumour imaging was the primary aim of these studies. Owing to the limited availability of the patients in the clinical centres, serum sampling was more frequent early on day 1 at the beginning of the distribution phase of 131I-mAbF19 than during the late distribution phase on days 1–2 and the elimination phase on days 2–7 (Figure 1). However, with the exception of the time period 6–24 h post infusion, the kinetic profile of 131I-mAbF19 was generally well covered and the NONMEM program could be used with advantage to pool data at different sampling times from all patients to obtain pharmacokinetic population estimates with good precision (Table 1). The mean value of V (3.1 l) is close to the physiological serum volume, and Vss is slightly higher (4.9 l), indicating limited extravascular distribution or binding. All parameters compare well with published clinical pharmacokinetic data on other murine monoclonal antibodies that do not bind to abundantly expressed normal tissue antigens or to blood cells [9, 10]. This therefore suggests that there is no accessible FAP antigen present in the circulation or on normal tissues to influence pharmacokinetics and tumour targeting.
The pharmacokinetic data reported here are based entirely on radioactivity derived from the 131I label of mAbF19, rather than measurement of mAbF19 protein via an immunological method. However, previously published data indicate that the radiolabel is stably attached to the antibody protein in such in vivo studies [11]. The long half-life determined for 131I-mAbF19 in the present study is consistent with these findings.
There was no difference in pharmacokinetic parameters of 131I-mAbF19 between patients with soft tissue sarcoma and colorectal carcinoma, nor was there any dose dependence of pharmacokinetics. These two factors were partially confounded, because two doses were used in the colorectal carcinoma study (0.2 and 2 mg) and an intermediate dose in the soft tissue sarcoma study (1 mg). Nevertheless, an important difference in pharmacokinetics between the colorectal carcinoma (n = 7) and soft tissue sarcoma patients (n = 9) would probably have been detected. In the colorectal carcinoma study, specific localization to the tumour and metastases occurred, but the amount of antibody bound was small compared with the dose administered [3]. The interindividual pharmacokinetic variability was somewhat higher in the soft tissue sarcoma study, with a 7.5-fold difference in clearance estimates across patients from 36 to 273 ml h−1, than in the colorectal carcinoma study with a 4.1 fold difference in CL from 58 to 238 ml h−1 (individual data not shown). In the soft tissue sarcoma study, the heterogeneity of the tumour types and the variability of their expression of the fibroblast activation protein antigen was also considerably higher than in the colorectal carcinoma study (data not shown). Soft tissue sarcomas are connective tissue tumours and, in contrast to colorectal carcinoma, fibroblast activation protein is a conventional tumour antigen in soft tissue sarcoma because it is expressed by the malignant cells in certain histological subtypes. Fibroblast activation protein heterogeneity is a characteristic feature in human soft tissue sarcoma [1], but the present data are not sufficient to allow an assessment of any correlation between pharmacokinetics and tumour FAP expression.
No other covariates could be identified that influenced pharmacokinetic parameters. The exact clearance mechanism of murine monoclonal antibodies in humans is not known, but may be mediated hepatically [12]. Significant renal or hepatic dysfunction, comedication and concomitant disease states were exclusion criteria in these studies and can therefore be excluded as sources of variability. Thus, the observed interindividual variability can at present only be attributed to relatively large random differences between the study patients. However, the total number of patients was small, and a larger study might reveal covariate effects that were not apparent in the present study population. Thus, data from more patients are required to identify potential sources of pharmacokinetic variability of mAbF19. This issue will be addressed in studies that are currently ongoing with the humanized form of mAbF19.
Acknowledgments
The authors gratefully acknowledge the Clinical Immunology Nursing group, particularly Lucy Dantis, for the careful collection of samples, the staff of the Nuclear Medicine Serivce for in vivo and ex vivo measurements of radioactivity, and the referring attendings, at the Memorial Sloan Kettering Cancer Centre; and the laboratory staff at the Ludwig Institute for Cancer Research, New York Branch.
References
- 1.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA. 1988;85:177–180. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welt S, Divgi CR, Scott AM, et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 4.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Boeckman AJ, Beal SL, Sheiner LB. 1998. NONMEM User's Guides, NONMEM. Project Group, University of California at San Francisco, San Francisco. [Google Scholar]
- 6.Maitre PO, Buehrer M, Thomson D, Stanski DR. A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm. 1991;19:377–384. doi: 10.1007/BF01061662. [DOI] [PubMed] [Google Scholar]
- 7.Ette EI, Ludden TM. Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res. 1995;12:1845–1855. doi: 10.1023/a:1016215116835. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 9.Welt S, Divgi CR, Real FX, et al. Quantitative analysis of antibody localization in human metastatic colon cancer: a phase I study of monoclonal antibody A33. J Clin Oncol. 1990;8:1894–1906. doi: 10.1200/JCO.1990.8.11.1894. [DOI] [PubMed] [Google Scholar]
- 10.Oosterwijk E, Bander NH, Divgi CR, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11:738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 11.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly RM, Sandhu J, Alvarez-Diez TM, Gallinger S, Kirsh J, Stern H. Problems of delivery of monoclonal antibodies: pharmaceutical and pharmacokinetic solutions. Clin Pharmacokin. 1996;28:126–142. doi: 10.2165/00003088-199528020-00004. [DOI] [PubMed] [Google Scholar]