Abstract
Aims
The study was carried out in order to assess the effects of gender and the use of oral contraceptives (OCs) on CYP2D6 and CYP2C19 activities in healthy volunteers.
Methods
Six hundred and eleven Caucasian volunteers (330 males and 281 females; age range 18–49 years) were phenotyped with respect to CYP2D6 and CYP2C19 by means of the probe drugs dextromethorphan and mephenytoin, respectively. Extensive metabolisers were selected for this study.
Results
The median dextromethorphan/dextrorphan metabolic ratio in non-OC using females was significantly lower than in males (0.067 vs 0.080; P = 0.033) (mean difference in ln dextromethorphan/dextrorphan metabolic ratio 0.023, 95% CI 0.03–0.43). For the mephenytoin S/R ratio, no such difference was observed. However, OC using females had a significantly higher median mephenytoin S/R ratio than non-OC using females (0.230 vs 0.090; P < 0.001) (mean difference in ln mephenytoin S/R ratio 0.082, 95% CI 0.60–1.04). Moreover, females using combined OCs had a significantly higher median ratio than females using OCs with progestins only (median 0.258 vs 0.135; P = 0.008) (mean difference in ln mephenytoin S/R ratio 0.82, 95% CI 0.21–1.34).
Conclusions
Given certain assumptions, the study indicates that females in the fertile age have a slightly higher CYP2D6 activity compared with males. There was no evidence of a gender difference in CYP2C19 activity. The use of combined OCs reduces the activity of CYP2C19, an effect that seems to be related to the ethinyloestradiol component.
Keywords: cytochrome P-450, CYP2D6, CYP2C19, ethinyloestradiol, gender, oral contraceptives, phenotype
Introduction
A large number of drugs are substrates of the polymorphic cytochrome P-450 (CYP) enzymes CYP2D6 and CYP2C19. Individual CYP2D6 activity can be evaluated with marker drugs such as sparteine, debrisoquine and dextromethorphan. The most common probe drug for CYP2C19 is mephenytoin.
Owing to genetic polymorphisms, considerable interindividual variability exists in the metabolic activity of these enzymes [1]. Gender is another factor that may affect CYP enzyme activity [2]. For example, the activity of CYP3A4 appears to be higher in females, whereas males seem to have a higher CYP1A2 activity. For CYP2D6 and CYP2C19, few data are available, and conflicting evidence exists regarding possible gender differences [2–5]. Furthermore sex hormones seem to be implicated in the regulation of CYP enzymes, but few conclusive data are available [2, 6]. The present study was undertaken in order to further examine the influence of gender and oral contraceptive use on CYP2D6 and CYP2C19 activities.
Methods
After giving their informed consent, 611 Caucasian volunteers (330 males and 281 females) between 18 and 49 (mean 26) years old were included in this study, which was approved by the Regional Ethics Committee of the University of Umeå. The health status of the subjects was assessed by medical history, physical examination and routine blood chemistry tests. Only healthy subjects (n = 472) or subjects with the following conditions/diseases were entered in the study: allergy (n = 108), asthma (n = 13), psoriasis (n = 8), migraine (n = 7), lactose intolerance (n = 4) and gluten intolerance (n = 1). None of the volunteers had a history of alcohol abuse or drug addiction. With the exception of oral contraceptives, inhaled β2-adrenoceptor agonists and glucocorticoids, and oral antihistamines, drug intake was not allowed during the 14 days before phenotyping. Drugs other than oral contraceptives were used by 30 subjects and herbal medications were used by 33 subjects. No subjects used St John's Wort. The subjects' body weights, estimated caffeine intake from dietary history and smoking status (daily smokers) are presented in Table 1.
Table 1.
Dextromethorphan/dextrorphan and mephenytoin S/R metabolic ratios in CYP2D6 and CYP2C19 extensive metabolisers, respectively, and demographic data divided according to gender and use of oral contraceptives (OCs).
| Dextromethorphan/dextrorphan (CYP2D6) | Mephenytoin S/R (CYP2C19) | |||||
|---|---|---|---|---|---|---|
| Men (n = 285) | Women not using OCs (n = 176) | Women using OCs (n = 82) | Men (n = 316) | Women not using OCs (n = 190) | Women using OCs (n = 82) | |
| Metabolic ratio | 0.080 | 0.067 | 0.062 | 0.089 | 0.090 | 0.230 |
| median (range) | 0.002–0.0857 | 0.00–0.079 | 0.01–0.083 | 0.006–0.530 | 0.006–0.650 | 0.010–0.659 |
| Ln metabolic ratio mean (s.d.) | −2.49 (1.04) | −2.72 (1.15)* | −2.78 (1.11) | −2.45 (0.86) | −2.49 (0.83) | −1.67 (0.84)** |
| Age (years) mean (s.d.) | 26.2 (6.5) | 27.6 (8.0) | 23.8 (4.4) | 26.2 (6.3) | 27.9 (8.2) | 24.0 (5.0) |
| Body weight mean (s.d.) | 76.5 (10.5) | 62.3 (9.3) | 61.8 (8.4) | 76.4 (10.5) | 62.2 (9.1) | 61.9 (8.7) |
| Caffeine intake mean (s.d.)(mg day−1) | 250 (190) | 280 (220) | 190 (170) | 240 (190) | 290 (220) | 190 (170) |
| Current smokers number (%) | 37 (13) | 14 (8) | 11 (14) | 38 (12) | 16 (8) | 9 (11) |
Men vs women not using OCs P = 0.033 (mean difference 0.23, 95% confidence interval 0.03–0.43).
Women using OCs vs women not using OCs P < 0.001 (mean difference 0.82, 95% confidence interval 0.60–1.04).
After intake of 50 mg dextromethorphan hydrobromide (Tussidyl mixture, 2 mg ml−1, Tika, Lund, Sweden) and 100 mg racemic mephenytoin (Mesantoin, Novartis, Basel, Switzerland), urine was collected for 10 h. Urinary dextromethorphan/dextrorphan and mephenytoin S/R ratios were used as estimates of CYP2D6 and CYP2C19 activity, respectively [7, 8]. Only individuals classified as extensive metabolisers (EMs) were included in the statistical evaluations of the metabolic ratios.
Dextromethorphan and dextrorphan were analysed by a capillary gas chromatography method described in detail previously [9]. Subjects with metabolic ratios < 0.9 were classified as EMs. The limits of quantification for these compounds were 1.0 and 2.5 ng ml−1, respectively. For dextromethorphan the intra-assay and interassay coefficients of variation were 9.1 and 15.6%, respectively (10 ng ml−1), and 5.1 and 11.5%, respectively (5000 ng ml−1). For dextrorphan the intra-assay and interassay coefficients of variation were 12.6 and 18.7%, respectively (10 ng ml−1), and 2.3 and 6.2%, respectively (5000 ng ml−1).
The mephenytoin hydroxylation phenotype was determined by a capillary gas chromatography method as described in detail previously [8, 10]. Subjects with S/R ratios < 0.8 were classified as EMs and included in the statistical evaluations. The limits of quantification were 20 ng ml−1 for both enantiomers. The intra-assay and interassay coefficients of variation for the S/R-mephenytoin ratio were 5.5 and 17.3%, respectively (20 ng ml−1), and 2.3 and 10.7%, respectively (160 ng ml−1).
Statistical evaluations were performed with SPSS version 9.0 (SPSS Inc, Chicago, IL, USA). Since the distributions of the metabolic ratios were highly skewed, data were log transformed (natural logarithm, ln) prior to analysis. This transformation successfully normalized the distribution as confirmed by Levene's test. For comparative statistics, Student's t-test was used. P values of less than 0.05 were considered statistically significant.
Results
With respect to CYP2D6, 543 subjects were extensive metabolisers (EMs) and 68 were poor metabolisers (PMs). The median metabolic ratio was 0.070 for EMs and 5.26 for PMs. With respect to CYP2C19, 588 subjects were EMs and 23 were PMs. The median metabolic ratio was 0.103 for EMs and 1.03 for PMs. There were no differences in the intake of drugs or herbal medications or in the distribution of diseases, caffeine intake or smoking status between males and females or between users and nonusers of oral contraceptives (OCs).
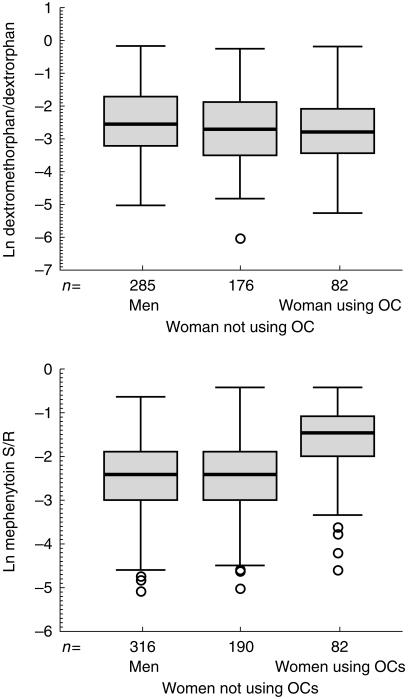
The distribution of the metabolic ratios for dextromethorphan and mephenytoin in EMs is shown in Figure 1. Females had a slightly, but statistically significantly lower, dextromethorphan/dextrorphan ratio compared to males (Table 1). No differences were revealed between OC using and non-OC using females. There were no differences in the mephenytoin S/R ratio between males and non-OC using females (Table 1). However, OC users had a significantly higher ratio than non-OC users. Moreover, females using combined OCs (n = 73) had a significantly higher mephenytoin S/R ratio than females using contraceptives with progestin only (n = 9) (median 0.244 vs 0.135; P = 0.008). The mean difference with ln mephenytoin S/R ratio was 0.82 (95% confidence interval 0.21–1.34).
Figure 1.
(a) Urinary dextromethorphan/dextrorphan ratios in 543 Caucasian volunteers between 18 and 49 years of age divided into three groups: males, non-OC using females and OC using females. The boxplot demonstrates median, interquartile range (box length) and outliers (cases with values at least 1.5 box lengths from the upper or lower edge of the box). (b) Urinary mephenytoin S/R ratios in 588 Caucasian volunteers between 18 and 49 years of age.
Discussion
In the present study, the median metabolic ratio for CYP2D6 was 16% lower in females than in males. This result contrasts with many previous studies (see [3]), where no gender effects have been found. In these studies, the number of subjects included has often been relatively low, and other CYP2D6 probes, such as sparteine and debrisoquine, have been employed. Although the probe drug in the present study was dextromethorphan, there is a high correlation between dextromethorphan and debrisoquine metabolic ratios [11]. The differences in body weight could not explain the finding because, if any effect was to occur, males would be expected to metabolize dextromethorphan faster than women due to their higher body weight. Our result is in accordance with another recent publication, also using dextromethorphan as the probe drug [5]. In that study, a 20% lower mean CYP2D6 metabolic ratio was reported in females. Nevertheless, given the considerable variability in CYP2D6 activity caused by factors other than gender, the differences observed most probably have a very limited clinical significance.
Gender related differences in renal clearance and urine flow may be potential confounders for urinary drug:metabolite ratios since the ratio is a function of both intrinsic clearance of the parent compound to metabolite and the renal clearances of the parent compound and the metabolite, respectively [14]. The effects of renal impairment on different indices used to mark CYP2D6 activity have been studied based on sparteine and dextromethorphan urinary recoveries, and urinary metabolic ratios. Twenty-four h fractional excretion of dextrorphan correlated well with creatinine clearance, but unfortunately dextromethorphan excretion and metabolic ratio were not available [12]. In the absence of conclusive data, it can not completely be excluded that gender differences in renal clearance and urine flow may explain the significantly lower dextromethorphan/dextrorphan metabolic ratio in females compared with males. Gender or sex hormone related differences in the activity of other metabolic routes may also be potential confounding factors. An increase in CYP3A4 activity may increase the dextromethorphan dextrorphan ratio [14]. However, since the mean CYP3A4 activity is higher in women [2] one should expect a higher ratio in women than in men, which is contrary to our result.
With respect to CYP2C19, our results indicate a reduced CYP2C19 activity in OC using females compared with non-OC using females, whereas no gender effect was observed. Some years ago, previous studies on this topic were reviewed [2] and it was concluded that CYP2C19 activity might be lower in females than in males. In contrast, in a recent study in a Chinese population [4], CYP2C19 activity was higher in female EMs than in male EMs. In another recent study in Caucasians [5], females had a 40% higher mephenytoin S/R ratio than males, indicating a lower CYP2C19 activity in females. However, in the latter investigation [5] subjects using OCs constituted a large part of the study population. Also, others have suggested that an age-specific effect of gender on CYP2C19 activity might be based on the use of OCs [16]. Thus, taken together, although the impact of gender on CYP2C19 activity is inconsistent and might differ according to ethnic group, OC use consistently decreases the metabolic activity of CYP2C19. As this effect was not found in subjects using OCs with progestin only, the effect is most likely caused by the ethinyloestradiol component. The mephenytoin S/R ratio may be confounded by metabolism via nonpolymorphic metabolic pathways [15]. Therefore, it cannot be completely excluded that the higher mephenytoin S/R ratio found in females using combined OCs compared with those using progestins only, is explained by the influence of oral contraceptives on these metabolic pathways. The renal clearances of the enantiomers of mephenytoin could also be confounding factors, but no relationship between the enantiomer ratio and creatinine clearance was found in a previous study [13].
Other CYP2C19 substrates have been studied with regard to the possible metabolic influence of OCs. The disposition of diazepam was compared in eight women using combined OCs with 50 µg ethinyloestradiol and eight women not using OCs [17]. The mean elimination half-lives of diazepam were 69 h and 47 h, respectively. Moreover, the metabolism of a single 80 mg oral dose of propranolol was investigated in nine young women before and after administration of ethinyloestradiol alone or in combination with norethindrone [18]. Administration of ethinyloestradiol alone significantly decreased the side chain oxidation of propranolol [18], a metabolic route which is in part mediated by CYP2C19 [19]. However the total clearance of propranolol was not significantly altered after administration of ethinyloestradiol.
The subjects were not genotyped in the present study, and we cannot conclude with certainty that the frequency of different alleles was the same in the groups studied. However, on the basis of previous studies, there is no reason to expect uneven distributions of alleles between genders [4], in particular because PMs, who only represent 3–7% of Caucasians, and therefore their alleles would be more likely to be unevenly distributed by chance, were not included in the present study.
In conclusion, the present study indicates that, given certain assumptions, females have a slightly higher CYP2D6 activity than males, and that the use of combined oral contraceptives reduces the activity of CYP2C19. Further studies are warranted in order to elucidate the impact of these factors on the metabolism of drugs known to be metabolized by CYP2D6 and CYP2C19.
Acknowledgments
The authors would like to thank Kerstin Granberg, Åke Norström and Hans-Åke Lakso for excellent clinical and technical assistance. This project was supported by grants from the Medical Faculty, Umeå University.
References
- 1.Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Clin Pharmacokin. 1995;29:169–173. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Llerena A, Cobaleda J, Martinez C, Benitez J. Interethnic differences in drug metabolism: influence of genetic and environmental factors on debrisoquine hydroxylation phenotype. Eur J Drug Metab Pharmacokin. 1996;21:129–138. doi: 10.1007/BF03190261. [DOI] [PubMed] [Google Scholar]
- 4.Xie HG, Huang SL, Xu Z, Xiao ZS, He N, Zhou HH. Evidence for the effect of gender on activity of (S) mephenytoin 4′-hydroxylase (CYP2C19) in a Chinese population. Pharmacogenetics. 1997;7:115–119. doi: 10.1097/00008571-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55:177–184. doi: 10.1007/s002280050615. 10.1007/s002280050615. [DOI] [PubMed] [Google Scholar]
- 6.Teichmann AT. Influence of oral contraceptives on drug therapy. Am J Obstet Gynecol. 1990;163:2208–2213. doi: 10.1016/0002-9378(90)90563-m. [DOI] [PubMed] [Google Scholar]
- 7.Hou ZY, Pickle LW, Meyer PS, Woosley RL. Salivary analysis for determination of dextromethorphan metabolic phenotype. Clin Pharmacol Ther. 1991;49:410–419. doi: 10.1038/clpt.1991.48. [DOI] [PubMed] [Google Scholar]
- 8.Tybring G, Bertilsson L. A methodological investigation on the estimation of the S-mephenytoin hydroxylation phenotype using the urinary S/R ratio. Pharmacogenetics. 1992;2:241–243. doi: 10.1097/00008571-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Spigset O, Granberg K, Hägg S, Norström Å, Dahlqvist R. Relationship between fluvoxamine pharmacokinetics and CYP2D6/CYP2C19 phenotype polymorphisms. Eur J Clin Pharmacol. 1997;52:129–133. doi: 10.1007/s002280050261. 10.1007/s002280050261. [DOI] [PubMed] [Google Scholar]
- 10.Sanz EJ, Villén T, Alm C, Bertilsson L. S-mephenytoin hydroxylation phenotypes in a Swedish population determined after coadministration with debrisoquin. Clin Pharmacol Ther. 1989;45:495–499. doi: 10.1038/clpt.1989.63. [DOI] [PubMed] [Google Scholar]
- 11.Schmid B, Bircher J, Preisig R, Kupfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985;38:618–624. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]
- 12.Kevorkian JP, Michel C, Hofmann U, et al. Assessment of individual CYP2D6 activity in extensive metabolizers with renal failure: comparison of sparteine and dextromethorphan. Clin Pharmacol Ther. 1996;59:583–592. doi: 10.1016/S0009-9236(96)90187-3. [DOI] [PubMed] [Google Scholar]
- 13.Pollock BG, Perel JM, Kirshner M, Altieri LP, Yeager AL, Reynolds Cf., III S-mephenytoin 4-hydroxylation in older Americans. Eur J Clin Pharmacol. 1991;40:609–611. doi: 10.1007/BF00279979. [DOI] [PubMed] [Google Scholar]
- 14.Rostami-Hodjegan A, Kroemer HK, Tucker GT. In-vivo indices of enzyme activity: the effect of renal impairment on the assessment of CYP2D6 activity. Pharmacogenetics. 1999;9:277–286. doi: 10.1097/00008571-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Jackson PR. Theoretical and experimental studies of variability in drug disposition. University of Sheffield; 1987. PhD thesis. [Google Scholar]
- 16.May DG. Genetic differences in drug disposition. J Clin Pharmacol. 1994;34:881–897. doi: 10.1002/j.1552-4604.1994.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 17.Abernethy DR, Greenblatt DJ, Divoll M, Arendt R, Ochs HR, Shader RI. Impairment of diazepam metabolism by low-dose estrogen-containing oral-contraceptive steroids. N Engl J Med. 1982;306:791–792. doi: 10.1056/NEJM198204013061307. [DOI] [PubMed] [Google Scholar]
- 18.Walle T, Fagan TC, Walle UK, Topmiller MJ. Stimulatory as well as inhibitory effects of ethinyloestradiol on the metabolic clearances of propranolol in young women. Br J Clin Pharmacol. 1996;41:305–309. doi: 10.1046/j.1365-2125.1996.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward SA, Walle T, Walle UK, Wilkinson GR, Branch RA. Propranolol's metabolism is determined by both mephenytoin and debrisoquin hydroxylase activities. Clin Pharmacol Ther. 1989;45:72–79. doi: 10.1038/clpt.1989.11. [DOI] [PubMed] [Google Scholar]