Abstract
Studies of novel antipsychotics in healthy volunteers are traditionally concerned with kinetics and tolerability, but useful information may also be obtained from biomarkers of clinical endpoints. A useful biomarker should meet the following requirements: a consistent response across studies and antipsychotics; a clear response of the biomarker to a therapeutic dose; a dose–response relationship; a plausible relationship between biomarker, pharmacology and pathogenesis. In the current review, all individual tests found in studies of neuroleptics in healthy volunteers since 1966 were progressively evaluated for compliance with these requirements. A MedLine search yielded 65 different studies, investigating the effects of 23 different neuroleptics on 101 different (variants of) neuropsychological tests, which could be clustered into seven neuropsychological domains. Subjective and objective measures of alertness, and of visual-visuomotor-auditory and motor skills were most sensitive to antipsychotics, although over half of all the studies failed to show statistically significant differences from placebo. The most consistent effects were observed using prolactin response and saccadic eye movements, where 96% and 83% of all studies resp. showed statistically significant effects. The prolactin inducing dose equivalencies relative to haloperidol of 19 different antipsychotic agents correlated with the lowest recommended daily maintenance dose (r2 = 0.52). This relationship could reflect the clinical practice of aiming for maximum tolerated levels, or it could represent a common basis behind prolactin release and antipsychotic activity (probably D2-receptor antagonism). The number of tests used in human psychopharmacology appears to be excessive. Future studies should look for the most specific and sensitive test within each of the domains that are most susceptible to neuroleptics.
Keywords: biomarker, antipsychotic, phase I, cognition, prolactin
Introduction
There is a growing pressure on the drug development process to enhance the relevance of studies at all stages. Traditionally, phase 1 studies were mainly concerned with kinetics and tolerability of a new compound in healthy volunteers, but increasing efforts are now made to include potential biomarkers of clinical endpoints. This approach is particularly useful in areas where phase 2 studies are cumbersome, for practical or ethical reasons. This is the case for many neuropsychiatric indications, including psychosis and schizophrenia. Patient studies can be complicated by factors associated with the disease, such as concomitant or previous treatments, adaptation of dose and duration of treatment to clinical responses, different types and severity of psychopathology and overlap between symptoms and side-effects of treatment. Also, a heterogenic patient population may augment individual variability, for example due to differences in intelligence and motivational aspects.
Studies in healthy volunteers lack most of these methodological and logistic problems, but are faced with others. Healthy volunteers are usually studied using single (ascending) doses, as opposed to chronic treatment in patients. They obviously also lack the disease characteristics that serve to measure the treatment effects, although some studies use healthy subjects with schizotypy-like personalities to approach clinical relevance [1–4]. The information derived from studies in healthy volunteers could also be enhanced with appropriate biomarkers, which can be defined as indicators of biologic, pathogenic or pharmacologic processes or responses to therapeutic interventions.
Currently, no validated biomarkers for psychosis or antipsychotics are available, but a useful marker should meet the following requirements:
1 – a clear, consistent response across studies (from different groups) and antipsychotics
2 – a clear response of the biomarker to a therapeutic dose of the antipsychotic
3 – a dose (concentration)-response relationship
4 – a plausible relationship between the biomarker, the pharmacology of the antipsychotic and the pathogenesis of the disease.
In the current review, these requirements were used to evaluate all potential biomarkers that have been used in healthy volunteer studies of antipsychotic agents over the past 30 years.
Methods
Structured literature evaluation
An extensive MedLine search (keywords: (antipsychotic or neuroleptic) and healthy) revealed a large number of individual tests, which differed widely in their sensitivity and specificity for detection of central nervous system (CNS) drug effects, with a lack of standardization between the studies even for the same tests. In addition, many studies used different antipsychotic dosages, usually at single doses. A structured procedure was adopted in order to obtain an overview. First, the results for all individual tests, drugs and dosages were put into a database. Most studies used different tests, which were all treated as independent measures of drug effect. The tests could then be roughly divided into neuropsychological/motor skills, subjective assessments, and neurophysiological and neuroendocrine measurements. This approach allowed the preservation of individual study data in early stages, followed by a progressive condensation of results in logical clusters.
The test results could not be recorded quantitatively, considering the large diversity of methods, parameters and treatments. Instead, the ability of a test to show a statistically significant difference from placebo was scored as + (improvement/increase), = (no significant effect) or − (impairment/decrease). Although statistical significance is not only determined by the test variance but also by other factors like group size, this approach at least allowed an evaluation of the applicability of a test as a biomarker. No efforts were made to further quantify the level of statistical significance. A more quantitative approach was possible only for prolactin, where the peak concentration relative to baseline could be determined from most studies.
The chance that a test will detect a difference from placebo is expected to grow with increasing dose. To investigate this possibility, for each individual neuroleptic and test it was determined whether the number of statistically significant results increased with the dose. In this way, the most frequently used tests and drug dosages could be compared for dose-dependency. In many cases however, the number of tests or doses was too small to determine a relationship. To obtain an overview of dose-effects across neuroleptics, drug dosages were pooled into ‘lower’, ‘medium’ and ‘higher’ dosages. The ‘medium’ dose was determined as the lowest recommended therapeutic starting dose [5, 6], as shown in Table 1. If the starting dose could not be retrieved, half the lowest recommended maintenance dose was used. The ‘lower’ and ‘higher’ doses were all dosages below or above this level.
Table 1.
PRL-inducing dose equivalencies for prolactin release, therapeutic dose and receptor affinities for antipsychotic drugs. (See text for explanation of PRL-inducing dose equivalence).
| Drug | Maintenance dose (mg day−1) | Reported study range (mg) | PRL-inducing dose equivalencies | D2 receptor affinityKi (nm) [49] | 5-HT2/D2ratio [49] |
|---|---|---|---|---|---|
| Amisulpride | 300–1200 | 20–400 | 56.6 | ||
| DU-29895 | ? | 3–10 | 2 | ||
| Remoxipride | 60–300 | 0.5–150 | 9.29 | 272 | > 40 |
| Sulpiride | 100–200 | 100–400 | 97.8 | 31 | 40 |
| Zetidoline | 10–30 | 10–40 | 0.81 | ||
| Raclopride | 4–8 | 0.1–16 | 1.72 | 7.0 | 1429 |
| Mazapertine | ? | 5–50 | – | ||
| Zotepine | 50–300 | 25–100 | 17.2 | 13 | 0.07 |
| Pimozide | 2–6 | 2–6 | 7.89 | 1.2 | 5 |
| Setoperone | 15–120 | 5–40 | 14 | ||
| Olanzapine | 5–20 | 3–5 | – | ||
| Clozapine | 150–300 | 12.5–50 | 309 | 152 | 0.02 |
| Risperidone | 4–8 | 1–2 | 0.1 | 3.1 | 0.05 |
| Chlorpromazine | 75–300 | 25–100 | 39.6 | 19 | 0.14 |
| Prochlorperazine | 25–50 | 2.5–5 | 8.3 | 3.1 | 2.4 |
| Trifluoperazine | 20–30 | 4 | 5.1 | 4.3 | 2 |
| Perphenazine | 16–64 | 1 | 2 | 6.5 | 0.66 |
| Haloperidol | 4–10 | 0.25–10 | 1.11 | 1.2 | 2.3 |
| Fluphenazine | 2.5–5 | 0.35 | 1.89 | 1.9 | 1.8 |
| Thiotixene | 20–30 | 0.25–0.5 | 0.56 | 2.5 | 39 |
| Thiethylperazine | 10 | 10 | 4.37 | 4.5 | 11 |
| Molindone | 30–100 | 5 | 1.13 | 25 | 94 |
| Thioridazine | 200–800 | 10–75 | – | 16 | 0.26 |
This approach allowed the identification of tests showing a consistent response across studies and antipsychotics, and those with a clear response to a therapeutic dose of the antipsychotic (requirements 1 and 2 from the introduction). All measurements fulfilling these criteria were further tested for compliance with requirements 3 and 4: the existence of dose–response relationship and the plausibility of a mechanistic relationship, by reference to the original publications and the neuropharmacological literature.
Neuropsychological/motor skill
In the first phase of the literature review, tests from different studies were only grouped if they were equal as judged from name and description or literature reference (e.g. all Digit Symbol Substitution Tests (DSST)), but all variants or related forms of the tests (DCCT, SDST, etc.) were treated separately.
Next, all tests that could be regarded as variants from a basic form were clustered as indicated in Table 2. Thus, all tests determining the ability to discriminate flash-or flicker frequencies were grouped as ‘flicker discrimination’. These data were used to determine the consistency of results within test clusters and to identify potential dose-effects.
Table 2.
Progressive condensation of all reported tests; from test to cluster to domain (after Spreen et al. 1998 [7]).
| Test | Cluster | Domain |
|---|---|---|
| WAIS vocabulair | Intelligence | Achievement |
| WAIS similarity | ||
| WAIS block design | ||
| WAIS picture | ||
| Blue-Brown visual inhibition | Inhibition task | Executive |
| H-mask visual inhibition | ||
| Auditory latent inhibition | ||
| Visual latent inhibition | ||
| Stroop colour word | ||
| Simple reaction (conflict task) | ||
| Cognitive set switching | ||
| Logical reasoning Decision making time | Complex info process | |
| Rapid info processing | ||
| Perceptual maze | ||
| Simulated driving | ||
| Visual search | ||
| Time estimation | Time estimation | |
| Time perception | ||
| Visual search | Search | Attention |
| Attentional search | ||
| Symbol copying | ||
| Letter cancellation | ||
| Alphabetic cross-out | ||
| D2 cancellation | ||
| Brickenkamp D2 | ||
| DCCT | DSST like | |
| SDST | ||
| DSST | ||
| Digit vigilance | Other vigilance | |
| Vigilance | ||
| Auditory vigilance test | ||
| Wesnes/Warburton vigilance task | ||
| Rapid info processing | ||
| Continuous attention | ||
| CRT +Tracking Divided attention | Divided attention | |
| Selective attention | ||
| Focused attention task | ||
| Emotional attention task | ||
| Auditory flutter fusion | Flicker discrimination | |
| Flash fusion | ||
| CFF | ||
| Paired associate learning | Learning | Memory |
| Word list learning | ||
| 15 word test | ||
| Introductory conditioning | ||
| Delayed word recall | Delayed recall | |
| Delayed word recognition | ||
| Delayed picture recognition | ||
| Word presentation | Immediate recall | |
| Word recognition | ||
| Numeric working memory | ||
| Numerical memory | ||
| Memory scanning | ||
| Auditory Brown/Peterson | ||
| Visual Brown/Peterson | ||
| Visual spatial memory | ||
| Fragmented picture test | ||
| Pauli test | Span tests | |
| Block span | ||
| Digit span | ||
| Digit span (forward) | ||
| Digit span (backward) | ||
| WAIS vocabulair | Language | Language |
| WAIS similarity | ||
| Word fluency | ||
| Verbal fluency | ||
| Performance time (Delayed word recognition) | Performance time | Visual, visuomotor and auditory |
| Performance time (Numeric working memory) | ||
| Performance time (Digit vigilance) | ||
| Performance time (Rapid info processing) | ||
| Performance time (Delayed picture recognition) | ||
| Performance time (Visual information processing) | ||
| Simple reaction time | Reaction time | |
| CRT | ||
| Complex RT visual | ||
| Visual 2 choice RT | ||
| VRT | ||
| Visual response speed | ||
| ART Acoustic RT | ||
| Wire maze tracing Archimedian spiral | Eye-hand coordination | |
| Critical tracking task | ||
| Trail making | ||
| Tracking | ||
| Complex tracking | ||
| Wiener Geraet | ||
| Flexibility of closure | Other | |
| WAIS block design | ||
| WAIS picture compilation | ||
| Digit copying | ||
| Manipulative motor | Manipulation | Motor |
| Feinmotorik | ||
| Graphological analysis | ||
| Tapping | ||
| Hand arm lateral reach coordination | Motor control | |
| Visual arm random reach | ||
| Motor control and coordination | ||
| Motor behaviour | ||
Although many different methods are used to evaluate the functional effects of neuroleptics, most actually measure a limited number of core features. Neuropsychological/motor skills-tests can be categorized according to a catalogue of neurocognitive tests (attention, executive, etc.) [7], as presented in Table 2. This catalogue divides tests according to different neuropsychological domains, assuming that the results of each test are mainly determined by one of these domains. To determine the domains that are most affected by neuroleptics in healthy subjects, all tests within a neurocognitive domain were bundled. The number of statistically significant differences from placebo was scored and compared with the total number of studies within the domain.
Subjective assessments
For the subjective assessments, most individual scales corresponded to individual lines for the subscales ‘alertness’, ‘mood’ and ‘calmness’, proposed by Norris and applied to CNS-drug evaluation by Bond & Lader [8, 9]. Other scales could be grouped under ‘anxiety’, ‘subjective (psychotropic) drug effects’ and ‘(extrapyramidal) side-effects’.
Neurophysiological assessments
Electroencephalography (EEG) EEG is sensitive to a wide range of centrally active substances, although the exact mechanism is hardly ever known [10–14]. EEG-studies differ in numbers of leads or technical settings, but they usually report effects per EEG-frequency band, which are divided into delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–11.5 Hz) and beta (above 11.5 Hz; sometimes subdivided into beta 1 (11.5–30 Hz) and beta 2 (above 30 Hz)). In the current review, statistically significant differences from placebo were scored for the four major frequency bands.
Eye movements Smooth pursuit and saccadic eye movements have been extensively validated to assess CNS-drug (side)-effects [15–20]. Saccadic eye movements provide information on the sedative properties of antipsychotic drugs. These effects are not specific for a class of drugs [21, 22], but rather quantify sleep/wake transition [23, 24]. Although there are different techniques to measure eye movements, most studies report peak velocity for visually guided saccades or sometimes antisaccades (where subjects are instructed to look away from the target). Smooth pursuit eye movements are reported as deviations from the time that the eyes closely followed the target. Statistically significant differences from placebo were reported, and dose–response relationships were investigated for consistent responses.
Evoked potentials Schizophrenic patients exhibit abnormalities in event related potentials (ERP) that are postulated to reflect characteristic changes in stimulus discriminability and decision making. Typically, these consist of a reduction in the amplitude and a prolongation of the latency of the P300 component [3, 4, 25–35].
There were not enough healthy volunteer studies to warrant (semi)quantitative evaluation of these tests, but the results are described because of the apparent relevance of this method in schizophrenia research.
Neuroendocrine assessments
Prolactin (PRL) Neuroendocrine tests and particularly the PRL response to antipsychotic agents have been reviewed in several publications [36–41]. PRL response to antipsychotics is clinically related to hyperprolactinaemia [42] and is therefore thought undesirable during drug development [43]. However, the prolactin response to antipsychotics is a direct consequence of dopamine antagonism, since pituitary PRL secretion is inhibited by dopamine. Dopamine antagonism is one of the core characteristics of antipsychotic agents [44, 45], and abnormal dopamine activity is a widely accepted central pathophysiological abnormality in psychosis [44–46]. The PRL-response to neuroleptics is frequently studied in healthy volunteers, and usually the maximum PRL-response is reported. This response is determined by the dose of a neuroleptic, and by its PRL-inducing potency. The value of prolactin as a biomarker would be particularly large, if for a range of neuroleptics the PRL-inducing potencies were closely related to the therapeutic doses. Such a comparison can only be made directly on the basis of well-defined PRL-inducing potencies determined from complete dose–response relationships for each neuroleptic. The literature did not provide this information for most neuroleptics; only haloperidol yielded enough data to plot a curve over a wide dose range, as described in the results-section. Therefore, an alternative approach was chosen where the PRL-inducing potency of each neuroleptic was expressed relative to this haloperidol dose–response curve [47, 48]. Neuroleptic doses that caused a larger PRL-response than observed with haloperidol were not plotted on this reference curve, i.e. data were not extrapolated beyond the extent of the curve. In this way, for each neuroleptic dose an equipotent haloperidol dose could be determined, that would theoretically cause the same peak PRL-response. Next, each dose was normalized to haloperidol 1 mg, and the mean of these values was calculated per neuroleptic. This constituted a PRL-inducing dose equivalence (relative to haloperidol) for each neuroleptic.
To examine the value of prolactin release as a biomarker for therapeutic efficacy, these mean PRL-inducing dose equivalencies were compared with the lowest recommended daily therapeutic maintenance doses ([5, 6], see Table 1). The relationships of individual PRL-inducing dose equivalencies with some key pharmacological features for the antipsychotics (D2 affinity (Ki) and 5-HT/D2 antagonism ratio) were examined. The Ki values (Table 1) were assessed using the same methods [49], allowing interdrug comparison.
Cortisol and growth hormone (GH) 5-HT agonists and antagonists have been found to have an effect on plasma cortisol and growth hormone levels, but the data are inconclusive [50–54]. These hormones have been used to evaluate antipsychotic drug action on serotonergic function, particularly 5-HT2 which may play a role in the mechanism of action of atypical neuroleptics [49]. The number of studies was too low to allow any quantitative analysis. The statistically significant differences from placebo were reported.
Statistical evaluation
To allow the calculation of average responses with confidence intervals for binomial proportions, responses were coded as follows. Impairment/decrease was coded as 0, no change was coded as 0.5 and improvement/increase was coded as 1. A cumulated response code was calculated by multiplying the number of occurrences for each response by the coding, and adding this over the three responses. A proportion was calculated by dividing the cumulated response code by the total number of responses. This yields an average response between 0 (impairment/decrease) and 1 (improvement/increase). For these proportions, exact confidence intervals for binomial proportions were calculated using the cumulated response code and the total number of responses. Exact confidence intervals were calculated using SAS for Windows V6.12 with the ExactPCI V1.2 procedure provided by SAS Inc (SAS Institute Inc, Cary, NC).
Results
The literature search yielded 65 different studies, published since 1966. These studies investigated 23 different neuroleptic agents, with 2.2 doses per study on average. Olanzapine was only given at slightly subtherapeutic dosages and mazapertine was not registered, but 76% of the doses of all other agents were at ‘medium’ or ‘higher’ levels. Thus, most studies were able to comply with the requirement that a useful biomarker should respond to therapeutic doses. Eighteen studies were solely devoted to haloperidol, and 12 studies used haloperidol as a reference for other neuroleptics. On average, there were 17 healthy participants (range 5–110) per study [2, 19, 47, 48, 52, 53, 55–107].
Neuropsychological/motor skill
There were 101 different test-(variants), as shown in Table 2; 51 of these were used only once. Six tests were used more than 10 times: critical flicker fusion (32 times), choice reaction times (32 times), finger tapping (18 times), time estimation (15 times), simple reaction times (15 times) and DSST (14 times). At least 33% of all tests that were used twice or more (by different groups) showed statistically nonsignificant or conflicting differences between neuroleptics and placebo. For the five most frequently used test, these percentages were 53%, 47%, 39%, 53%, 40% and 43%, respectively.
Fifteen individual tests showed statistically significant impairment in all cases (100%), but nine of these were only used once (one dose of one antipsychotic), and the six other tests were only used by a single research group: alphabetic cross-out (8 times), wire maze tracing (3 times), Pauli test (3 times), delayed picture recognition (2 times), delayed word recognition (2 times), and performance time for digit vigilance (2 times).
Subsequently, comparable tests or variants were clustered as shown in Table 2. Reaction times showed significant prolongation in 46% of the 52 times this method was used. Complex information processing tasks were used 39 times, showing significant impairment in 46%. Flicker fusion was employed 38 times, demonstrating significant impairment in 45%. The 21 DSST-like tests showed statistically significant impairment in 48%, no change in 48% and an improvement in 4%. Significant impairment on search tasks was found in 70% of 20 cases. Medium or higher doses were used in all cases except two. Manipulative motor tasks were performed 31 times, and showed significant impairment in 48%. A significant impairment was found in 41% of the 34 times that eye hand coordination was studied. Clustering of comparable tests thus did not increase the number of significant results.
However, the larger number of studies within clusters allowed a better estimation of dose-dependency. In most cases, consideration of only ‘medium’ or ‘higher’ doses did not appreciably increase the percentages of significant results. Only flicker fusion and complex information processing showed modest increases in the percentages of tests demonstrating impairment, when the lower dosages were omitted (from 45% to 57%, and from 46% to 51%, respectively).
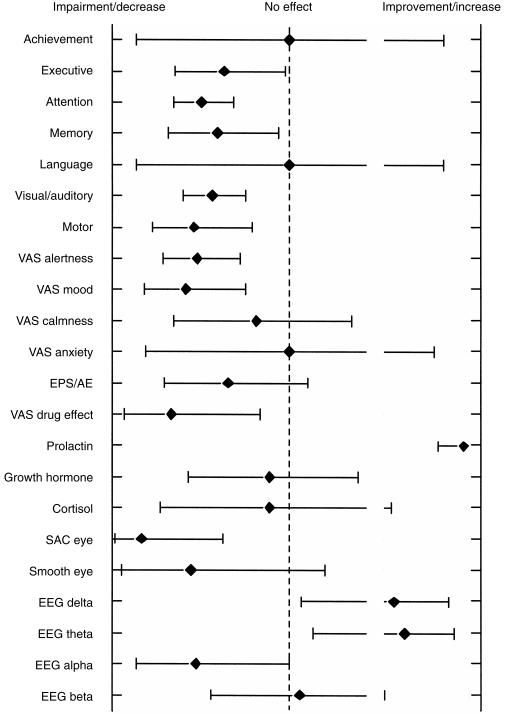
No individual neuropsychological/motor skill-test or cluster of related test variants showed a consistent response to antipsychotics, and this did not improve to any extent when a dose-effect relationship was taken into account. To evaluate which neuropsychological domains are most clearly affected by neuroleptics in healthy volunteers, tests were categorized as indicated in Table 2. The percentages of statistically significant test results are presented in Figure 1. These results show that the most sensitive neuropsychological domains are attention, visual/auditory/visuomotor skills, and motor function.
Figure 1.
The averaged significant effects of antipsychotics on neurophysiological domains, subjective assessment, neuroendocrine and neurophysiological parameters.
It was subsequently determined for these most sensitive areas, whether there were systematic differences between effects of ‘classic’ (haloperidol, thioridazine and chlorpromazine) and ‘atypical’ neuroleptics (all others see Table 1). In addition, an overview was obtained for differences between individual agents, although this effort was restricted by the limited number of assessments per drug. Such differences did not appear to exist. In each of the most sensitive areas, at least 48% of the ‘atypical’ antipsychotics caused impairment. Similar or even lower percentages were found the ‘classical’ neuroleptics.
Subjective assessments
Thirty-one different subjective assessment scales were employed; five of which only once. The scales used most often (by more than one research group) were: simple visual analogue scales for alertness (17 times), mood (13 times) and attention (10 times), and the combined scales from Bond & Lader (11 times) and the Von Zerssen Befindlichkeitsskala (10 times). The latter test was most consistent (significant results in 8 cases), but these were all from the same group [70, 74, 107]; the only other group using this method obtained nonsignificant results [82]. The other frequently used tests showed impairment in 38–59% of cases. Thus, none of the individual neuropsychological/motor skill tests or subjective assessments exhibited a consistent response across studies and antipsychotics. None of the subjective assessments showed an improvement, except one positive mood change with 2 mg haloperidol.
Assessments were clustered into scales for ‘alertness’ (57 measurements; significant deterioration in 53%), ‘mood’ (28 times; 50%), ‘calmness’ (16 times; 19%), ‘anxiety’ (5 times; 0%), ‘subjective (psychotropic) drug effects’ (14 times; 57%) and ‘extrapyramidal side-effects’ (21 times; 29%). Most subjective assessments showed indications for dose-dependency. After deletion of ‘lower’ doses, scales for ‘alertness’ became significant in 64%, ‘mood’ in 70%, ‘calmness’ in 33%,‘subjective (psychotropic) drug effects’ in 80% and ‘extrapyramidal side-effects’ in 43%.
Neurophysiological parameters
Electroencephalogram (EEG) EEG was measured 17 times employing six different antipsychotics. The observed trend is an increase in delta (59%) and theta (65%) and a decrease in alpha (59%) and beta (29%) frequencies, as shown in Figure 1. These effects can be observed with a number of other psycho-active drugs and generally indicate sedation. Consideration of only ‘medium’ and ‘higher’ doses did not appreciably change these results.
Eye movements Saccadic eye movements were used more frequently than smooth pursuit eye movements (18 times vs 9 times) (Figure 1). No more than three different antipsychotics were evaluated by saccadic eye movement. Saccadic peak velocity showed significant impairment compared with placebo in 83%. Only 56% of the smooth pursuit eye movement recordings showed impairment (increased saccadic intrusions). These percentages increased slightly to 85% and 57% after discarding the ‘lower’ doses. However the effects of the neuroleptics on eye movements were found to be indistinguishable from the effects of benzodiazepines [19]. Saccadic eye movements appear to remain a sensitive nonspecific marker for the sedative properties of a drug.
Evoked potentials The effects of oral sulpiride 150 and 300 mg on ERPs have been studied recently in healthy volunteers [89]. Sulpiride induced an increase in P200 and P300 latencies. The amplitude response to sulpiride of ERP parameters was bidirectional; the amplitude of subjects with a high initial value decreased while those with low initial values increased. It is remarkable that comparable results were obtained with the dopamine agonist bromocriptine [108]. However, a recent study showed that the dopamine agonist apomorphine (0.75 mg s.c.) had no effect on the P300 [12]. Assessing the potential of ERP as a biomarker is difficult. First of all, no clear quantitative relationship between abnormalities in ERP components and schizophrenic symptomatology exists. Secondly, the relationship between the latency/amplitude and stimulus perception/processing is speculative. Also, the P300 is markedly influenced by the subjective expectancy of a stimulus by an individual subject. Given that the effect of antipsychotic drugs on ERP in healthy volunteers has been assessed in very few studies, ERP is as yet unsuitable as a biomarker in the development of antipsychotic drugs.
Neuroendocrine parameters
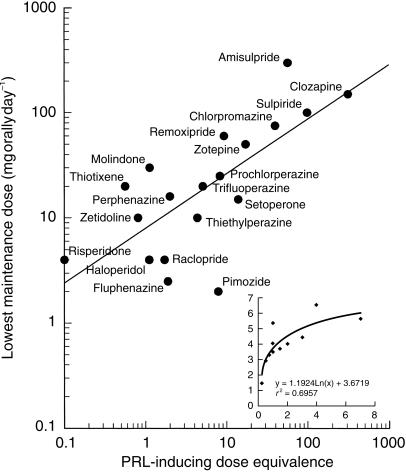
Prolactin (PRL) Plasma prolactin response to antipsychotic agents was assessed 79 times using 21 different antipsychotics. Three statistically nonsignificant responses were measured, for the lowest dose (0.1 mg orally) of raclopride and for clozapine 12.5 and 50 mg. ‘Lower’ doses showed 96% statistically significant PRL responses. Consideration of only the ‘medium’ and ‘higher’ dosages increased these percentages to 97%. These uniform PRL responses allowed an examination of the relationship between PRL response and therapeutic effect of antipsychotics, as described in the methods-section. The normalization of different doses was accomplished by reference to a logarithmic dose–response curve for haloperidol, constructed using 11 haloperidol dosages reported in the literature (range 0.25–7 mg; relative PRL increase=1.192ln(dose) + 3.672; r2 = 0.70; see insert in Figure 2. This range of haloperidol doses caused peak PRL-increases of 1.4–6.6 times baseline. All doses of the other neuroleptics that did not exceed the maximum PRL-increase observed with haloperidol were plotted on this curve. For each neuroleptic, the geometric mean of the equivalent haloperidol-doses was calculated as a measure of PRL-inducing dose equivalence. Nineteen neuroleptic doses caused PRL responses beyond the range of the haloperidol reference line (range 7.6–10.6 times PRL elevation). This completely excluded mazapertine from the analysis, as well as several doses of amisulpride, raclopride, remoxipride, risperidone, sulpiride and zetidoline.
Figure 2.
PRL-inducing dose equivalecies compared with lowest daily dose therapeutic maintenance dose for various antipsychotics (see text for explanation). Insert: reference curve for haloperidol dose (x axis) and PRL increase relative to baseline (y axis).
The PRL-inducing dose equivalencies and their concomitant lowest therapeutic maintenance doses are shown in Table 1 and Figure 2. The neuroleptics showed a good correlation between these two characteristics (r2 = 0.52, P < 0.001). Compounds to the right of haloperidol in the graph are less potent PRL-releasers than haloperidol, although higher doses may still cause more PRL-release. Clinically potent drugs with a minimal PRL-releasing propensity are expected in the lower right hand corner of the graph, which only includes pimozide. There is no clear distinction between ‘classic’ and ‘atypical’ neuroleptics.
Prolactin release is generally attributed to inhibition of the D2-receptor [48], whereas the antipsychotic effect may be more related to the ratio of 5-HT2/D2-antagonism [109, 110]. This was further investigated by correlating these parameters (shown in Table 1) with the therapeutic and PRL-inducing dose equivalencies. There were no significant relationships between the 5-HT2/D2-ratio and the PRL-inducing dose equivalence (r2 = 0.05) or the therapeutic dose (r2 = 0.03). Weak correlations were found between D2-Ki-values and PRL-release (r2 = 0.34, P < 0.05) or the recommended maintenance dose (r2 = 0.63, P < 0.001).
Cortisol and growth hormone (GH) It seems that 5-HT function is reflected by cortisol and GH release; agonists elevate hormone levels and antagonists reduce the hormone response. Decreased levels of both hormones are expected after neuroleptics, since most antipsychotic agents have some 5-HT antagonistic properties (particularly 5-HT2 and 5-HT1A). Cortisol response to antipsychotic drugs was measured 11 times; GH was evaluated in 18 instances. Significant changes from baseline were rare. Cortisol levels were changed in only two studies with antipsychotics in healthy volunteers. Only 11% of the GH responses to neuroleptics showed significant decreases (2 out of 18). This percentage decreased if only ‘medium’ and ‘higher’ doses are taken into consideration. Decreased levels from baseline of both hormones are difficult to measure due to detection limits. Baseline levels can be increased using heat stress (cortisol) or exercise (GH). Both methods were used once with neuroleptics and both yielded significant decreases. For now, cortisol and GH responses are unreliable biomarkers, but may become measures of 5-HT antagonism in studies using baseline-induction techniques.
Discussion
The aim of this review was to evaluate the usefulness of methods used to assess neuroleptic effects in healthy subjects. A striking number of different neurocognitive tests was identified, and only very few methods were used frequently enough to allow individual evaluation. Consequently, tests had to be grouped, to observe trends for relationships with neuroleptic effects. Several different meaningful ways to group tests were used in this review, although each method inevitably led to a loss of information. Even grouping tests with the same name and/or description bypasses differences among research groups or test variants. Some methods used by individual research groups may have all the characteristics of ideal biomarkers, but this may have been missed in this review. Some of these tests consistently showed effects of different neuroleptics (e.g. alphabetic cross-out, wire maze tracing, Pauli test), but it is difficult to evaluate their usefulness in drug development if they are not generally applied, and more studies are needed to allow a judgement on these tests.
Even after clustering of comparable tests (DSST-like, flicker discrimination-like, etc., as shown in Table 2), most methods were still applied relatively infrequently. Six of 20 test clusters were performed more than 20 times, and the effects of neuroleptics were inconsistent in over half of these cases. Thus, no single widely applied test or test-cluster appeared to stand out.
Despite the large number of test forms, most primarily address a single neuropsychological function, or a limited number of functional domains (Table 2). Therefore, tests were further grouped according to their primary neuropsychological domain. This showed that certain functional and subjective drug effects were more consistently affected by neuroleptics than others (Figure 1), notably attention (DSST like, flicker discrimination and search clusters), visual/auditory visuomotor responses (reaction time cluster), motor skills (manipulative motor skill cluster), and subjective effects (mood, alertness, and ‘drug effect’). Tests aiming for achievement, executive function, memory, language and (extrapyramidal) side-effects showed little or no change, although they were used quite regularly. This information is useful for planning future studies with neuroleptics in healthy subjects, because it allows the targeted selection of a few specific tests within each sensitive domain. Attention for instance is one of the most sensitive domains to single dose neuroleptics, and some 24 different test clusters (or more than 50 different individual tests) were used within this domain. Not all of these tests have been systematically compared, but whenever comparisons were made, saccadic eye movement (peak velocity) was the most sensitive measure of alertness/attention caused by a wide range of drugs or circumstances [21, 23]. The sensitivity of saccadic eye movements to neuroleptics was confirmed in the current review, as shown in Figure 1. More comparative studies are necessary to determine the most useful tests for the other neuropsychological domains.
Electroencephalography (EEG) has also been claimed to be sensitive to antipsychotic medication. On average, the EEG showed a decrease in alpha, and an increase in delta and theta frequencies, but the sensitivity was not as large as for saccadic eye movements. The evaluation of evoked potentials as potentially useful biomarkers was severely impaired by the small number of studies using this technique.
Baseline levels of growth hormone and cortisol are relatively low, and decreases therefore rarely reach significance in small groups. Significant changes were only detected after predrug growth hormone and cortisol levels were elevated by exercise or heat. Prolactin response showed a pronounced consistent effect across studies, antipsychotics and dosages. The relative dose equivalence to induce a PRL-response was clearly related to the affinity for D2-receptors and to the recommended therapeutic starting dose. Theoretically, this information could be used to predict a likely therapeutic (starting) dose for a new neuroleptic, by plotting its PRL-inducing dose equivalence on the curve of Figure 2. In practice, this application could be limited by the logarithmic scaling, and by the lack of reference data for (potential) neuroleptics that cause more PRL-release than haloperidol. Also, the data were derived from a large variation in studies and methods, and the applicability could benefit from a systematic characterization of dose-PRL-response curves for a range of antipsychotics.
Despite these practical limitations, the findings clearly show that PRL response is the best validated biomarker for ‘clinical’ effects of antipsychotic drugs, although it is unclear what these effects are. At first glance, Figure 2 suggests that PRL-release directly reflects the antipsychotic potency, because both may be related to D2-antagonism [47, 48]. Our review indicates that D2-affinity is significantly (albeit weakly) related to clinical potency. Neither relationship showed a difference between older/‘classic’ and newer/‘atypical’ neuroleptics. This is in agreement with a postulated common action of antipsychotics on cortical D2-receptors, irrespective of class [110]. There may also be another explanation for the close relationship between the PRL-inducing and therapeutic potencies. Both in drug development and medical treatment it is common practice to look for a maximum tolerated dose, to increase the chance of a therapeutic success. Consequently, many recommended antipsychotic doses are too high [111]. By increasing the dose to maximum tolerated levels, any therapeutic selectivity that may exist between ‘classic’ and ‘atypical’ neuroleptics (or amongst novel antipsychotics [112]) could disappear. In this case, the PRL-inducing dose equivalence is as much a measure of tolerability as of clinical efficacy. This would explain why no differences were found between the two classes in any of the more sensitive neuropsychological or subjective domains, including motor skills reflecting extrapyramidal side-effects. Ideally, this suggestion can be examined by comparing the effects on a biomarker for efficacy with the prolactin dose equivalence and/or therapeutic dose. However, no validated biomarker for efficacy is currently available.
Obviously, the clear relationship between PRL-inducing and therapeutic potencies does not imply that all mentioned antipsychotics will necessarily cause clinical cases of hyperprolactinaemia. Clinical hyperprolactinaemia typically develops during prolonged treatment, and is usually characterized by higher levels of prolactin than measured in the single-dose experiments reported here [42, 43]. Thus, chronic and acute prolactin elevation may differ, and we cannot exclude that ‘classic’ and ‘atypical’ neuroleptics have different long-term effects on PRL-release. The maximum extent to which neuroleptics can cause prolactin release (Emax) cannot be determined from the PRL-inducing dose equivalence relative to haloperidol, shown in Table 1 and Figure 2. This can only be derived from fully characterized individual dose–response relationships.
In conclusion, the number of different neuropsychological, subjective, neurophysiological and neuroendocrine tests that are used to measure effects of antipsychotic agents in healthy volunteers, far outweigh the number of studies. This greatly impairs the usefulness of these tests in drug development. Only a few neuropsychological domains appear to be sensitive to neuroleptics in clinically relevant single doses, notably subjective and objective measures of decreased alertness, and of reduced visual-visuomotor-auditory and motor skills. Most studies used several methods, which in part overlapped in these domains, and in part were aimed at insensitive areas. Useful biomarkers should be particularly sought in the most specific and sensitive tests within each of these susceptible domains. All neuroleptics caused an increase in prolactin, which was closely related to the therapeutic dose. This relationship could reflect the clinical practice of aiming for maximum tolerated levels, or it could represent proximity of pathways involved in prolactin release and antipsychotic activity. The number of tests used in human psychopharmacology appears to be excessive and reduction of the number of tests as well as further evaluation and validation is long overdue.
References
- 1.Leonard JP, Lehr E, Meyer T, Beck J. A phase-overlapping anhedonia-model (animal-volunteer-patient) to predict the effects of neuroleptics. Clin Neuropharmacol. 1992;15(Suppl 1):554–554. doi: 10.1097/00002826-199201001-00288. A A Part A. [DOI] [PubMed] [Google Scholar]
- 2.Williams JH, Wellman NA, Geaney DP, Feldon J, Cowen PJ, Rawlins JN. Haloperidol enhances latent inhibition in visual tasks in healthy people. Psychopharmacology (Berl) 1997;133:119–132. doi: 10.1007/s002130050400. [DOI] [PubMed] [Google Scholar]
- 3.Trestman RL, Horvath T, Kalus O, et al. Event-related potentials in schizotypal personality disorder. J Neuropsychiatry Clin Neurosci. 1996;8:33–40. doi: 10.1176/jnp.8.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Frangou S, Sharma T, Alarcon G, et al. The Maudsley Family Study, II. Endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. doi: 10.1016/S0920-9964(96)00089-8. 10.1016/s0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 5.van der Kuy A. Vol. 16. Amstelveen: Ziekenfonds Raad; 1999. Farmacotherapeutisch Kompas. 1999. [Google Scholar]
- 6.de Prins L. Denmark: H. Lundbeck A/S; 1996. Psychotropics 95/96. [Google Scholar]
- 7.Spreen O, Strauss E. A compendium of neuropsychological tests; Administration, norms, and commentary. 2. New York: Oxford University Press, Inc.; 1998. (ISBN 0-19-510019-0) [Google Scholar]
- 8.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- 9.Bond AJ, Lader MH. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 10.Gerez M, Tello A. Selected quantitative EEG (QEEG) and event-related potential (ERP) variables as discriminators for positive and negative schizophrenia. Biol Psychiatry. 1995;38:34–49. doi: 10.1016/0006-3223(94)00205-H. 10.1016/0006-3223(94)00205-h. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann WM, Scharer E, Wendt G, Delini-Stula A. Pharmaco-EEG profile of levoprotiline: second example to discuss the predictive value of pharmaco-electroencephalography in early human pharmacological evaluations of psychoactive drugs. Pharmacopsychiatry. 1991;24:206–213. doi: 10.1055/s-2007-1014470. [DOI] [PubMed] [Google Scholar]
- 12.Luthringer R, Rinaudo G, Toussaint M, et al. Electroencephalographic characterization of brain dopaminergic stimulation by apomorphine in healthy volunteers. Neuropsychobiology. 1999;39:49–56. doi: 10.1159/000026560. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann WM, Scharer E, Delini-Stula A. Predictive value of pharmaco-electroencephalography in early human-pharmacological evaluations of psychoactive drugs. First example: savoxepine. Pharmacopsychiatry. 1991;24:196–205. doi: 10.1055/s-2007-1014469. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann WM, Scharer E, Wendt G, Delini-Stula A. Pharmaco-EEG profile of Maroxepine: Third example to discuss the predictive value of pharmaco-electroencephalography in early human pharmacological evaluations of psychoactive drugs. Pharmacopsychiatry. 1991;24:214–224. doi: 10.1055/s-2007-1014471. [DOI] [PubMed] [Google Scholar]
- 15.Bittencourt PRM, Wade P, Smith AT, Richens A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 1983;15:259–262. doi: 10.1111/j.1365-2125.1983.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Steveninck AL, Cohen AF, Ward T. A microcomputer based system for recording and analysis of smooth pursuit and saccadic eye movements. Br J Clin Pharmacol. 1989;27:712P–713P. [Google Scholar]
- 17.Van Steveninck AL. Thesis: State University Leiden; 1993. Methods of assessment of central nervous system effects of drugs in man. [Google Scholar]
- 18.Szymanski S, Kane JM, Lieberman JA. A selective review of biological markers in schizophrenia. Schizophr Bull. 1991;17:99–111. doi: 10.1093/schbul/17.1.99. [DOI] [PubMed] [Google Scholar]
- 19.King DJ. Psychomotor impairment and cognitive disturbances induced by neuroleptics. Acta Psychiatr Scand Suppl. 1994;380:53–58. doi: 10.1111/j.1600-0447.1994.tb05833.x. [DOI] [PubMed] [Google Scholar]
- 20.King DJ. Guidelines for the use of antipsychotic. Drug studies in healthy volunteers. J Psychopharmacol (Oxf) 1997;11:201–209. doi: 10.1177/026988119701100302. [DOI] [PubMed] [Google Scholar]
- 21.Van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50:172–180. doi: 10.1038/clpt.1991.122. [DOI] [PubMed] [Google Scholar]
- 22.Van Steveninck AL, Verver S, Schoemaker HC, et al. Effects of temazepam on saccadic eye movements: concentration–effect relationships in individual volunteers. Clin Pharmacol Ther. 1992;52:402–408. doi: 10.1038/clpt.1992.162. [DOI] [PubMed] [Google Scholar]
- 23.Van Steveninck AL, van Berckel BNM, Schoemaker RC, Breimer DD, van Gerven JMA, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:11–18. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson MO, Schoemaker RC, Kemp B, et al. A pharmacodynamic Markov mixed-effect model for temazepam's effect on sleep. Clin Pharmacol Ther. 2000;68:175–188. doi: 10.1067/mcp.2000.108669. [DOI] [PubMed] [Google Scholar]
- 25.Frodl-Bauch T, Gallinat J, Meisenzahl EMM, Müller HJ, Hegerl U. P300 subcomponents reflect different aspects of psychopathology in schizophrenia. Biol Psychiatry. 1999;45:116–126. doi: 10.1016/s0006-3223(98)00108-5. 10.1016/s0006-3223(98)00108-5. [DOI] [PubMed] [Google Scholar]
- 26.Flaum M, Andreasen NC. More choices for treating voices. Lancet. 1997;350:22–22. doi: 10.1016/s0140-6736(97)90055-6. [DOI] [PubMed] [Google Scholar]
- 27.Verbaten MN. Aandacht, bewustzijn en psychofarmaca. Pharm Weekblad. 1995;130:34–41. [Google Scholar]
- 28.Rockstroh B, Muller M, Wagner M, Cohen R, Elbert T. Event-related and motor responses to probes in a forewarned reaction time task in schizophrenic patients. Schizophr Res. 1994;13:23–34. doi: 10.1016/0920-9964(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 29.Adams J, Faux SF, Nestor PG, et al. ERP abnormalities during semantic processing in schizophrenia. Schizophr Res. 1993;10:247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- 31.Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophr Res. 1991;6:9–13. doi: 10.1016/0920-9964(91)90015-j. [DOI] [PubMed] [Google Scholar]
- 32.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 33.Blackwood DH, St Clair DM, Muir WJ, Duffy JC. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry. 1991;48:899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- 34.Shajahan PM, O'Carroll RE, Glabus MF, Ebmeier KP, Blackwood DH. Correlation of auditory ‘oddball’ P300 with verbal memory deficits in schizophrenia. Psychol Med. 1997;27:579–586. doi: 10.1017/s0033291796004692. [DOI] [PubMed] [Google Scholar]
- 35.Roxborough H, Muir WJ, Blackwood DH, Walker MT, Blackburn IM. Neuropsychological and P300 abnormalities in schizophrenics and their relatives. Psychol Med. 1993;23:305–314. doi: 10.1017/s0033291700028385. [DOI] [PubMed] [Google Scholar]
- 36.Sachar EJ, Gruen PH, Altman N, Halpern FS, Frantz AG. Use of neuroendocrine techniques in psychopharmacological research. In: Sachar EJ, editor. Hormones, behavior, and psychopathology. New York: Raven Press; 1976. pp. 161–176. [Google Scholar]
- 37.Sachar EJ, Gruen PH, Altman N, Langer G, Halpern FS, Liefer M, Usdin E, et al. Neuroregulators and psychiatric disorders. New York: Oxford University Press; 1977. Prolactin responses to neuroleptic drugs: an approach to the study of brain dopamine blockade in humans; pp. 242–249. [Google Scholar]
- 38.Rubin RT. Neuroregulators and Psychiatric Disorders. New York: Oxford University Press; 1976. Strategies of neuroendocrine research in psychiatry; pp. 233–241. [Google Scholar]
- 39.Clark D, Hjorth S, Carlsson A. Dopamine receptor agonists: mechanisms underlying autoreceptor selectivity. II. Theoretical considerations. J Neural Transm. 1985;62:171–207. doi: 10.1007/BF01252236. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer HY. Dopamine autoreceptor stimulation: clinical significance. Pharmacol Biochem Behav. 1982;17(Suppl 1):1–10. doi: 10.1016/0091-3057(82)90504-4. [DOI] [PubMed] [Google Scholar]
- 41.Kolakowska T, Braddock L, Wiles D, Franklin M, Gelder M. Neuroendocrine tests during treatment with neuroleptic drugs I. Plasma prolactin response to haloperidol challenge. Br J Psychiatry. 1981;139:400–404. doi: 10.1192/bjp.139.5.400. [DOI] [PubMed] [Google Scholar]
- 42.Chung YC, Eun HB. Hyperprolactinaemia induced by risperidone. Int J Neuropsychopharmacology. 1998;1:93–94. doi: 10.1017/S1461145798001011. [DOI] [PubMed] [Google Scholar]
- 43.Anonymous. Hyperprolactinaemia associated with effective antipsychotic treatment no longer inevitable. Drugs Ther Perspective. 1999;14:11–14. [Google Scholar]
- 44.Mackay AVP, Iversen LL, Rossor M, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 1982;39:991–997. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds GP. Dopamine receptors, antipsychotic action and schizophrenia. J Psychopharmacol. 1999;13:202–203. doi: 10.1177/026988119901300220. [DOI] [PubMed] [Google Scholar]
- 46.Emilien G, Maloteaux JM, Geurts M, Owen MJ. Dopamine receptors and schizophrenia: contribution of molecular genetics and clinical neuropsychology. Int J Neuropsychopharmacology. 1999;2:197–227. doi: 10.1017/S1461145799001479. [DOI] [PubMed] [Google Scholar]
- 47.Langer G, Sachar EJ, Halpern FS, Gruen PH, Solomon M. The prolactin response to neuroleptic drugs. A test of dopaminergic blockade: neuroendocrine studies in normal men. J Clin Endocrinol Metab. 1977;45:996–1002. doi: 10.1210/jcem-45-5-996. [DOI] [PubMed] [Google Scholar]
- 48.Gruen PH, Sachar EJ, Langer G, et al. Prolactin responses to neuroleptics in normal and schizophrenic subjects. Arch Gen Psychiatry. 1978;35:108–116. doi: 10.1001/archpsyc.1978.01770250110011. [DOI] [PubMed] [Google Scholar]
- 49.Leysen JE, Janssen PM, Schotte A, Luyten WH, Megens AA. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl) 1993;112:40–54. doi: 10.1007/BF02245006. [DOI] [PubMed] [Google Scholar]
- 50.Laakmann G, Wittmann M, Gugath M, et al. Effects of psychotropic drugs (desimipramine, chlorimipramine, sulpiride and diazepam) on the human HPA axis. Psychopharmacology (Berl) 1984;84:66–70. doi: 10.1007/BF00432027. [DOI] [PubMed] [Google Scholar]
- 51.Schürmeyer T, Brademann G, von zur Mühlen A. Effect of fenfluramine on episodic ACTH and cortisol secretion. Clin Endocrinol (Oxf) 1996;45:39–45. doi: 10.1111/j.1365-2265.1996.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 52.de Koning P, de Vries MH. A comparison of the neuro-endocrinological and temperature effects of DU 29894, flesinoxan, sulpiride and haloperidol in normal volunteers. Br J Clin Pharmacol. 1995;39:7–14. doi: 10.1111/j.1365-2125.1995.tb04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holland RL, Wesnes K, Dietrich B. Single dose human pharmacology of umespirone. Eur J Clin Pharmacol. 1994;46:461–468. doi: 10.1007/BF00191912. [DOI] [PubMed] [Google Scholar]
- 54.van Praag HM, Lemus C, Kahn R. Hormonal probes of central serotonergic activity: Do they really exist? Biol Psychiatry. 1987;22:86–98. doi: 10.1016/0006-3223(87)90134-x. [DOI] [PubMed] [Google Scholar]
- 55.Berger HJ, van Hoof JJ, van Spaendonck KP, et al. Haloperidol and cognitive shifting. Neuropsychologia. 1989;27:629–639. doi: 10.1016/0028-3932(89)90109-7. [DOI] [PubMed] [Google Scholar]
- 56.Lee C, Frangou S, Russell MA, Gray JA. Effect of haloperidol on nicotine-induced enhancement of vigilance in human subjects. J Psychopharmacol (Oxf) 1997;11:253–257. doi: 10.1177/026988119701100309. [DOI] [PubMed] [Google Scholar]
- 57.King DJ, Henry G. The effect of neuroleptics on cognitive and psychomotor function. A preliminary study in healthy volunteers. Br J Psychiatry. 1992;160:647–653. doi: 10.1192/bjp.160.5.647. [DOI] [PubMed] [Google Scholar]
- 58.Rammsayer T, Gallhofer B. Remoxipride versus haloperidol in healthy volunteers: psychometric performance and subjective tolerance profiles. Int Clin Psychopharmacol. 1995;10:31–37. doi: 10.1097/00004850-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Rammsayer TH. A cognitive-neuroscience approach for elucidation of mechanisms underlying temporal information processing. Int J Neurosci. 1994;77:61–76. doi: 10.3109/00207459408986019. [DOI] [PubMed] [Google Scholar]
- 60.Peretti CS, Danion JM, Kauffmann-Muller F, Grange D, Patat A, Rosenzweig P. Effects of haloperidol and amisulpride on motor and cognitive skill learning in healthy volunteers. Psychopharmacology (Berl) 1997;131:329–338. doi: 10.1007/s002130050300. 10.1007/s002130050300. [DOI] [PubMed] [Google Scholar]
- 61.McClelland GR, Cooper SM, Pilgrim AJ. A comparison of the central nervous system effects of haloperidol, chlorpromazine and sulpiride in normal volunteers. Br J Clin Pharmacol. 1990;30:795–803. doi: 10.1111/j.1365-2125.1990.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saarialho-Kere U. Psychomotor, respiratory and neuroendocrinological effects of nalbuphine and haloperidol, alone and in combination, in healthy subjects. Br J Clin Pharmacol. 1988;26:79–87. doi: 10.1111/j.1365-2125.1988.tb03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams JH, Wellman NA, Geaney DP, Rawlins JN, Feldon J, Cowen PJ. Intravenous administration of haloperidol to healthy volunteers: lack of subjective effects but clear objective effects. J Psychopharmacol (Oxf) 1997;11:247–252. doi: 10.1177/026988119701100308. [DOI] [PubMed] [Google Scholar]
- 64.Lambert GW, Horne M, Kalff V, et al. Central nervous system noradrenergic and dopaminergic turnover in response to acute neuroleptic challenge. Life Sci. 1995;56:1545–1555. doi: 10.1016/0024-3205(95)00120-u. 10.1016/0024-3205(95)00120-u. [DOI] [PubMed] [Google Scholar]
- 65.Hennig J, Rzepka U, Mai B, Netter P. Suppression of HPA-axis activity by haloperidol after experimentally induced heat stress. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:603–614. doi: 10.1016/0278-5846(95)00105-5. 10.1016/0278-5846(95)00105-5. [DOI] [PubMed] [Google Scholar]
- 66.Leigh TJ, Link CG, Fell GL. Effects of granisetron and haloperidol, alone and in combination, on psychometric performance and the EEG. Br J Clin Pharmacol. 1992;34:65–70. doi: 10.1111/j.1365-2125.1992.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coull JT, Sahakian BJ, Middleton HC, et al. Differential effects of clonidine, haloperidol, diazepam and tryptophan depletion on focused attention and attentional search. Psychopharmacology (Berl) 1995;121:222–230. doi: 10.1007/BF02245633. [DOI] [PubMed] [Google Scholar]
- 68.Rammsayer T. Is there a common dopaminergic basis of time perception and reaction time? Neuropsychobiology. 1989;21:37–42. doi: 10.1159/000118549. [DOI] [PubMed] [Google Scholar]
- 69.Rammsayer T. Dopaminergic and serotoninergic influence on duration discrimination and vigilance. Pharmacopsychiatry. 1989;22(Suppl 1):39–43. doi: 10.1055/s-2007-1014623. [DOI] [PubMed] [Google Scholar]
- 70.Saletu B, Grunberger J, Linzmayer L, Dubini A. Determination of pharmacodynamics of the new neuroleptic zetidoline by neuroendocrinologic, pharmaco-EEG, and psychometric studies – Part I. Int J Clin Pharmacol Ther Toxicol. 1983;21:489–495. [PubMed] [Google Scholar]
- 71.Frey S, Bente G, Fuchs A, Preiswerk G, Glatt A, Imhof P. Spontaneous motor activity in healthy volunteers after single doses of haloperidol. Int Clin Psychopharmacol. 1989;4:39–53. doi: 10.1097/00004850-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Kleinbloesem CH, Jaquet-Muller F, al-Hamdan Y, et al. Incremental dosage of the new antipsychotic mazapertine induces tolerance to cardiovascular and cognitive effects in healthy men. Clin Pharmacol Ther. 1996;59:675–685. doi: 10.1016/S0009-9236(96)90008-9. [DOI] [PubMed] [Google Scholar]
- 73.Hartigan-Go K, Bateman DN, Nyberg G, Martensson E, Thomas SH. Concentration-related pharmacodynamic effects of thioridazine and its metabolites in humans. Clin Pharmacol Ther. 1996;60:543–553. doi: 10.1016/S0009-9236(96)90150-2. [DOI] [PubMed] [Google Scholar]
- 74.Saletu B, Grünberger J, Linzmayer L, Dubini A. Determination of pharmacodynamics of the new neuroleptic zetidoline by neuroendocrinologic, pharmaco-EEG, and psychometric studies: Part II. Int J Clin Pharmacol Ther Tox. 1983;21:544–551. [PubMed] [Google Scholar]
- 75.Rammsayer TH. On dopaminergic modulation of temporal information processing. Biol Psychol. 1993;36:209–222. doi: 10.1016/0301-0511(93)90018-4. [DOI] [PubMed] [Google Scholar]
- 76.Magliozzi JR, Mungas D, Laubly JN, Blunden D. Effect of haloperidol on a symbol digit substitution task in normal adult males. Neuropsychopharmacology. 1989;2:29–37. doi: 10.1016/0893-133x(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 77.King DJ, Best P, Lynch G, et al. The effects of remoxipride and chlorpromazine on eye movements and psychomotor performance in healthy volunteers. J Psychopharmacol. 1995;9:143–149. doi: 10.1177/026988119500900209. [DOI] [PubMed] [Google Scholar]
- 78.Mattila MJ, Aranko K, Mattila ME, Paakkari I. Effects of psychotropic drugs on digit substitution: comparison of the computerized symbol-digit substitution and traditional digit-symbol substitution tests. J Psychopharmacol. 1994;8:81–87. doi: 10.1177/026988119400800202. [DOI] [PubMed] [Google Scholar]
- 79.Callaghan JT, Cerimele BJ, Kassahun KJ, Nyhart E, Jr, Hoyes-Beehler PJ, Kondraske GV. Olanzapine. Interaction study with imipramine. J Clin Pharmacol. 1997;37:971–978. doi: 10.1002/j.1552-4604.1997.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 80.Cooper SM, Jackson D, Loudon JM, McClelland GR, Raptopoulos P. The psychomotor effects of paroxetine alone and in combination with haloperidol, amylobarbitone, oxazepam, or alcohol. Acta Psychiatr Scand Suppl. 1989;350:53–55. doi: 10.1111/j.1600-0447.1989.tb07174.x. [DOI] [PubMed] [Google Scholar]
- 81.Schwinn G, Schwarck H, McIntosh C, Milstrey HR, Willms B, Kubberling J. Effect of the dopamine receptor blocking agent pimozide on the growth hormone response to arginine and exercise and on spontaneous growth hormone fluctuations. J Clin Endocrinol Metab. 1976;43:1183–1185. doi: 10.1210/jcem-43-5-1183. [DOI] [PubMed] [Google Scholar]
- 82.Wetzel H, Wiesner J, Hiemke C, Benkert O. Acute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteers. J Psychiatr Res. 1994;28:461–473. doi: 10.1016/0022-3956(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 83.Barbieri C, Parodi M, Bruno S, et al. Effects of acute administration of zetidoline, a new antidopaminergic drug, on plasma prolactin and aldosterone levels in man. Eur J Clin Pharmacol. 1984;26:29–32. doi: 10.1007/BF00546704. [DOI] [PubMed] [Google Scholar]
- 84.Mattila MJ, Mattila ME. Effects of remoxipride on psychomotor performance, alone and in combination with ethanol and diazepam. Acta Psychiatr Scand Suppl. 1990;358:54–55. doi: 10.1111/j.1600-0447.1990.tb05288.x. [DOI] [PubMed] [Google Scholar]
- 85.Szabadi E, Bradshaw CM, Gaszner P. The comparison of the effects of DL-308, a potential new neuroleptic agent, and thioridazine on some psychological and physiological functions in healthy volunteers. Psychopharmacology (Berl) 1980;68:125–134. doi: 10.1007/BF00432129. [DOI] [PubMed] [Google Scholar]
- 86.Farde L, Grind M, Nilsson MI, Ogenstad S, Sedvall G. Remoxipride – a new potential antipsychotic drug. Pharmacological effects and pharmacokinetics following repeated oral administration in male volunteers. Psychopharmacology (Berl) 1988;95:157–161. doi: 10.1007/BF00174501. [DOI] [PubMed] [Google Scholar]
- 87.Fagan D, Scott DB, Mitchell M, Tiplady B. Effects of remoxipride on measures of psychological performance in healthy volunteers. Psychopharmacology (Berl) 1991;105:225–229. doi: 10.1007/BF02244314. [DOI] [PubMed] [Google Scholar]
- 88.von Bahr C, Wiesel FA, Movin G, et al. Neuroendocrine responses to single oral doses of remoxipride and sulpiride in healthy female and male volunteers. Psychopharmacology (Berl) 1991;103:443–448. doi: 10.1007/BF02244242. [DOI] [PubMed] [Google Scholar]
- 89.Takeshita S, Ogura C. Effect of the dopamine D2 antagonist sulpiride on event-related potentials and its relation to the law of initial value. Int J Psychophysiol. 1994;16:99–106. doi: 10.1016/0167-8760(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 90.Beuzen JN, Taylor N, Wesnes K, Wood A. A comparison of the effects of olanzapine, haloperidol and placebo on cognitive and psychomotor functions in healthy elderly volunteers. J Psychopharmacol. 1999;13:152–158. doi: 10.1177/026988119901300207. [DOI] [PubMed] [Google Scholar]
- 91.Bartfai A, Wiesel FA. Effect of sulpiride on vigilance in healthy subjects. Int J Psychophysiol. 1986;4:1–5. doi: 10.1016/0167-8760(86)90045-0. [DOI] [PubMed] [Google Scholar]
- 92.Ramaekers JG, Louwerens JW, Muntjewerff Nd, et al. Psychomotor, Cognitive, extrapyramidal, and affective functions of healthy volunteers during treatment with an atypical (amisulpride) and a classic (haloperidol) antipsychotic. J Clin Psychopharmacol. 1999;19:209–221. doi: 10.1097/00004714-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka O, Kondo T, Otani K, Yasui N, Tokinaga N, Kaneko S. Single oral dose kinetics of zotepine and its relationship to prolactin response and side effects. Ther Drug Monit. 1998;20:117–119. doi: 10.1097/00007691-199802000-00021. [DOI] [PubMed] [Google Scholar]
- 94.Green JF, King DJ. The effects of chlorpromazine and lorazepam on abnormal antisaccade and no-saccade distractibility. Biol Psychiatry. 1998;44:709–715. doi: 10.1016/s0006-3223(97)00452-6. 10.1016/s0006-3223(97)00452-6. [DOI] [PubMed] [Google Scholar]
- 95.Liu YJ, Stagni G, Walden JG, Shepherd AM, Lichtenstein MJ. Thioridazine dose-related effects on biomechanical force platform measures of sway in young and old men. J Am Geriatr Soc. 1998;46:431–437. doi: 10.1111/j.1532-5415.1998.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 96.Meyer-Lindenberg A, Rammsayer T, Ulferts J, Gallhofer B. The effects of sulpiride on psychomotor performance and subjective tolerance. Eur Neuropsychopharmacol. 1997;7:219–223. doi: 10.1016/s0924-977x(97)00407-0. 10.1016/s0924-977x(97)00407-0. [DOI] [PubMed] [Google Scholar]
- 97.Williams JH, Wellman NA, Geaney DP, Cowen PJ, Feldon J, Rawlins JN. Antipsychotic drug effects in a model of schizophrenic attentional disorder: a randomized controlled trial of the effects of haloperidol on latent inhibition in healthy people. Biol Psychiatry. 1996;40:1135–1143. doi: 10.1016/S0006-3223(95)00629-X. 10.1016/s0006-3223(95)00629-x. [DOI] [PubMed] [Google Scholar]
- 98.Farde L, von Bahr C, Wahlen A, Nilsson L, Widman M. The new selective D2-dopamine receptor antagonist raclopride – pharmacokinetics, safety and tolerability in healthy males. Int Clin Psychopharmacol. 1989;4:115–126. doi: 10.1097/00004850-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Galderisi S, Mucci A, Bucci P, Mignone ML, Maj M. Multilead quantitative EEG profile of clozapine in resting and vigilance-controlled conditions. Psychiatry Res. 1996;67:113–122. doi: 10.1016/0925-4927(96)02883-1. [DOI] [PubMed] [Google Scholar]
- 100.Meco G, Lestingi L, Buzzi MG, et al. Neuroendocrine effects of setoperone: a new neuroleptic drug. Int J Clin Pharmacol Res. 1986;6:465–468. [PubMed] [Google Scholar]
- 101.Hughes AM, Lynch P, Rhodes J, Ervine CM, Yates RA. Electroencephalographic and psychomotor effects of chlorpromazine and risperidone relative to placebo in healthy volunteers. Br J Clin Pharmacol. 1999;48:323–330. doi: 10.1046/j.1365-2125.1999.00021.x. 10.1046/j.1365-2125.1999.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Movin-Osswald G, Karlsson P, Hammarlund-Udenaes M, Farde L. Influence of rate of administration of raclopride on akathisia and prolactin response. Psychopharmacology (Berl) 1994;114:248–256. doi: 10.1007/BF02244845. [DOI] [PubMed] [Google Scholar]
- 103.Herbert M, Standen PJ, Short AH, Birmingham AT. A comparison of some psychological and physiological effects exerted by zetidoline (DL308) and by oxazepam. Psychopharmacology (Berl) 1983;81:335–339. doi: 10.1007/BF00427573. [DOI] [PubMed] [Google Scholar]
- 104.Grind M, Nilsson MI, Nilsson L, Oxenstierna G, Sedvall G, Wahlen A. Remoxipride – a new potential antipsychotic compound. Tolerability and pharmacokinetics after single oral and intravenous administration in healthy male volunteers. Psychopharmacology (Berl) 1989;98:304–309. doi: 10.1007/BF00451679. [DOI] [PubMed] [Google Scholar]
- 105.Movin-Osswald G, Nordstrom AL, Hammarlund-Udenaes M, Wahlen A, Farde L. Pharmacokinetics of raclopride formulations. Influence of prolactin and tolerability in healthy male volunteers. Clin Pharmacokinet. 1992;22:152–161. doi: 10.2165/00003088-199222020-00006. [DOI] [PubMed] [Google Scholar]
- 106.Huang ML, Van Peer A, Woestenborghs R, et al. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54:257–268. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- 107.Saletu B, Grunberger J, Linzmayer L, Anderer P. Comparative placebo-controlled pharmacodynamic studies with zotepine and clozapine utilizing pharmaco-EEG and psychometry. Pharmacopsychiatry. 1987;20:12–27. doi: 10.1055/s-2007-1017125. [DOI] [PubMed] [Google Scholar]
- 108.Nishimura N, Ogura C, Ohta I. Effects of the dopamine-related drug bromocriptine on event-related potentials and its relation to the law of initial value. Psychiatry Clin Neurosci. 1995;49:79–86. doi: 10.1111/j.1440-1819.1995.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 109.Abuzzahab FSSr, Zimmerman RL. Psychopharmacological correlates of post-psychotic depression: a double-blind investigation of haloperidol vs thiothixene in outpatient schizophrenia. J Clin Psychiatry. 1982;43:105–110. [PubMed] [Google Scholar]
- 110.Lidow MS, Williams GV, Goldman-Rakic PS. The cerebral cortex: a case for a common site of action of antipsychotics. Tips. 1998;19:136–140. doi: 10.1016/s0165-6147(98)01186-9. [DOI] [PubMed] [Google Scholar]
- 111.Meltzer HY, Matsubara S, Lee J. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 112.Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. 10.1016/s0893-133x(97)00112-7. [DOI] [PubMed] [Google Scholar]