Introduction
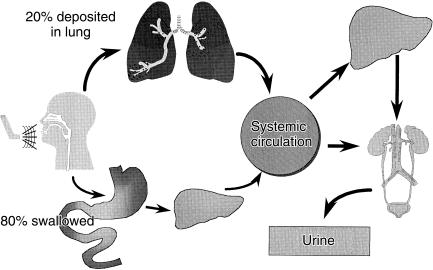
Inhalation of bronchodilators and anti-inflammatory agents enables direct delivery to the therapeutic sites in the airways for the management of asthma and chronic obstructive pulmonary disease. Figure 1 shows that following inhalation up to 20% of the dose is delivered to the lungs whilst the majority is swallowed [1, 2]. The proportion of the dose delivered to the lungs, following inhalation, will be cleared either by the mucociliary convenor belt or by absorption through the airway wall into the systemic circulation. The latter is the fraction of the dose that will exert the clinical effect within the airway wall. The total amount of drug which enters the systemic circulation via the pulmonary and gastrointestinal routes, has the potential to cause extra-pulmonary side-effects. The high first pass effect of some corticosteroids (e.g. fluticasone) limits this potential.
Figure 1.
Pharmacokinetic description of an inhaled dose.
Drug particles less than 5 µm have the greatest probability of deposition in the lung [3], whereas those less than 2 µm tend to be concentrated in the alveoli [4, 5]. The dose emitted from an inhaled product contains a large proportion of particles within the 2–5 µm range ensuring a fairly even distribution throughout the lungs [2]. When comparing inhaled products, especially generic against innovator or different devices, emphasis is placed on the in-vitro aerodynamic particle size characteristics measured using cascade impactors. Regulatory authorities view these as important quality-control procedures but for equivalence they focus on the amount delivered to the airways and to the body.
Table 1 lists the pharmacokinetic and scintigraphic methods that have been developed to identify the amount of drug delivered to the lungs following an inhalation. Pharmacokinetic methods are indirect measurements because they utilize measurements from serum [6–8] or urine [9, 10]. Figure 1 illustrates how these methods identify the amount of drug delivered to the systemic circulation via the lungs and gastrointestinal tract; thus procedures to differentiate between these two routes of absorption are required. These procedures are mentioned in Table 1. Although these methods do not differentiate between the distribution of drug into different zones of the lungs they do provide an index of the relative amount of the inhaled dose which has the potential to produce a clinical effect within the airways. The methods are therefore useful in crossover studies to investigate the equivalence between two inhaled products or differences between inhalation techniques. Following inhalation, drug is distributed to all parts of the airway and that the total dose, rather than pulmonary distribution, is related to the clinical effect [2]. This is consistent with reports that suggest that the receptors for the β-adrenoceptor agonists [4, 5, 11–13] and the inflammatory markers [14–18] are distributed throughout the airways.
Table 1.
Summary of the methods used to identify the bioequivalence of inhaled products.
| Pharmacokinetic (using plasma or urine samples) |
| Relative lung deposition (drugs with an extensive first pass, charcoal block, absorption lag times) |
| Total systemic delivery |
| Gamma scintigraphy |
| Two dimensional |
| SPECT |
| PET |
| Clinical studies |
| Spirometry (crossover or parallel design) |
| Bronchoprovocation for lung desposition |
| Multiple dosing for extra-pulmonary effects |
| In vitro |
| Determination of the in vitro particle size distribution, fine particle (respirable) dose, emitted dose |
Scintigraphic methods following inhalation using two [1, 19, 20] and three [21–23] dimensional imaging are direct methods for the determination of lung deposition. These techniques, which have been extensively described elsewhere [24], can highlight the zones of the lungs into which the drug is deposited. However, they do not differentiate between the removal of drug from the lungs by systemic absorption or by mucociliary clearance, and also require modification to the original drug formulation.
Pharmacokinetic methods
Pharmacokinetic methods can be used to estimate the total systemic delivery via the oral and inhaled routes and thus provide valuable data which predict extrapulmonary effects. Estimation of delivery can be achieved by comparing area-under-the-curve data or urinary drug excretion (to infinity) for inhaled products. To identify the effective lung dose, methods which differentiate between drug delivered to the systemic circulation via the oral and pulmonary routes are needed. This approach is not necessary if oral absorption is poor (e.g. sodium cromoglycate) or when the first pass effect is substantial (e.g. fluticasone). To separate systemic delivery via the gastrointestinal and pulmonary routes, oral charcoal to block all gastrointestinal absorption or sampling during the lag time of the absorption phase has been used.
(a) Identification of total systemic delivery
Inhaled drugs with a high extraction ratio are extensively metabolized on first pass through the liver and thus will enter the body via the oral route as metabolites which may (e.g. beclomethasone) or may not (e.g. fluticasone) be active. The oral bioavailability of inhaled corticosteroids is < 1% for fluticasone [25, 26] and 11% for budesonide [27]. Thus, for fluticasone propionate virtually all the systemic delivery of the intact molecule is by the pulmonary route. Mollman et al. [28] highlighted this in a study of plasma fluticasone propionate concentrations following inhalation of 500 µg with and without oral charcoal administration. There was no statistically significant difference in the pharmacokinetic parameters with and without charcoal administration.
Studies have measured the area under the curve for fluticasone following inhalation and intravenous administration to identify the absolute systemic (and hence lung) bioavailability. Mackie et al. [29] measured serial plasma fluticasone concentrations following a 250-µg intravenous dose and after separate inhalations of 1000 µg, in healthy volunteers using a Diskhaler and a Diskus (otherwise known as an Accuhaler in the UK). The mean (90% confidence interval) bioavailability was 11.9 (9.0–15.7)% and 16.6 (13.6–20.3)% for the Diskhaler and the Diskus, respectively. A similar bioavailability for the Diskhaler was reported by Thorsson et al. [26]. Following intravenous administration of 200 µg of fluticasone and a single inhalation of 1000 µg from a Diskhaler, in 12 healthy adults, these authors reported a mean (90% confidence interval) absolute bioavailability of 15.6 (13.6–18.4)% of the nominal dose.
Plasma fluticasone concentrations following inhalation of 500 µg twice daily by asthmatic patients using a Diskhaler and Diskus [30] were lower than those obtained in healthy volunteers [29]. All these asthmatic patients had mild to moderate asthma (FEV1 50–80% of predicted). The lower values indicate a reduced delivery of drug to the lungs. In asthmatic patients, area-under-the-curve data for the Diskus was greater than those for the Diskhaler, with a mean relative bioavailability of 115.1% [30] which compares with 119.7% reported in the study using the healthy volunteers [29].
The area under the curve measured after inhalation for drugs that do not have a high first pass effect gives some indication of total systemic delivery via the oral and pulmonary routes. Comparison of this parameter from crossover studies for different inhaled methods or products gives some indication of relative safety. Derendorf et al. [31] measured plasma triamcinolone concentrations following intravenous (2 mg), oral (5 mg) and inhaled (2 mg) administration. The oral bioavailability was 23% and after inhalation the average lung bioavailability was 20%.
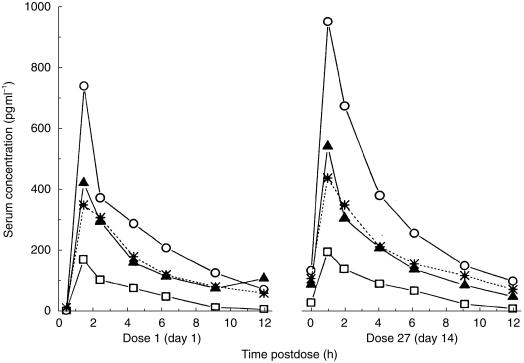
Metered dose inhaler (MDI) formulations of beclomethasone dipropionate using hydrofluoroalkane (HFA) propellants instead of chlorofluorocarbon (CFC)-based propellants are now available. Figure 2 indicates increased systemic delivery via the pulmonary route has been shown for new formulations of beclomethasone dipropionate emitted from a metered dose inhaler (MDI) [32–34]. The increased area under the curve (AUC) for the HFA formulations indicates that the dose should be halved. The particles in the beclomethasone HFA-MDI currently available, are ultrafine [35] because alcohol is included in the formulation and beclomethasone is soluble in this excipient. All other MDI formulations are suspensions of a dry powder in a propellant and thus the particle characteristics are determined by manufacturing processes. Particles emitted from solution aerosols are usually less than 2 µm (ultrafine) and thus have the potential for greater deposition in the alveoli. This has been shown using gamma scintigraphy [36] with 50% of the inhaled dose from the beclomethasone HFA-MDI deposited in the lungs and only 28% in the oropharyngeal region. In contrast, the particles emitted from the CFC-MDI formulation are bigger and only 4% is delivered to the lungs with 94% deposited in the oropharyngeal region [36]. The greater proportion of the inhaled dose delivered via the lungs rather than the gastrointestinal tract results in a high systemic delivery from the HFA-MDI formulations of beclomethasone. The AUC following inhalation of 200 µg beclomethasone HFA-MDI was 13.4% greater than that after 400 µg from a CFC-MDI. The respective time to maximum plasma beclomethasone concentration (tmax) of 0.6 h compared with 2 h indicates that the majority of the systemic delivery from the HFA product is via the pulmonary route [33]. The longer tmax for the CFC-MDI formulation indicates that most of its inhaled dose is delivered to the body via the gastrointestinal tract. The finding of doubled systemic delivery from the beclomethasone HFA-MDIs led to parallel group clinical studies which suggest that the inhaled dose from these should be halved (compared with those from the CFC-MDI) [37, 38]. However this conclusion may not be justified owing to the relatively high corticosteroid doses used in these studies. Other clinical studies do not support this conclusion [39–41]. One of these studies [39] involved parallel groups taking three separate daily doses of beclomethasone from the HFA and CFC propellant-based MDI products (i.e. six different parallel groups of patients were used). Owing to the shallow dose-response relationships a Finney Bioassay was used to demonstrate that the two relationships for each preparation were parallel. From this analysis it was found that HFA product was 2.6 times more potent than the CFC formulation although the 95% confidence interval was wide (1.1,11.1). Thus, at one extreme the ratio supports the lack of a clinically significant difference between each dose while at the other extreme the ratio supports the lung deposition data [36].
Figure 2.
Mean total beclomethasone concentrations following inhalation after the first dose and twice daily dosing in steady state (day 27);100 µg HFA (beclomethasone dipropionate in CFC-free propellant); ▵, 200 µg HFA; δ, 400 µg HFA;  , 400 µg CFC (beclomethasone dipropionate in CFC propellant). (reproduced from [34] with permission).
, 400 µg CFC (beclomethasone dipropionate in CFC propellant). (reproduced from [34] with permission).
Total systemic delivery can also be assessed using urinary excretion, especially if the molecules are polar and basic. The latter property ensures that ionization is unaffected by changes in the pH of urine. Both physicochemical properties prevent passive tubular reabsorption within the kidney and thus urine pH does not have to be controlled. Hindle & Chrystyn [10] collected urine up to 24 h postdose following inhaled and oral salbutamol administration. The mean (s.d.) urinary excretion of salbutamol and its metabolite 24 h postoral and inhaled dosing was 63.3 (10.9) and 57.4 (4.4)% of the nominal dose. This index, the relative bioavailability of salbutamol to the body, provides a comparison of systemic delivery between two inhaled products or methods.
(b) Assessment of pulmonary deposition using charcoal block
To identify lung deposition using pharmacokinetic methods, methods to separate absorption via the pulmonary and oral routes are required except for drugs with a high first pass effect such as fluticasone (Figure 1). A charcoal block method was described in a study of 13 healthy patients who received budesonide [42]. On separate occasions, these subjects inhaled 1 mg of budesonide from a Turbohaler and an MDI, with and without oral co-administration of charcoal. They also received an oral dose of 4 mg budesonide with the concurrent oral administration of charcoal and on a separate occasion they were given 50 µg budesonide intravenously. Plasma budesonide concentrations showed that oral availability with the concurrent charcoal administration was 2.5%. The preventative effect of charcoal on budesonide absorption was estimated to be approximately 80%. However, the oral dose was four times that inhaled and so if 1 mg had been swallowed the preventative effect would have been complete. The studies with charcoal showed that the pulmonary deposition from the Turbohaler was 32% and for the MDI was 18%. In the absence of charcoal the systemic availability was 38% and 20%, respectively, highlighting the relatively high first-pass metabolism of budesonide. A similar ratio with and without charcoal for the Turbohaler using AUC data up to 4 h postdose was previously reported in eight children (< 13 years) [43].
Urinary excretion of drug following the oral administration of charcoal has also been reported to identify the total effective lung dose. This method was first proposed by Borgström & Nilsson [9]. Eleven volunteers inhaled four doses of 250 µg terbutaline from an MDI with concurrent oral administration of 50 g charcoal suspended in 500 ml of water. The mean (s.d.) amount of terbutaline excreted in the urine up to 48 h postinhalation was 9.1 (30.1)% of the nominal dose. Following oral terbutaline and charcoal administration (50 g over 4 h) in five of the individuals the mean (s.d.) amount of terbutaline excreted in the urine was 0.30 (0.21)% of the nominal dose. A similar study in six volunteers, inhaling from a Turbohaler, reported that the total effective lung dose was 21.1 (3.2)% of the nominal dose [44]. Similar urinary excretion studies have also been carried out using salbutamol and charcoal but only data on salbutamol rather than salbutamol and its metabolite have been reported [45], thereby underestimating the total effective lung dose. For the MDI the mean (s.d.) amount of unchanged salbutamol excreted in the 24 h postinhalation with oral charcoal was 13.3 (3.6)% of the nominal dose [45].
(c) Assessment of pulmonary deposition utilizing absorption lag times
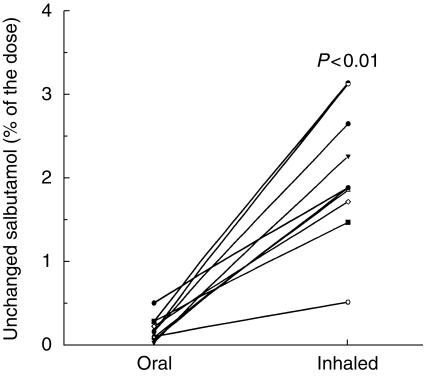
Most drugs are absorbed in the small intestine and thus after oral administration there is a time lag before drug enters the body as indicated in Figure 1. In contrast salbutamol following inhalation is delivered to the lungs within 1–3 s and is instantly absorbed [46]. Salbutamol delivered to the systematic circulation is very rapidly eliminated in the urine. The lag time for oral absorption of salbutamol into the systematic circulation is described in Figure 3 [10]. This figure shows that following oral administration there are negligible amounts of salbultamol excreted in the urine within the first 30 min postdosing. In contrast, following inhalation there are significantly greater amounts of salbulatmol excreted in the urine [10].
Figure 3.
Individual values of urinary unchanged salbutamol recovery 0.5 h after oral and inhaled administration (from [10] with permission).
Lipworth and coworkers have shown that plasma salbutamol concentrations taken at 5, 10 and 20 min post inhalation are a useful index to compare lung deposition of inhaled products or methods [6, 45, 47]. Using this method the relative bioavailability of salbutamol to the lungs following inhalation can be determined by comparing either Cmax concentrations or the average of the three plasma salbutamol concentrations. Multiple doses are inhaled (e.g. 12 doses) to enable quantification of plasma salbutamol concentrations. Whether or not the actuation of 12 consecutive doses from an MDI affects the aerodynamic particle size characteristics of the emitted dose is not clear because consecutive dosing will alter the temperature of the valve in the MDI. Furthermore, the authors have yet to evaluate their method using oral administration or simultaneous oral administration of charcoal to block any gastrointestinal absorption.
Comparing plasma salbutamol taken at 5, 10, 20, 30 and 60 min postinhalation for an MDI and a novel MDI incorporating a modified actuator provided a AUC(0,60 min) which was 25% more for the latter [6]. Simultaneous extra-pulmonary responses were also greater although the differences were not significant. This study highlights the issue of bioequivalence between inhaled products but missing from the study was the in vitro determination of the emitted dose and the fine particle dose for each product.
Clark & Lipworth [48] have compared their plasma salbutamol concentration technique with the 30 min urinary salbutamol method pursued by Hindle & Chrystyn [10]. They reported that a CFC (chlorofluorocarbon)-free formulation in an MDI (Airomir, 3 M, UK) produced significantly higher plasma salbutamol concentrations than following the original MDI containing a CFC propellant and those from a Diskhaler. Urinary salbutamol could not distinguish between the three products. These workers obtained similar results comparing generic innovator MDIs [47]. However, they collected urine up to 30 min after the last of 12 doses rather than after the start of the first (of four) dose as used by Hindle & Chrystyn [10]. The 12 doses used by Clark & Lipworth [48] would have been inhaled over 6 min and thus a urine sample taken in the 36 min after the start of the first inhalation would have contained the drug, which had been delivered via the gastrointestinal route [56, 57].
Figure 3 shows that following oral and inhaled salbutamol the mean (s.d.) salbutamol excreted in the urine in the first 30 min (after the start of the first inhalation) was 0.18 (0.14) and 2.06 (0.80)% of the nominal dose (P < 0.01) [10]. This index, termed the relative bioavailability of salbutamol to the body, could be used to compare lung deposition between two inhaled products and has subsequently been shown to be sufficiently sensitive to demonstrate differences between inhalation techniques [58], spacer devices [59, 60], dry powder inhalers [61–65], formulations [65] and nebulisers [66]. A linear relationship for the 30 min urinary excretion between 1, 2, 3, 4 and 5 doses has been shown for a salbutamol MDI [67]. It is not necessary to swallow oral charcoal when using the 30 min urinary salbutamol excretion method [68]. Following oral administration of 25 g activated charcoal, no salbutamol was detected following oral administration. The mean (s.d.) salbutamol excreted 30 min postdose was 13.90 (4.89) µg and 13.53 (4.73) µg with and without charcoal administration with a mean difference (95% confidence interval) of 0.45 (−0.48, 1.37) µg. For the 24 h urinary excretion of salbutamol the mean (s.d.)% of the nominal dose with charcoal administration was 14.5 (5.8)%, which compares with a value of 13.3 (3.6)% reported by Olsson et al. [45]. However, these values do not include the metabolite. The mean (s.d.)% of the nominal dose for the excretion of salbutamol and its metabolite for the 24 h postdose with charcoal administration was 20.0 (9.1)% of the nominal dose [68]. This indicates the total effective lung dose. The 30 min urinary method has recently been shown to determine the relative bioavailability of sodium cromoglycate [69], nedocromil [70] and gentamicin [71] to the lungs following inhalation.
(d) Total effective lung dose rather than distribution
One disadvantage of the pharmacokinetic methods to evaluate lung deposition is that they do not highlight differences in regional deposition. However, following inhalation, using techniques according to the manufacturer's instructions by clinically stable subjects, it has been demonstrated that the total lung deposition can vary but the percentage of the total lung dose distributed into the zones of the lungs remains constant [2].
Lipworth & Clark have suggested that an even distribution of drug throughout the lungs may not occur when the airway calibre alters, especially in severe asthma [51]. Following nebulization of salbutamol over 8 min in normal subjects and patients with mild and with severe asthma, they reported lower plasma salbutamol concentrations (up to 30 min postend of the 8 min nebulization) in the severe asthmatic group. Concentrations in the mild asthmatics were also lower than in normals. It was postulated that their concentrations postinhalation reflect absorption mainly from the peripheral alveolar rather than the proximal bronchial sites. However, the results are more likely due to a decrease in the total lung dose as the calibre of the airways declines, even though deposition in the central zones could be increased.
Although either plasma or urine drawn soon after inhalation could reflect alveolar deposition it should be borne in mind that distribution of drug throughout the airways is not affected by the total lung dose when a normal inhalation technique is used [2]. Furthermore, drug delivered to the alveolar region can be redistributed to the large airways either by the pulmonary circulation or via mucociliary clearance. A recent study comparing the 30 min urinary excretion of salbutamol to the dose of methacholine to reduce the FEV1 by 20% (PD20) [72] has shown a correlation between the pharmacokinetic and pharmacodynamic method. Twelve asthmatics inhaled 0, 1 and 2 doses of 100 µg salbutamol from a breath-activated MDI on separate study days. The mean (s.d.) salbutamol 30 min postdose was 0, 2.25 (0.65) and 5.18 (1.68) µg, respectively, with a ratio between 1:2 doses of 2.29. The mean (s.d.) PD20 was 1.07 (1.24), 3.77 (3.56) and 8.76 (8.16) µmol, respectively, with a ratio of 2:30 between 1 and 2 doses. The response to methacholine would be from the upper and central airways. Hence the correlation suggests that the urinary salbutamol method reflects total lung deposition rather than deposition only in the alveolar region. Recently we have compared urinary sulbutamol excretion 30 min post inhalation to PD20 following different inhalation techniques from the same product by 12 asthmatics [73]. The urinary sulbutamol excretion 30 min postdose mean ratio (90% confidence interval), between the two inhalation methods, was 76.8 (61.5, 93.1)% and for PD20 it was 84.9 (66.5,103.4)%. This indicates that during bioequivalence studies the inhalation technique used can influence the results and raises issues about what is being compared during these studies.
Gamma scintigraphy
(a) Two-dimensional gamma scintigraphy
Gamma scintigraphy was first used during diagnostic testing, and its use was extended to pharmaceuticals in the 1970s. Originally, radiolabelled Teflon particles [19] were used but more recently techniques have been developed to adhere the radionuclide (usually 99 m Technetium) to either the formulation or the drug molecule [74]. The imaging of a radionuclide relies on stable activity and distribution. The processes of mucociliary clearance and coughing together with rapid permeability through the airways into the blood stream mean that the imaging period is limited and should be over a short period of time. Also, the absolute amount of radioactivity within the respiratory tract is complicated by attenuation and scatter within the tissues. Thus, tissue attenuation corrections have to be made and these values validated [24]. Furthermore, since deposition occurs in three dimensions, for planar imaging a correction for the distance from the imaging apparatus is required. The technique provides a two-dimensional image of the oropharynx, lungs and the stomach together with the inhalation device. A krypton ventilation or a transmission scan is also carried out, usually some time before the study, to outline the lung fields. The method enables deposition into ‘central’, ‘intermediate’ and ‘peripheral’ zones to be quantified together with a penetration index (peripheral/central zone ratio) but the clinical relevance of these is not proven.
Two-dimensional gamma scintigraphy has been the most commonly used method to determine the lung deposition following inhalations. Using this method, if two inhalation products deliver the same amount of drug and have similar whole lung and regional deposition patterns then their clinical effect within the airways should be the same. All scintigraphic methods involve changes to the original formulation to incorporate the radiolabel. Thus in vitro assessment of the emitted dose and its aerodynamic particle size distribution should be carried out. Some recommendations for these in vitro measurements have been made [24]. For MDIs an Andersen Cascade Impactor method has been recommended. This method allows extensive characterization of the particle size distribution emitted from an inhaler. However, for dry powder inhalers the multistage liquid impinger has been recommended [24]. This method has limitations in the determination of the particle size range especially the respirable fraction. A previous report has shown that when using the Andersen Cascade Impactor the mass median aerodynamic diameter of a labelled drug was larger than the original product and that there was a difference in the homogeneity of the size distribution [76]. Furthermore, particle size ranges should be quoted as amounts emitted rather than percentage, and in vitro determinations should use the same number of doses that were used in the scintigraphic study. More careful consideration of these in vitro recommendations should therefore be made.
Amongst the numerous studies in the literature are those which focus on inhalation technique [76–80], inhaled products [1, 44, 81–84], spacers [19, 85–88] and nebulizers [89–93]. The planar images obtained with this method may be insensitive to the relative deposition in the different zones of the lungs because of the three dimensional structure of the lungs. To overcome this problem three-dimensional imaging methods (SPECT and PET) have recently been introduced.
(b) SPECT (single photon emission computed tomography)
This method [21, 22, 94, 95] is similar to two-dimensional gamma scintigraphy except that the gamma camera rotates through 360 °. This allows a full three-dimensional reconstruction of the lungs but takes much longer so the radiation dose has to be higher. Furthemore, the technique is difficult to apply to multidose inhaled products. The increased imaging time may affect the actual distribution due to mucociliary clearance, coughing and absorption into the systemic circulation. Using this method it is possible to create a three-dimensional penetration index which provides more sensitivity than two-dimensional imaging [1, 96]. To date lung deposition has only been reported for the cromones [28].
(c) PET (Positron emission tomography)
It is now possible to directly incorporate a radiolabel into the drug molecule. The ones recently used are positron emitters such as 11C or 18F. Disadvantages of this new method are that the positron emitters used so far have short half-lives and the method is very expensive. 11C has been introduced into triamcinolone acetonide and studies have highlighted the greater peripheral deposition when a spacer is attached to an MDI [23]. This was mainly due to a substantial increase in the total amount of drug deposited in the lungs (13.6% with and 4.9% without the spacer). This technique has recently been used for fluticasone [97].
Comparison between pharmacokinetic methods and gamma scintigraphy
The charcoal block method using urinary excretion of terbutaline [9] has been compared with total lung deposition measured by gamma scintigraphy [44]. The mean (s.d.) terbutaline excreted in the urine postinhalation with concurrent charcoal administration was 21.1 (3.2)% of the nominal dose whilst gamma scintigraphy showed the total lung deposition was 26.9 (3.8)%. Gamma scintigraphy identifies total lung deposition of a drug that is cleared from the lungs either by absorption into the systemic circulation (effective lung dose) or by mucociliary clearance. The difference obtained is because pharmacokinetic methods only identify the former. This has been highlighted by Newman et al. [77] who compared the two methods using different flow rates when inhaling from an MDI and when using a large volume spacer. Table 2 shows that following inhalation at 15 l min−1 from the MDI when it was attached to a Nebuhaler and at 30 l min−1 for the MDI alone there was little difference between the urinary and scintigraphy methods. Following fast inhalation with the MDI using a flow rate of 180 l min−1, total lung deposition was greater when measured by gamma scintigraphy. The fraction deposited onto the central airways was increased (39.4% of the total lung dose compared with 26.6% for the nebuliser and 26.2% for the MDI at 30 l min−1). The increased mucociliary clearance, because of greater deposition in the central airways, would not have been detected by the pharmacokinetic method.
Table 2.
Mean (s.d.) whole lung deposition data expressed as percentages of the metered dose determined by gamma scintigraphy and by the charcoal-block method. Inhaled flows are targeted values (reproduced from [77] with permission).
| MDI at 30 l min−1 | MDI at 180 l min−1 | MDI +Nebuliser spacer at 15 l min−1 | |
|---|---|---|---|
| Charcoal block | 11.2 (4.0) | 7.2 (2.2) | 33.8 (10.6) |
| Gamma scintigraphy | 10.7 (2.6) | 10.4 (5.0) | 31.6 (10.1) |
Using gamma scintigraphy Melchor et al. [1] have shown that total lung deposition following inhalation from an MDI, MDI + spacer and a dry powder inhaler (DPI) was lower in asthmatic subjects and that the tendency was for more drug to be deposited into the central airways of the asthmatic subjects. Again, more of the dose would be removed by mucociliary clearance as it would be deposited more centrally. These two factors would explain the lower plasma salbutamol [51] and fluticasone [29, 30] concentrations reported in asthmatics compared with healthy volunteers. It is most likely that the difference in the total effective lung dose between asthmatics and normals is due to decreased total lung deposition and greater mucociliary clearance because more drug could be deposited in the central zone. This would also explain the failure of LUDEP, a computerized model, to predict lung deposition in asthmatics [98].
Conclusion
Planar (two-dimensional) gamma scintigraphy is the most widely used method to identify lung deposition. Although it is not as sensitive as the new three-dimensional methods to quantify regional deposition there are no conclusive studies that show the importance of targeting drug to different regions of the lungs. The lung deposition identified by gamma scintigraphy includes the effective lung dose and the amount removed by mucociliary clearance, whereas the pharmacokinetic methods only identify the former. This may be why gamma scintigraphy studies between an MDI and a Gentlehaler showed no difference [83] whereas a pharmacokinetic method using plasma salbutamol, drawn during the lag phase of oral absorption, revealed greater lung deposition from the Gentlehaler with corresponding increased extrapulmonary effects [6]. Pharmacokinetic methods do not differentiate between deposition into different zones of the lungs but as mentioned above the relevance of distribution is not proven. When using pharmacokinetic methods modification/reformulation of the inhaled product is not necessary and by incorporating an intravenous study absolute values may be obtained. These pharmacokinetic methods can identify the safety and efficacy of inhaled products, methods or techniques. For identification of the effective lung dose techniques to differentiate between the inhaled and swallowed fractions are required. This can be achieved by oral charcoal administration, but patient studies may be limited on ethical grounds if they are prescribed other oral medications. Studies using samples during the lag phase of oral absorption are useful for comparisons with the innovator product. These would be suitable for patient studies.
To identify bioequivalence between two inhaled products Regulatory Authorities favour the demonstration of clinical equivalence together with in vitro characterization of the emitted dose. For clinical studies, owing to maximal spirometric response from therapeutic inhaled doses, bronchoprovocation challenge has been advocated [99]. However the interpatient variability is high [72] and thus sensitivity to detect a difference is low. Thus, a large number of subjects need to be studied. Comparing the urinary salbutamol method to bronchoprovocation [73] revealed that the former was more sensitive. This study also showed that small differences in the inhalation technique affect the results and thus raises the question ‘What is compared during these clinical studies – the product’s performance or the patient's technique?'. The bronchoprovocation agents stimulate different receptors to those of the drug studied, cause deterioration of lung function (owing to their action together with the changing osmolarity and pH of the solutions nebulized) and these are very expensive studies to commission. Other problems include strict inclusion criteria to identify responsive subjects and poor dosage consistency for the emitted dose from the methods used to deliver the bronchoprovocating agents. All these lead to wide confidence intervals. The advantages and disadvantages of pharmacokinetic methods and gamma scintigraphy are highlighted in Table 3.
Table 3.
Advantages and disadvantages of pharmacokinetic methods and gamma scintigraphy.
| Advantages | Disadvantages |
|---|---|
| Pharmacokinetics methods | |
| Good for comparison of | Need to differentiate |
| relative lung deposition | between the swallowed |
| relative systemic delivery | and inhaled doses |
| Identifies the effective lung | Does not identify dose deposition into different zones of the lungs |
| Original product used | Some assays do not have the sensitivity to measure the low concentrations |
| Simple crossover design for studies. | |
| No washout period (can substitute another drug for continued management) | |
| Can use urine samples | |
| Shows a dose response relationship | |
| Gamma scintigraphy | |
| Widely used – good for comparisons | Reformulation of the product |
| Identifies the total lung dose | Long-term safety of radionuclide |
| Differentiates between deposition | In vitro validation of |
| into different zones of the lungs | reformulated product required and methods are not standardized |
| Striking visual images | Correction for tissue attenuation |
| Simple crossover design | Tissue attenuation |
| No wash-out period required | Does not identify systemic delivery |
| Demonstrates dose-response | Does not identify the |
| relationships | effective lung dose |
| Expensive study to commission | |
The Regulatory Authorities regard pharmacokinetic and gamma scintigraphy methods as useful for supporting data. Both of these methods demonstrate dose-response relationships. Also, these methods are easier to carry out than bronchoprovocation studies, are ideal for a cross-over design and do not require special criteria to select responsive patients. Unlike bronchoprovocation they produce therapeutic benefit from the study dose. Recently the two methods, especially the pharmacokinetic approaches, have been extensively developed and further validated and viewed with less scepticism by the Regulatory Authorities. In future their use will increase.
References
- 1.Melchor R, Biddiscomb MF, Mak VHF, Spiro SG. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993;48:289–299. doi: 10.1136/thx.48.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrystyn H. Is total particle dose more important than particle distribution? Resp Med. 1997;91(Suppl A):17–19. doi: 10.1016/s0954-6111(97)90100-1. [DOI] [PubMed] [Google Scholar]
- 3.Rees PJ, Clark TJ, Morén F. The importance of particle size in responses to inhaled bronchodilators. Eur J Respir Dis. 1982;63(Suppl):73–78. [PubMed] [Google Scholar]
- 4.Zanen P, Go TL, Lammers J-WJ. Optimal particle size for β2-agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax. 1996;51:977–980. doi: 10.1136/thx.51.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanen P, Go TL, Lammers J-WJ. The optimal particle size for β2-adrenergic aerosols in mild asthmatics. Int J Pharm. 1994;107:211–217. [Google Scholar]
- 6.Newnham DM, McDevitt DG, Lipworth BJ. Comparison of the extrapulmonary β2-adrenoceptor responses and pharmacokinetics of salbutamol given by standard metered dose inhaler and modified actuator devices. Br J Clin Pharmacol. 1993;36:445–450. doi: 10.1111/j.1365-2125.1993.tb00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seale JP, Harrison LI. Effect of changing the fine particle mass of inhaled beclomethasone dipropionate on intrapulmonary deposition and pharmacokinetics. Resp Med. 1998;91(Suppl A):9–16. doi: 10.1016/s0954-6111(98)90212-8. [DOI] [PubMed] [Google Scholar]
- 8.Derendorf H, Huchhaus G, Meibohm B, Möllmann H, Barth J. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. J Allergy Clin Immunol. 1998;101:S440–S446. doi: 10.1016/s0091-6749(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 9.Borgström L, Nilsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–1070. doi: 10.1023/a:1015951402799. [DOI] [PubMed] [Google Scholar]
- 10.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311–315. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carstairs JR, Nimmo AJ, Barnes PJ. Radiographic visualisation of beta-adrenoceptor subtype in the human lung. Am Rev Resp Dis. 1989;132:541–547. doi: 10.1164/arrd.1985.132.3.541. [DOI] [PubMed] [Google Scholar]
- 12.Hamid QA, Mak JC, Sheppard MN, et al. Localisation of beta2-adrenoceptor messenger RNA in human and rat lungs using in situ hybridisation: correlation with receptor autoradiography. Eur J Pharmacol. 1991;206:133–138. doi: 10.1016/0922-4106(91)90021-9. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Beta-adrenergic receptors and their regulations. Am J Respir Crit Care Med. 1995;152:838–860. doi: 10.1164/ajrccm.152.3.7663795. [DOI] [PubMed] [Google Scholar]
- 14.Faul J, Leonard C, Tormey VJ. Lung immunopathology in cases of sudden asthmatic death. Eur Respir J. 1997;10:301–307. doi: 10.1183/09031936.97.10020301. [DOI] [PubMed] [Google Scholar]
- 15.Dunnill MS. The pathology of asthma with specific reference to change in bronchial mucosa. J Clin Path. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll N, Cooke C, James A. The distribution of eosinophils and lymphocytes in the large and small airways of asthmatics. Eur Resp J. 1997;10:292–300. doi: 10.1183/09031936.97.10020292. [DOI] [PubMed] [Google Scholar]
- 17.Hamid QA, Ying LLS, Minshall E, Elliott M, Hogg JC. Immunocytochemical study of inflammation in airways of surgically resected lungs from asthmatic and non-asthmatic subjects. J Allergy Clin Immunol. 1996;97:355. [Google Scholar]
- 18.Kraft M, Djukanovic R, Witson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505–1510. doi: 10.1164/ajrccm.154.5.8912772. [DOI] [PubMed] [Google Scholar]
- 19.Newman SP, Pavia D, Morén F, Sheahun NF, Clarke SW. Deposition of aerosol in the human respiratory tract. Thorax. 1981;36:52–55. doi: 10.1136/thx.36.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman SP, Clark AR, Talaee N, Clarke SW. Pressurised aerosol deposition in the human lung with and without an ‘open’ spacer device. Thorax. 1987;44:706–710. doi: 10.1136/thx.44.9.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming JS, Nassim N, Hashish AH, et al. Description of pulmonary deposition of radiolabelled aerosol by airway generation using a conceptual three dimensional model of lung morphology. J Aerosol Med. 1995;8:341–356. [Google Scholar]
- 22.Summers AA, Fleming JS, Dai Y, et al. The pulmonary deposition of two aerosol preparations of nedcromil sodium delivered by MDI assessed by single photon emission computed tomography. J Aerosol Med. 1996;9:S93–S104. doi: 10.1089/jam.1996.9.suppl_1.s-93. [DOI] [PubMed] [Google Scholar]
- 23.Heald DL, Berridge MS, Lee Z, Leisure GP. In vivo lung deposition of triamcinolone acetonide (Azmacort) with and without the integrated spacer. In: Dalby RN, Byron PR, Farr SY, editors. Respiratory drug delivery. Interpharm Press Inc.; 1997. pp. 345–347. [Google Scholar]
- 24.Snell JL, Ganderton D. Assessing lung deposition of inhaled medications: Consensus statement from a workshop of the British Association of Lung Research. Respir Med. 1999;93:123–133. doi: 10.1016/s0954-6111(99)90302-5. [DOI] [PubMed] [Google Scholar]
- 25.Falcoz C, Mackie A, McDowall J, et al. Oral bioavailability of fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:459P–460P. doi: 10.1046/j.1365-2125.1996.36110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorsson L, Dahlström K. Edsbäcker S, Källen A, Paulson J, Wirén J-E, editors. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryrfeldt A, Andersson P, Edsbäcker S, Toensson M, Davies D, auwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis. 1982;63:86–95. [PubMed] [Google Scholar]
- 28.Möllman H, Wagner M, Meibohm B, et al. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53:459–467. doi: 10.1007/s002280050407. 10.1007/s002280050407. [DOI] [PubMed] [Google Scholar]
- 29.Mackie AE, Falcoz C, McDowall JF, Moss J, Ventresca GP, Bye A. Pharmacokinetics of fluticasone propionate inhaled from the Diskhaler® and the Diskus® powder devices in healthy subjects. Br J Clin Pharmacol. 1997;43:540P. [Google Scholar]
- 30.Falcoz C, Mackie AE, Horton J, et al. Pharmacokinetics of fluticasone propionate inhaled from the Diskhaler® and the Diskus® powder devices in asthmatic patients. Br J Clin Pharmacol. 1997;43:541P–542P. [Google Scholar]
- 31.Derendorf H, Hochhaus G, Rohatogi S, et al. Pharmacokinetics of triamcinolone acetonide after intravenous, oral and inhaled administration. J Clin Pharmacol. 1995;35:302–305. doi: 10.1002/j.1552-4604.1995.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 32.Seale JP, Harrison LJ. Effect of changing the fine particle mass of inhaled beclomethasone dipropionate on intrapulmonary deposition and pharmacokinetics. Resp Med. 1998;92(Suppl A):9–15. doi: 10.1016/s0954-6111(98)90212-8. [DOI] [PubMed] [Google Scholar]
- 33.Harrison L, Soria I, Machacek J, et al. Pharmacokinetics of beclomethasone dipropionate from CFC-free ultrafine aerosol and conventional CFC inhalers in asthmatics. Thorax. 1996;51(Suppl 3):A74. [Google Scholar]
- 34.Harrison LJ, Colice GL, Donnell D, Soria I, Dockhorn R. Adrenal effects and pharmacokinetics of CFC-free beclomethasone dipropionate; a 14-day dose–response study. J Pharm Pharmacol. 1999;51:263–269. doi: 10.1211/0022357991772439. [DOI] [PubMed] [Google Scholar]
- 35.June DS, Schultz DL. Improved performance characteristics of CFC-free aerosols MDIs. J Aerosol Med. 1995;8:91. [Google Scholar]
- 36.Leach CL. Improved delivery of inhaled steroids to the large and small airways. Resp Med. 1998;92(Suppl A):3–8. doi: 10.1016/s0954-6111(98)90211-6. [DOI] [PubMed] [Google Scholar]
- 37.Gross G, Thompson PJ, Chervinsky P, Burgt JV. and the study group. Hydrofluoroalkane – 134a beclomethasone dipropionate, 400 µg, is as effective as chlorofluorocarbon beclomethasone dipropionate, 800 µg for the treatment of modonile asthma. Chest. 1999;115:343–351. doi: 10.1378/chest.115.2.343. [DOI] [PubMed] [Google Scholar]
- 38.Davies RJ, Stampone P, O'Connor BJ. Hydrofluoroalkane-134a beclomethasone dipropionate extrafine aerosol provides equivalent asthma control as chlorofluorocarbon beclomethasone dipropionate at approximately half the total daily dose. Resp Med. 1998;92(Suppl A):23–31. doi: 10.1016/s0954-6111(98)90214-1. [DOI] [PubMed] [Google Scholar]
- 39.Busse W, Colice G, Hannon S. CFCBDP requires 2.6 times the dose to achieve equivalent improvement in FEV1 as HFA BDP. Am J Respir Crit Care Med. 1998;157:A405. [Google Scholar]
- 40.Dahl R, Ringdal N, Ward SM, Stampone P, Donnell D. Equivalence of asthma control with new CFC-free formulation HF-134a beclomethasone dipropionate and CFC beclomethasone dipropionate and CFC beclomethasone dipropionate. Br J Clin Pharmacol. 1997;51:11–15. [PubMed] [Google Scholar]
- 41.Milanowski J, Qualtrough J, Perrin VL. Inhaled beclomethasone (BDP) with non-CFC propellent (HFA134a) is equivalent to BDP-CFC for the treatment of asthma. Respir Med. 1999;93:245–251. doi: 10.1016/s0954-6111(99)90020-3. [DOI] [PubMed] [Google Scholar]
- 42.Thorsson L, Edsbäcker S, Conradson T-B. Lung deposition of budesonide from Turbohaler is twice that from a pressurised metered dose inhaler pMDI. Eur Respir J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen S, Steffensen G, Ohlsson SV. The influence of orally deposited budesonide on the systemic availability of budesonide after inhalation from a Turbohaler®. Br J Clin Pharmacol. 1993;36:211–214. doi: 10.1111/j.1365-2125.1993.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgström L, Newman S, Weisz A, Morén F. Pulmonary deposition of inhaled terbutaline: comparison of scanning gamma camera and urinary excretion methods. J Pharm Sci. 1992;81:753–755. doi: 10.1002/jps.2600810807. [DOI] [PubMed] [Google Scholar]
- 45.Olsson B, Asking L, Borgström L, Bondesson E. Effect of inlet throat on the correlation between the fine particle dose and lung deposition. In: Dalby RN, Byron PR, Farr SY, editors. Respiratory drug delivery V. USA: Interpharm Press, Inc.; 1996. pp. 273–281. [Google Scholar]
- 46.Shenfield GM, Evans ME, Paterson JW. Absorption of drugs by the lungs. Br J Clin Pharmacol. 1976;3:583–589. doi: 10.1111/j.1365-2125.1976.tb04879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark DJ, Gordon-Smith J, McPhale G, Clark G, Lipworth BJ. Lung bioavailability of generic and innovator salbutamol metered dose inhalers. Thorax. 1996;51:325–332. doi: 10.1136/thx.51.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark DJ, Lipworth BJ. Lung bioavailability of chlorofluorocarbon free, dry powder and chlorofluorocarbon containing formulations of salbutamol. Br J Clin Pharmacol. 1996;41:247–249. doi: 10.1111/j.1365-2125.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 49.Clark DJ, Lipworth BJ. Effect of multiple acutations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer device. Thorax. 1996;51:981–983. doi: 10.1136/thx.51.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipworth BJ, Clark DJ, Koch P, Arbeeny C. Pharmacokinetics and extrapulmonary β2 adrenoceptor activity of nebulised racemic salbutamol and its R and S isomers in healthy volunteers. Thorax. 1997;52:849–852. doi: 10.1136/thx.52.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipworth BJ, Clark DJ. Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax. 1997;52:1036–1039. doi: 10.1136/thx.52.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark DJ, Tan KS, Lipworth BJ. Evaluation of plasma and urinary salbutamol levels in COPD. Eur J Clin Pharmacol. 1996;51:91–93. doi: 10.1007/s002280050166. 10.1007/s002280050166. [DOI] [PubMed] [Google Scholar]
- 53.Lipworth BJ, Clark DJ. Comparative lung delivery of salbutamol given via Turbohaler and Diskus dry powder inhaler devices. Eur J Clin Pharmacol. 1997;53:47–49. doi: 10.1007/s002280050335. 10.1007/s002280050335. [DOI] [PubMed] [Google Scholar]
- 54.Lipworth BJ, Clark DJ. Lung delivery of non-CFC salbutamol via small volume metal spacer and large volume plastic spacer devices compared with an open vent jet nebuliser. Br J Clin Pharmacol. 1998;45:160–163. doi: 10.1046/j.1365-2125.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipworth BJ, Clark DJ. Lung delivery of salbutamol given by breath activated pressurised aerosol and dry powder inhaler devices. Pulm Pharmacol Ther. 1997;10:211–214. doi: 10.1006/pupt.1997.0093. 10.1006/pupt.1997.0093. [DOI] [PubMed] [Google Scholar]
- 56.Chrystyn H. Lung bioavailability of chlorofluorocarbon-free dry powder and chlorofluorocarbon-containing formulations of salbutamol. Br J Clin Pharmacol. 1996;42:257. [PubMed] [Google Scholar]
- 57.Silkstone V, Corlett SA, Chrystyn H. Lung bioavailability of generic and innovator salbutamol MDIs. Thorax. 1996;51:658. doi: 10.1136/thx.51.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hindle M, Newton DAG, Chrystyn H. Investigations of an optimal inhaler technique using urinary salbutamol excretion as a measure of relative bioavailability to the lung. Thorax. 1993;48:607–611. doi: 10.1136/thx.48.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hindle M, Chrystyn H. Relative bioavailability of salbutamol to the lung following inhalation using metered dose inhalation methods and spacer devices. Thorax. 1994;49:549–553. doi: 10.1136/thx.49.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chege JK, Chrystyn H. Volumatic usage: some generic salbutamol metered dose inhalers can be used. Thorax. 1994;49:1162–1163. doi: 10.1136/thx.49.11.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hindle M, Newton DAG, Chrystyn H. Dry powder inhalers are bioequivalent to metered dose inhalers: a study using a new urinary albuterol (salbutamol) assay technique. Chest. 1995;107:629–633. doi: 10.1378/chest.107.3.629. [DOI] [PubMed] [Google Scholar]
- 62.Hindle M, Parry-Billings M, Peers EM, Chrystyn H. Relative bioavailability of salbutamol to the lungs following inhalation via a novel dry powder inhaler and a standard metered dose inhaler. Br J Clin Pharmacol. 1997;43:336–338. doi: 10.1046/j.1365-2125.1997.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chege JK, Chrystyn H. Evaluation of in vivo lung deposition following inhalation from a Rotahaler and Cyclohaler using urinary salbutamol excretion. Pharm Sci. 1996;2:569–570. [Google Scholar]
- 64.Chege JK, Parry-Billings M, Chrystyn H. Relative bioavailability of salbutamol to the lung following inhalation via two versions of a novel dry powder inhaler (Clickhaler) and a standard pressurized metered dose inhaler (Ventolin) Pharm Pharmacol Comm. 1995;4:107–109. [Google Scholar]
- 65.Chege JK, Chrystyn H. The relative bioavailability of salbutamol to the lung using urinary excretion following inhalation from a novel dry powder inhaler: the effects of inhalation rate and formulation. Respir Med. 2000;94:51–56. doi: 10.1053/rmed.1999.0692. 10.1053/rmed.1999.0692. [DOI] [PubMed] [Google Scholar]
- 66.Silkstone VL, Corlett SA, Chrystyn H. The relative lung bioavailability of salbutamol following administration by metered dose inhaler and nebuliser. Thorax. 1997;52:A82. [Google Scholar]
- 67.Tomlinson HS, Corlett SA, Chrystyn H. Effect of dose on the relative lung bioavailability of salbutamol. J Aerosol Med. 1995;8:P193. [Google Scholar]
- 68.Chrystyn H, Corlett SA, Tomlinson HS. Further validation of the use of using urinary salbutamol excretion to evaluate the relative bioavailability of salbutamol to the lung following inhalation. Thorax. 1997;52:A82. [Google Scholar]
- 69.Aswania OA, Corlett SA, Chrystyn H. Determination of the relative bioavailability of nedocromil sodium to the lung following inhalation using urinary excretion. Eur J Clin Pharmacol. 1998;54:475–478. doi: 10.1007/s002280050496. 10.1007/s002280050496. [DOI] [PubMed] [Google Scholar]
- 70.Aswania OA, Corlett SA, Chrystyn H. Development of an ion pair liquid chromatography method for the quantification of sodium cromoglycate in urine following inhalation. J Chromatog Biomed Appl. 1997;690:377–378. doi: 10.1016/s0378-4347(96)00382-9. [DOI] [PubMed] [Google Scholar]
- 71.Nasr H, Chrystyn H. Relative bioavailability of gentamicin to the lungs following inhalation. Eur Resp J. 1997;10:129s. [Google Scholar]
- 72.Chrystyn H, Allen MD, Corlett SA, Tomlinson HS. Simultaneous measurement of pharmacodynamic and pharmacokinetic parameters which can be used to evaluate the equivalence of inhaled salbutamol. Am J Respir Crit Care Med. 1998;157(3):A636. [Google Scholar]
- 73.Tomlinson HS, Allen MD, Corlett SA, Chrystyn H. Comparison of urinary salbutamol 30 minutes post-inhalation (USAL) at the methacholine dose to reduce the FEV1 by 20% (PD20) to identify the equivalence of inhaled salbutamol products. Eur Respir J. 1999;14:328s. [Google Scholar]
- 74.Kohler D, Fleischer W, Mathys H. New method for easy labelling of beta-2-agonists in the metered dose inhaler with technetium 99m. Respiration. 1988;53:65–73. doi: 10.1159/000195399. [DOI] [PubMed] [Google Scholar]
- 75.Aydin M, Meakin BJ, Staniforth JN, Woodhouse CE. Comparative deposition of 99m Tc labelled and unlabelled terbutaline in a 5-stage liquid impinger. Pharm Res. 1997;14:S134. [Google Scholar]
- 76.Newman S, Steed K, Hooper G, Källen A, Borgström L. Comparison of gamma scintigraphy and a pharmacokinetic technique for assessing pulmonary deposition of terbutaline sulphate delivered by pressurized metered dose inhaler. Pharm Res. 1995;12:231–236. doi: 10.1023/a:1016278926231. [DOI] [PubMed] [Google Scholar]
- 77.Newman SP, Pavia D, Garland N, Clarke SW. Effect of various inhalation modes on the deposition of radioactive pressurised aerosols. Eur J Respir Dis. 1982;119(Suppl):57–65. [PubMed] [Google Scholar]
- 78.Farr S, Rowe AM, Rubsamen R, Taylor C. Aerosol deposition in the human lung following administration from a microprocessor-controlled pressurised metered dose inhaler. Thorax. 1995;40:639–644. doi: 10.1136/thx.50.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newman SP, Morén F, Trufast E, Talaee N, Clarke SW. Terbutaline sulphate Turbohaler; effect of inhaled flow rate on drug deposition and efficacy. Int J Pharm. 1991;74:209–213. [Google Scholar]
- 80.Newman SP, Weisz AW, Talaee N, Clarke SW. Improvement of drug delivery with a breath activated pressurised aerosol for patients with poor intake technique. Thorax. 1991;46:712–716. doi: 10.1136/thx.46.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Newman SP, Morén F, Trufast E, Talaee N, Clarke SW. Deposition and clinical efficacy of terbutaline sulphate from Turbohaler, a new multi-dose powder inhaler. Eur Respir J. 1989;2:247–252. [PubMed] [Google Scholar]
- 82.Borgström L, Derom E, Stähl E, Wählin-Bul E, Pauwals R. The inhalation device influences lung deposition and bronchodilatory effect of terbutaline. Am J Respir Crit Care Med. 1996;153:1436–1460. doi: 10.1164/ajrccm.153.5.8630614. [DOI] [PubMed] [Google Scholar]
- 83.Newman SP, Clarke SW. Bronchodilator delivery from Gentlehaler, a low-velocity pressurized aerosol inhaler. Chest. 1993;103:1442–1446. doi: 10.1378/chest.103.5.1442. [DOI] [PubMed] [Google Scholar]
- 84.Newman SP, Morén F, Pavia D, Little F, Clarke SW. Deposition of pressurised suspension aerosols inhaled through extension devices. Am Rev Respir Dis. 1981;124:317–320. doi: 10.1164/arrd.1981.124.3.317. [DOI] [PubMed] [Google Scholar]
- 85.Newman SP, Millar AB, Lennard-Jones TR, Morén F, Clarke SW. Improvement of pressurised aerosol deposition with Nebuhaler spacer device. Thorax. 1984;39:935–941. doi: 10.1136/thx.39.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman SP, Woodman G, Clarke SW, Sackner MA. Effect of InspirEase on the deposition of metered dose aerosols in the human respiratory tract. Chest. 1986;89:551–556. doi: 10.1378/chest.89.4.551. [DOI] [PubMed] [Google Scholar]
- 87.Newman SP, Pellow PG, Clarke SW. Droplet size distributions of nebulised aerosols for inhalation therapy. Clin Phys Physiol Meas. 1986;7:139–146. doi: 10.1088/0143-0815/7/2/004. 10.1088/0143-0815/7/2/004. [DOI] [PubMed] [Google Scholar]
- 88.Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered dose inhalers with and without spacer devices. Chest. 1998;113:957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 89.Newman SP, Pellow PG, Clay MM, Clarke SW. Evaluation of jet nebulisers for use with gentamicin solution. Thorax. 1985;40:671–676. doi: 10.1136/thx.40.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zainudin BMZ, Biddiscombe M, Tolfree SEJ, Spiro SG. Comparison of the bronchodilator response and deposition patterns of salbutamol from a pressurized metered dose inhalers, as a dry powder and as a nebulized solution. Thorax. 1990;45:469–473. doi: 10.1136/thx.45.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardy JG, Newman SP, Knoch M. Lung deposition from four nebulisers. Respir Med. 1993;87:461–465. doi: 10.1016/0954-6111(93)90074-a. [DOI] [PubMed] [Google Scholar]
- 92.Johnson MA, Newman SP, Bloom R, Talaee N, Clarke SW. Delivery of albuterol and ipratopium bromide from two nebuliser systems in chronic stable asthma: efficacy and pulmonary deposition. Chest. 1989;96:1–10. doi: 10.1378/chest.96.1.6. [DOI] [PubMed] [Google Scholar]
- 93.Newman SP, Pitcairn GR, Hooper G, Knoch M. Efficient drug delivery to the lungs from a continuously open-vent nebulizer and low pressure compressor system. Eur Respir J. 1994;7:1177–1181. [PubMed] [Google Scholar]
- 94.Phipps P, Gonda I, Bailey D, Borham P, Bautovich G, Anderson S. Comparisons of planar and tomographic gamma scintigraphy to measure the penetration index of inhaled aerosols. Am Rev Respir Dis. 1989;139:1516–1523. doi: 10.1164/ajrccm/139.6.1516. [DOI] [PubMed] [Google Scholar]
- 95.Conway J, Fleming J, Holgate S. Three-dimensional imaging of inhaled aerosols. Eur Respir Rev. 1997;44:180–183. [Google Scholar]
- 96.Fleming JS, Hashish AH, Conway JH, et al. Assessment of deposition of inhaled aerosol in the respiratory tract of man using three dimensional multi-modality imaging and mathematical modelling. J Aerosol Med. 1996;9:317–232. doi: 10.1089/jam.1996.9.317. [DOI] [PubMed] [Google Scholar]
- 97.Berridge MS, Lee Z, Leisure GP, Moraldi F, Heald DL. Nasal delivery and kinetics of fluticasone propionate using positron tomography (PET) Pharm Sci. 1998;1(Suppl 4):S208–S209. [Google Scholar]
- 98.Price AC, Rowland M, Aarons L, Pritchard JN, Falcoz C. Drug Delivery to the Lungs IX. The Aerosol Society; 1998. The use of Ludep, as a tool, in the prediction of total and regional lung deposition: advantages, limitations and possible developments of the package; pp. 51–56. [Google Scholar]
- 99.Adams WP, Poochikian G, Taylor AS, Patel RM, Burke GP, Williams RL. Regulatory aspects of modifications to innovator bronchodilator metered dose inhalers and development of generic substitutes. J Aerosol Med. 1994;7:119–134. doi: 10.1089/jam.1994.7.119. [DOI] [PubMed] [Google Scholar]