Abstract
Aims
To investigate the effect on the hypothalamo-pituitary-adrenal (HPA) axis of treatment with budesonide, 400 µg once daily, morning or evening, or 200 µg twice daily, and 800 µg twice daily via Turbuhaler in a randomized, placebo-controlled, double-blind, double-dummy crossover study.
Methods
Healthy men received budesonide, 400 µg in the morning (08.00–09.00 h) or evening (20.00–21.00 h), budesonide, 200 µg twice daily, 800 µg twice daily, and placebo twice daily, for 1 week each. Plasma and urine samples were obtained over 24 h on day 7 for cortisol determination. Twenty-five subjects completed all treatments, and 27 were included in the analysis.
Results
The 24 h plasma cortisol concentrations vs placebo (95% CI) were 98% (89, 108) for 400 µg in the morning, 92% (83, 100) for 400 µg in the evening, 95% (86, 104) for 200 µg twice daily, and 76% (70, 84) for 800 µg twice daily.
Conclusions
Budesonide at a dose of 400 µg day−1 via Turbuhaler had no statistically significant effect on 24 h cortisol production, irrespective of whether treatment is given once or twice daily, whereas a dose of 800 µg twice daily resulted in a statistically significant suppression vs placebo. Neither could a significant difference be found between morning and evening dosing.
Keywords: budesonide, cortisol, inhaled corticosteroids, once daily
Introduction
Inhaled glucocorticosteroids are now recommended as a first line preventative treatment for patients with all but the mildest asthma [1, 2]. Such treatment is normally given twice daily, but more frequent dosing regimens can be used if required. However, multiple dosing regimens can lead to poor compliance with treatment [3], which is a major cause of asthma-related morbidity [4]. A simple, once daily dosage might be expected to promote compliance with treatment and thereby improve standards of asthma management, even if a once daily dosing regimen is not indicated in all patients.
Studies with the inhaled corticosteroid budesonide (Pulmicort, AstraZeneca R&D Lund, Sweden) have shown that once daily treatment with doses of up to 400 µg delivered via the dry powder inhaler Turbuhaler® has comparable effects on lung function and asthma symptoms as the same total dose given in two daily administrations [5, 6]. However, in addition to efficacy measures comparisons of inhaled corticosteroid regimens should include safety assessments, such as that of the incidence of local adverse effects and the effect of treatment on the hypothalamo-pituitary-adrenal (HPA) axis [7]. In a previous study, there was no significant difference between the effects on plasma cortisol of budesonide 800 µg day−1, given twice daily or once daily in the morning or evening. However, all three regimens resulted in a statistically significant suppression vs placebo [8]. The present study was performed to investigate the effect of once and twice daily dosing with inhaled budesonide, 400 µg day−1, on the HPA axis in healthy volunteers. In addition, possible diurnal effects were studied by giving the once daily dose both in the morning and in the evening.
Methods
The study was a randomized, placebo-controlled, double-blind, double-dummy, crossover trial conducted at a single centre in the UK. It was approved by the District Research Ethics Committee, St Bartholomew's Hospital, London, UK.
Subjects
Subjects were eligible for inclusion in the study if they were men, aged between 18 and 40 years, and in good health as determined by a medical history, physical examination, ECG and routine clinical laboratory screening (including a negative narcotics screen). Subjects with a known allergy or idiosyncrasy to steroids were excluded, as were subjects who had experienced a respiratory tract infection or other significant illness within 2 weeks prior to entering the study. Other exclusion criteria included requirement for regular medication, evidence of severe illness, donation of blood or participation in a drug trial within the previous 3 months, substance abuse, or high risk of human immunodeficiency virus infection. All subjects received full verbal and written information about the study, and provided written consent before inclusion.
Study procedures
Eligible subjects received five treatments in random order: budesonide, 400 µg in the morning (08.00–09.00 h) and placebo in the evening (20.00–21.00 h); placebo in the morning and budesonide, 400 µg in the evening; budesonide, 200 µg in the morning and evening; budesonide, 800 µg in the morning and evening; placebo in the morning and evening. Each treatment was given for 1 week, with wash-out periods of at least 1 week between study phases. All treatments were given via a Turbuhaler dry powder inhaler; which was identical in appearance for budesonide and placebo. Inhaler technique was checked at the start of the study and at the beginning of each study period (the morning inhalation on day 1). Both inhalations on day 7 were performed under supervision.
Blood samples (5 ml) were obtained via a venous catheter before the first dose in each treatment period and immediately before and 1, 2, 4, 6, 8, 10, and 12 h after the morning and evening doses on day 7. The 12 h sample after the morning dose acted as the baseline sample for the evening dose. Samples were centrifuged at 3000 g within 30 min of collection, and the plasma was stored at −20 °C prior to analysis. Urine samples were collected over 0–12 and 12–24 h after the morning dose on day 7. The samples were weighed, and the volume calculated, assuming a density of 1.02 g l−1. Samples were inverted three times and three 10 ml portions stored at −20 °C prior to analysis.
Plasma concentrations of cortisol were measured by high performance liquid chromatography at AstraZeneca R & D Lund, Sweden. The limit of quantification of the assay was 25 nmol l−1, and the coefficient of variation was 6.0% at a sample concentration of 52 nmol l−1. Urinary cortisol concentrations were measured by gas chromatography plus mass spectrometry. Here, the limit of quantification was 5 nmol l−1 and the coefficient of variation 16.5% at a sample concentration of 15 nmol l−1.
Information about adverse events was obtained on the first day and the day after the end of each treatment period (day 8). In addition, routine clinical chemistry and haematology screening was performed at the beginning and end of the study.
Statistical analysis
The primary study variables were 24 h urinary cortisol excretion and the area under the curve of plasma cortisol concentration vs time curve over 24 h (AUC(0,24 h)). Because the observed cortisol concentrations were not normally distributed, geometric means and coefficients of variation were used, rather than arithmetic means and standard deviations. For both urine and plasma concentrations, statistical analysis was performed by means of analysis of variance (anova), with factors for subject, period, and treatment, followed by pairwise comparisons; principally between the two 400 µg once daily regimens and the 200 µg twice daily regimen. The average plasma cortisol concentration (CAv) during the last 24 h of dosing, calculated from AUC(0,24 h)/24, was used for statistical analyses.
It was estimated that a sample size of 24 subjects would provide 80% power to detect a significant difference in urinary cortisol between treatments, using a paired t-test at the 5% significance level, if the true difference was 24% of the placebo urinary cortisol level.
Results
A total of 33 subjects were recruited, of whom 27 were included in the analysis. The mean age (s.d.) of the subjects included was 24.8 (3.2) years, and their mean weight was 71.4 (7.8) kg.
Three subjects discontinued during the first treatment period and were lost to follow-up: In addition, five subjects discontinued during the subsequent course of the study. The reasons for discontinuation in these latter subjects were personal reasons (n = 2), adverse events (influenza), use of prohibited medication, and refusal to continue (n = 1 each). A total of six major protocol violations, which invalidated the actual treatments, occurred during the course of the study. These were plasma cortisol concentration outside specified ranges during placebo treatment (n = 2), incorrect dose or duration of treatment (n = 3), or incorrect urine collection period (n = 1).
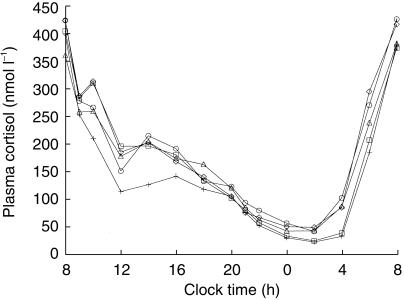
Mean plasma cortisol concentrations during the treatments with budesonide or placebo are shown in Figure 1. Morning dosing with budesonide resulted in a small decrease in plasma cortisol concentrations, compared with placebo. Concentrations returned to normal within 6 h, except for the 800 µg twice daily dose, where concentrations did not return to normal until after approximately 12 h. Evening dosing with 400 µg and 800 µg reduced nocturnal cortisol production little in absolute amounts, since cortisol concentrations are low during the night.
Figure 1.
Geometric mean plasma cortisol concentrations (nmol l−1) in healthy volunteers after inhalation of budesonide (○ 400 µg morning, □ 400 µg evening, ▵ 200 µg twice daily, + 800 µg twice daily) and placebo (⋄) via Turbuhaler.
The effects of budesonide on 24 h plasma and urine cortisol concentrations are summarized in Table 1. There were no significant differences between the two 400 µg once daily regimens and the 200 µg twice daily regimen. Significant decreases were seen only during treatment with budesonide, 800 µg twice daily.
Table 1.
24 h average plasma cortisol and urinary cortisol concentrations. Results are presented as geometric means and coefficients of variation (CVs), and as percentages of placebo treatment (95% CI).
| Plasma cortisol 24 h Cav (nmol l−1) | 24 h urinary cortisol (nmol l−1) | |||
|---|---|---|---|---|
| Dose regimen | Geometric mean (CV %) | % of placebo (95% CI) | Geometric mean (CV %) | % of placebo (95% CI) |
| 400 µg once daily (morning) | 182.2 (25.6) | 98.3 (89.5, 108.1) | 71.4 (53.7) | 98.1 (77.9, 123.6) |
| 400 µg once daily (evening) | 174.0 (18.0) | 91.5 (83.4, 100.4) | 69.6 (54.3) | 95.3 (75.9, 119.7) |
| 200 µg twice daily | 177.1 (13.5) | 94.6 (86.4, 103.6) | 66.7 (49.9) | 93.9 (75.0, 117.6) |
| 800 µg twice daily | 140.9 (16.0) | 76.3 (69.5, 83.8) | 52.4 (73.5) | 74.2 (59.0, 93.2) |
| Placebo | 185.7 (16.0) | 100 | 72.2 (53.4) | 100 |
Although the magnitude of suppression vs placebo was similar for both HPA-axis measurements, the coefficient of variation was about 40% for urinary cortisol concentration, which was very close to the assumption made in the power calculation, and 16% for plasma cortisol concentration, indicating that plasma cortisol concentration AUC was a more reproducible parameter. The highest budesonide dose (800 µg twice daily) produced a 24% and 26% suppression of plasma and urine cortisol concentration vs placebo.
The adverse event profile of budesonide in this study was comparable with that of placebo. Only one patient withdrew from the study because of an adverse event (influenza).
Discussion
Integrated measurements of plasma cortisol and 24 h urinary cortisol concentrations, and stimulation tests using low dose tetracosactrin or ACTH are widely accepted as being among the most sensitive markers of HPA axis function [9]. The sensitivity of integrated plasma measurements is underlined by the results in a previous study, in which a daily dose of 400 µg (200 µg twice daily) produced a statistically significant suppression of 24 h plasma cortisol concentration [10]. The same daily dose of budesonide had no significant effect on the HPA axis in the present study, whereas a dose of 800 µg twice daily (considerably higher than that required by the majority of asthma patients) produced statistically significant reductions in plasma and urinary cortisol concentrations. The plasma sampling technique used in the present study caused minimum disturbance to the subject, and has previously been shown not to affect the HPA-axis [11].
The clinical significance of these relatively small changes is questionable. Nevertheless, measurement of inhaled corticosteroid effects on the HPA axis remains a preferred safety assessment, because of the well established nature of the procedure [12]. Administration of exogenous corticosteroids decreases plasma cortisol concentrations via normal feedback control mechanisms [13], and the total exposure of the body to corticosteroids can remain within physiological limits [9]. Thus, statistically significant changes in laboratory measures may simply reflect the normal functioning of the HPA axis rather than clinically relevant abnormalities. Indeed, even treatment with high doses of systemic corticosteroids for long periods does not consistently produce clinically significant changes in HPA axis function [14].
A once-daily dose in the evening of oral corticosteroids [15–17] and of inhaled beclomethasone dipropionate [18] have shown a tendency to produce a greater effect than morning dosing or twice daily administration. However, more recent studies with inhaled corticosteroids, including the present study have shown that the effects on the HPA axis are similar, irrespective of the time of administration [19, 20].
Although the use of healthy volunteers in studies of corticosteroid effects on HPA axis function, rather than patients with moderate or severe asthma, may be questioned, this approach has the advantage of eliminating the potential confounding effects of previous or concurrent inhaled steroid therapy, and of variation in the degree of airway inflammation and obstruction. Indeed, the use of healthy volunteers is recommended in current guidelines for the assessment of the efficacy and safety of inhaled corticosteroids [12].
In conclusion, this study has shown that budesonide had no statistically significant effect on the HPA axis in healthy volunteers when given at a dose of 400 µg day−1 via Turbuhaler, irrespective of whether treatment is given once daily or twice daily, whereas a dose of 800 µg twice daily resulted in a statistically significant suppression vs placebo. Furthermore, no significant difference was found between morning and evening dosing.
References
- 1.British Thoracic Society. British guidelines on asthma management. 1995 review and position statement. Thorax. 1997. pp. S1–S21.
- 2.National Heart Lung and Blood Institute. Expert Panel Report II. Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Institutes of Health; 1997. [Google Scholar]
- 3.Mann M, Eliasson O, Patel K, ZuWallack RL. A comparison of the effects of bid and qid dosing on compliance with inhaled flunisolide. Chest. 1992;101:325–328. doi: 10.1378/chest.101.2.496. [DOI] [PubMed] [Google Scholar]
- 4.Horn CR, Clark TJH, Cochrane GM. Compliance with inhaled therapy and morbidity from asthma. Respir Med. 1990;84:67–70. doi: 10.1016/s0954-6111(08)80097-2. [DOI] [PubMed] [Google Scholar]
- 5.Jones AH, Langdon CG, Lee PS, et al. Pulmicort® Turbohaler® once daily as initial prophylactic therapy for asthma. Respir Med. 1994;88:293–299. doi: 10.1016/0954-6111(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 6.Campbell LM, Gooding TN, Aitchison WR, et al. Initial loading (400 µg twice daily) versus static (400 µg nocte) dose budesonide for asthma management. Int J Clin Pract. 1998;52:361–370. [PubMed] [Google Scholar]
- 7.Rogers DF, Ganderton D, editors. Respir Med. Vol. 89. London: Royal Brompton National Heart and Lung Institute; 1995. Consensus statement from a workshop of the British Association for Lung Research; pp. 253–261. [DOI] [PubMed] [Google Scholar]
- 8.Thorsson L, Källén A. A randomized controlled assessment of the systemic activity of budesonide when given once or twice daily via Turbuhaler. Eur J Clin Pharmacol. 2000;56:207–210. doi: 10.1007/s002280000134. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen S, O'Byrne P. A comparison of the efficacy and safety of inhaled corticosteroids in asthma. Allergy. 1997;52(Suppl 39):1–34. doi: 10.1111/j.1398-9995.1997.tb05047.x. [DOI] [PubMed] [Google Scholar]
- 10.Grahnén A, Brundin RM, Ling-Andersson A, Lönnebo A, Eckernäs S-Å. The systemic potency of fluticasone propionate from Diskhaler vs budesonide from Turbuhaler®. Eur J Clin Pharmacol. 1997;52:261–267. doi: 10.1007/s002280050287. 10.1007/s002280050287. [DOI] [PubMed] [Google Scholar]
- 11.Jennings BH, Andersson K-E, Johansson S-Å. Assessment of the systemic effects of inhaled glucocorticosteroids: the influence of blood sampling technique and frequency on plasma cortisol and leucocytes. Eur J Clin Pharmacol. 1990;39:127–131. doi: 10.1007/BF00280045. [DOI] [PubMed] [Google Scholar]
- 12.Boulet L-P, Cockcroft DW, Toogood J, Lacasse Y, Baskerville J, Hargreave FE. Comparative assessment of safety and efficacy of inhaled corticosteroids: report of a Committee of the Canadian Thoracic Society. Eur Respir J. 1998;11:1194–1210. doi: 10.1183/09031936.98.11051194. [DOI] [PubMed] [Google Scholar]
- 13.Keller-Wood ME, Dallmen MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–23. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 14.Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326:226–230. doi: 10.1056/NEJM199201233260403. [DOI] [PubMed] [Google Scholar]
- 15.Myles AM, Bacon PA, Daly JR. Single daily dose corticosteroid treatment. Ann Rheum Dis. 1971;30:149. doi: 10.1136/ard.30.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayek A, Crawford JD, Bode HH. Single dose dexamethasone in treatment of congenital adrenocortical hyperplasia. Metabolism. 1971;20:897–901. doi: 10.1016/0026-0495(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 17.Reinberg A, Smolensky MH, D'Alonzo GE, McGovern JP. Chronobiology and asthma. III. Timing corticotherapy to biological rhythms to optimize treatment goals. J Asthma. 1988;25:219–248. doi: 10.3109/02770908809071368. [DOI] [PubMed] [Google Scholar]
- 18.Tabachnik E, Zadik Z. Diurnal cortisol excretion during therapy with inhaled beclomethasone dipropionate in children with asthma. J Pediatr. 1991;118:294–297. doi: 10.1016/s0022-3476(05)80506-2. [DOI] [PubMed] [Google Scholar]
- 19.Pincus DJ, Szefler SJ, Ackerson LM, Martin RJ. Chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1995;95:1172–1178. doi: 10.1016/s0091-6749(95)70073-0. [DOI] [PubMed] [Google Scholar]
- 20.Pincus DJ, Humeston TR, Martin RJ. Further studies on the chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1997;100:771–774. doi: 10.1016/s0091-6749(97)70272-0. [DOI] [PubMed] [Google Scholar]