Abstract
Aims
Allopurinol improves endothelial function in chronic heart failure by reducing oxidative stress. We wished to explore if such an effect would attenuate autonomic dysfunction in CHF in line with many other effective therapies in CHF.
Methods
We performed a prospective, randomized, double-blind cross-over study in 16 patients with NYHA Class II-IV chronic heart failure (mean age 67 ± 10 years, 13 male, comparing allopurinol (2 months) at a daily dose of 300 mg (if creatinine < 150 µmol l−1) or 100 mg (if creatinine > 150 µmol l−1) with matched placebo. Mean heart rate and dysrhythmia counts were recorded from 24 h Holter tapes at monthly intervals for 6 months. We assessed autonomic function using standard time domain heart rate variability parameters (HRV): SDNN, SDANN, SDNN index, rMSSD and TI.
Results
Allopurinol had no significant effect on heart rate variability compared with placebo; the results are expressed as a difference in means ± s.d. with 95% confidence interval (CI) between allopurinol and placebo: SDNN mean=6.5 ± 4.8 ms, P = 0.18 and 95% CI (−3.7, 17); TI mean=−2.1 ± 1.4, P = 0.16 and 95% CI (−5.2, 0.8); SDANN mean=−2.8 ± 7 ms, P = 0.68 and 95% CI (−18, 12); SDNNi mean=2 ± 6.6, P = 0.7 and 95% CI (−12, 16); RMSSD mean=−0.9 ± 2, P = 0.68 and 95% CI (−5.6, 3.7). For mean heart rate the corresponding results were 0.9 ± 1.4, P = 0.5 and 95% CI (−2, 3.8). Log 24 h ventricular ectopic counts (VEC) were 0.032 ± 0.37, P = 0.7 and 95% CI (−0.1, 0.2). Patient compliance with study medication was good since allopurinol showed its expected effect of reducing plasma uric acid (P < 0.001).
Conclusions
Allopurinol at doses, which are known to reduce oxidative stress appear to have no significant effect on resting autonomic tone, as indicated by time domain heart rate variability or on dysrhythmia count in stable heart failure patients.
Keywords: allopurinol, dysrhythmia, heart failure, heart rate variability, ischaemic stress, mean heart rate, mortality, NO, oxidant stress, uric acid
Introduction
There have been major advances in the management of chronic heart failure over the last 10 years yet the disease remains a major cause of morbidity and mortality. Novel treatment strategies are therefore still required. A traditional way to develop new treatments is to block the mechanisms that are known to be associated with poor survival. Autonomic dysfunction is one such mechanism producing poor outcome.
Abnormalities in heart rate variability are a way of measuring autonomic imbalance and have repeatedly been shown to be a powerful prognostic indicator in patients with heart failure [1–3]. For example, at any given ejection fraction, a reduced heart rate variability (HRV) increases subsequent mortality twofold to threefold [4]. Furthermore, for most treatments in heart failure, there is a strong concordance between their effects on HRV and their effects on survival [5, 6]. These HRV effects are thought to be largely due to promoting vagal tone and to a lesser extent reducing sympathetic activity [7]. Medications that improve HRV are promising therapeutically as they also usually improve mortality [8–11]. However directly acting parasympathomimetic drugs (distigmine; carbachol; pirenzipine) are not easy to use in cardiac medicine. An alternative approach would be to indirectly stimulate parasympathetic activity by preventing free radical-induced nitric oxide (NO) breakdown. This latter effect could be achieved by inhibiting xanthine oxidase that produces free radicals or by mopping up free radicals with antioxidants [12–14]. The reason why increased NO bioactivity should improve parasympathetic activity is based on a collection of experimental studies in animals and very recently human data, all of which show that endogenous nitric oxide augments cardiac vagal effects on heart rate in humans [15, 16].
Allopurinol is thus a promising option since there are already several studies that show xanthine oxidase inhibitors improving nitric oxide-dependent endothelial dysfunction [17–21]. Based on the experimental evidence quoted above the ability of allopurinol to improve nitric oxide and endothelial dysfunction might well translate into ability for allopurinol to also improve heart rate variability. We aimed to explore if allopurinol would attenuate autonomic dysfunction and increase vagal activity in chronic heart failure (CHF) in line with many other effective therapies in CHF.
Methods
Subjects and measurements
A randomized, double-blind crossover study was performed. Patients continued on their normal medication during an 8-week run-in and through the 16-week study period. At the end of interim-in period only those patients with stable heart failure were eligible to continue. Patients then were randomized to receive allopurinol (300 mg daily or 100 mg daily if blood creatinine < 150 µmol l−1 or > 150 µmol l−1, respectively) or matched placebo daily for an 8-week period. Patients then crossed over to receive the alternative, randomly allocated allopurinol or placebo for a further 8 weeks.
Sixteen patients with chronic heart failure were recruited. All patients were stable (34 months since established diagnosis) at the time of recruitment on the basis of medical history, symptoms and physical examination. The diagnosis of systolic dysfunction was confirmed by radionuclide ventriculography in all cases. Details of patient clinical characteristics and current cardiac therapies are given in Table 1. Patients were recruited on the basis of their ejection fraction and history of admission to hospital because of heart failure decompensation, and were recruited either from general medical outpatient clinics or a specialized heart failure clinic. Patients were excluded if they were currently on allopurinol or had a previously documented sensitivity to allopurinol. The local research and ethics committee approved the study prior to recruitment. All patients gave written and informed consent to participate in the study.
Table 1.
Clinical characteristics of study subjects and baseline therapy.
| Parameter | Mean or number | s.d. |
|---|---|---|
| Total number | 16 | |
| Male/female | 13/3 | |
| Age (years) | 68 | 10 |
| DBP (mm Hg) | 71 | 10 |
| SBP (mm Hg) | 126 | 18 |
| Heart rate (beats min−1) | 66 | 13 |
| 24 h SDNN | 142 | 57 |
| 24 h SDANN | 121 | 44 |
| 24 h SDNNi | 69 | 56 |
| 24 h rMSSD | 45 | 36 |
| Triangular index | 34 | 15 |
| 24 h MHR (beats min−1) | 69 | 12 |
| Weight (kg) | 74 | 13 |
| BMI | 30 | 5 |
| Plasma urea (mmol l−1) | 6 | 2 |
| Plasma creatinine (µmol l−1) | 144 | 46 |
| Plasma urate (mmol l−1) | 0.52 | 0.11 |
| Plasma potassium (mmol l−1) | 4 | 0.5 |
| Plasma sodium (mmol l−1) | 136 | 2 |
| Plasma ACE (U l−1) | 9 | 2 |
| Plasma BNP (pg ml−1) | 52 | 47 |
| Urinary urate | 0.62 | |
| NYHA class | ||
| II/III/IV | 9/5/2 | |
| LVEF% | 26 | 7 |
| Previous MI | 14 | |
| Dilated cardiomyopathy | 2 | |
| Baseline therapy | ||
| Frusemide (mg day−1) | 73 (16) | 59 |
| ACEI (mean dose mg enalapril equivalent day−1) | 17 (16) | 12 |
| Nitrate (n =) mg day−1 | ||
| Digoxin (n=) µg day−1 | 208 (6) | 102 |
| Aspririn (n=) mg day−1 | 152 (12) | 100 |
DBP, diastolic blood pressure; SBP, systolic blood pressure; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; MHR, mean heart rate.
Figures in parentheses indicate number of patients.
Patients were seen at monthly intervals and at each visit venous blood was drawn for assessment of plasma uric acid in order to assess compliance as well as urea and electrolytes. At baseline and at 4 week intervals thereafter, patients underwent standard Holter monitoring (Tracker2, Reynolds Medical Ltd, Hertford, and UK). The 24 h tapes were analysed using a Reynolds pathfinder 600 series workstation. All tapes were subjected to standard Holter analysis with artefacts and beat classification confirmed manually and corrected. Subsequently, an automated analysis was conducted for time domain heart rate variability using RR intervals that had normal morphology and cycles length between 80% and 120% of preceding cycle duration [4]. Cumulative 24 h incidence of premature and or aberrant beats; supraventricular tachycardia (> 5 beats at > 130 beats min−1); bigeminy; couplets; triplets ventricular salvos (4 beats > 100 beats min−1) or nonsustained ventricular tachycardia (> 5 beats at > 100 beats min−1) were documented for each visit. All tapes were analysed and their data files were reviewed and edited by an investigator (AS) blinded to the individual patient's clinical status. The length of recording was generally 24 h in each patient. Recordings < 18 h and patients in atrial fibrillation were excluded from the analysis. On average, 97% of the data for each patient was available for further analysis after editing.
Patients were visited in their home and were studied after having taken all their routine cardiac medications. Any changes in drug therapy from the previous visit were noted after documentation of body weight; the patient rested supine for at least 20 min to allow a 12 lead ECG to be documented and a 24 h ambulatory ECG monitor to be applied and calibrated. Blood pressure was documented and blood samples were taken. The patient was visited the following day at the completion of the 24 h ECG recording, patients were instructed to take the study medication in the morning with their daily anti heart failure medication.
Heart rate variability parameters
Analysis of heart rate variability was performed according to standard guidelines [22]. Time domain measures included the overall heart rate variability of the entire recording were calculated as the s.d. of all normal RR intervals (SDNN) and the triangular index, a geometric measure obtained by dividing the total number of all R-R intervals by the height of the histogram of all R-R intervals measured on a discrete scale with bins of 7.8 ms. The height of the histogram equals the total number of intervals found in the modal bin. Long-term HRV was estimated by the s.d. of mean RR values from all 5 min segments (SDANN). Short-tem HRV in 5 min periods were measured by the SDNN index, a mean of 5 min s.d.s of RR intervals or RMSSD, a root-mean-square of the successive normal sinus RR interval difference. The energy within short-term oscillations has been shown to be positively related to vagal activity [6].
Statistical analysis
The results were analysed in accordance with ‘the simple cross-over design’, as described by Armitage & Berry [23], taking treatment, period, and carry-over effects into account. Differences were evaluated by means of paired and unpaired t-tests and log transformed as appropriate. Changes in 24 h (HRV, MHR and ventricular ectopic counts) related to other variables by means of anova and the Pearson correlation test. Statistical calculations were performed with the use of the SPSS statistical program package, version 10. The level of significance was 5% (two-sided).
Results
Of 19 patients who agreed to take part 16 patients fulfilled all study criteria and completed recordings (Table 1). Eighty per cent were male and 60% had mild heart failure (NYHA II). Coronary artery disease (previous myocardial infarction) was the most common aetiology of heart failure. Patient compliance with study medication was good as assessed by the effect of active treatment on plasma uric acid (P < 0.001).
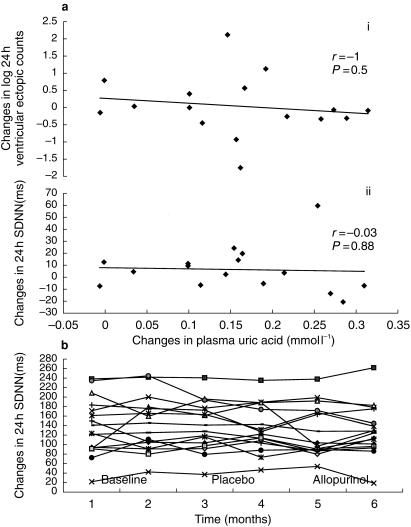
Neither allopurinol nor placebo had any statistically significant effect on heart rate variability parameters, mean heart rate, or dysrhythmia counts with P values ranging between 0.18 and 0.76 (Table 2 and Figure 1b). In addition we found no significant linear correlation between changes in serum uric acid concentrations and changes in parameters of heart rate variability, mean heart rate and dysrhythmia (r ≥ 0.3 and P ≥ 0.3), as illustrated in Figure 1a and b. To investigate whether allopurinol exhibited an effect only in particular subgroups, we looked for any effect of allopurinol on heart rate variability in subgroups of patients with (1) low heart rate variability at baseline, (2) high plasma uric acid at baseline, or (3) both high plasma uric acid and low heart rate variability at baseline. Allopurinol treatment again had no significant effect, P values ranging between 0.2 and 1 (Table 3).
Table 2.
The effect of allopurinol and placebo on the mean plasma uric acid, indices of heart rate variability, mean heart rate and arrhythmias in the study groups and on heart rate variability in sub-groups of patients with significantly low baseline HRV (i), high plasma uric acid baseline (ii) or both (iii).
| Parameters | Baseline (mean ± s.d.) | Placebo (mean ± s.d.) | Allopurinol (mean ± s.d.) | P-value placebo vs. allopurinol | 95% CI placebo vs. allopurinol |
|---|---|---|---|---|---|
| Groups | |||||
| SDNN (ms) | 139 ± 56 | 137 ± 49 | 131 ± 52 | 0.18 | (− 3.4, 16.5) |
| SDANN (ms) | 118 ± 44 | 110 ± 37 | 113 ± 38 | 0.68 | (− 17, 11) |
| SDNNi | 68 ± 56 | 62 ± 42 | 66 ± 51 | 0.76 | (− 12, 16) |
| RMSSD (ms) | 45 ± 36 | 46 ± 33 | 45 ± 33 | 0.68 | (− 5.6, 3.7) |
| Triangular index | 35 ± 16 | 34 ± 13 | 31 ± 14 | 0.5 | (− 5.2, 0.8) |
| Log ventricular ectopies | 5.9 ± 1.7 | 5.7 ± 1.9 | 5.8 ± 1.8 | 0.7 | (− 0.5, 0.3) |
| Mean heart rate (beats min−1) | 68 ± 12 | 69 ± 10 | 70 ± 13 | 0.5 | (− 2, 3.8) |
| Plasma uric acid (mmol l−1) | 0.52 ± 0.08 | 0.52 ± 0.08 | 0.34 ± 0.05 | <0.0001 | (0.11, 0.22) |
| Subgroups | |||||
| (i) | |||||
| SDNNi (ms) | 32 ± 12 | 34 ± 10 | 34 ± 8 | 0.4 | (− 7.6, 3.7) |
| RMSSD (ms) | 26 ± 15 | 27 ± 13 | 30 ± 14 | 0.2 | (− 12, 3.4) |
| Triangular index | 20 ± 8 | 20 ± 9 | 20 ± 8 | 1 | (− 5.1, 4.8) |
| (ii) | |||||
| SDNNi (ms) | 67 ± 55 | 65 ± 53 | 66 ± 51 | 0.87 | (− 5.01, 5.7) |
| RMSSD (ms) | 47 ± 38 | 49 ± 37 | 50 ± 36 | 0.27 | (− 9.3, 2.8) |
| Triangular index | 35 ± 17 | 34 ± 16 | 32 ± 15 | 0.08 | (− 0.4, 6.4) |
| (iii) | |||||
| SDNNi (ms) | 32 ± 12 | 33 ± 10 | 34 ± 8 | 0.4 | (− 7.6, 3.7) |
| RMSSD (ms) | 22 ± 10 | 25 ± 9 | 27 ± 12 | 0.2 | (− 15, 12) |
| Triangular index | 19 ± 9 | 19 ± 88 | 20 ± 9 | 0.8 | (− 6.2, 5.5) |
HRV, heart rate variability; rMSSD, root-mean-square of the successive normal sinus RR interval difference; SDANN, standard deviation of the averaged normal sinus RR intervals for all 5 min segments; SDNN, standard deviation of all normal sinus RR intervals over 24 h; SDNNi, mean of the standard deviations of all normal sinus RR intervals for all 5 min segments; TI, triangular index; CI, confidence intervals.
Figure 1.
a) There is no significant correlation between plasma uric acid and both (i) changes in log 24 h ventricular ectopics and (ii) changes in 24 h HRV (SDNN). b) The effect of allopurinol and placebo on individual 24 h HRV (SDNN).
Discussion
The main finding of this study was that allopurinol had no effect on heart rate variability, mean heart rate or dysrhythmias in our cohort of heart failure patients. The patient group exhibited the expected baseline abnormalities of both uric acid and HRV. Ninety percent of our patients had an abnormally high baseline plasma uric acid, which has been shown to be an independent predictor of mortality in heart failure [21]. While allopurinol clearly produced reductions of plasma uric acid as expected, there was no significant effect on time domain HRV parameters and thus on autonomic tone. This could be due to the size of our sample and/or to the sensitivity of HRV as a marker of autonomic tone but many previous controlled studies have shown beneficial effects on HRV was effective treatments of CHF.
Our patients had effective therapy in place, which may explain why only half had significantly low baseline HRV (as judged by a value < 50 ms for RMSSD and SDNNi or a value < 25 for TI). Even in this sub group with poor autonomic tone and a worse prognosis, allopurinol had no effect (Table 3) despite a marked reduction in uric acid. This might be regarded as evidence against uric acid as an independent marker of poor prognosis in CHF [23]. It could equally suggest that uric acid might exert harmful effects independent of both autonomic tone or dysrhythmia.
We hypothesized that allopurinol might help in heart failure either by reducing free radicals and boosting nitric oxide and/or by reducing uric acid [17, 20]. If such effects attenuate autonomic dysfunction in CHF then this would strongly underpin the tantalizing possibility that allopurinol might improve mortality in CHF. Allopurinol has been shown to have generally favourable antiarrhythmic effects in other clinical situations such as in patients following CABG and during reperfusion injury [24, 25]. While we saw no effect in treated chronic heart failure, this may be because free radicals are only small contributors to dysrhythmias or autonomic tone during periods of stability and in the place of other treatments in stable patients. It remains possible that free radical oxidant stress assumes a greater role in arrhythmogenesis, for example during periods of ischaemic injury.
Recent reports show that endogenous nitric oxide can augment vagal control of heart rate in humans [15, 16]. These data were based on changes in heart rate variability in response to pressor stimuli. It is possible that in patients with heart failure changes in nitric oxide balance contribute little to baseline autonomic tone yet may still be important during periods of acute stress, such as during pressor stimuli or during ischaemia (as above). Again this could explain our negative findings with baseline HRV data. An effect of allopurinol might become evident during baroreflex testing with pressor stimuli.
We can have reasonable confidence that this small study did not miss an important clinical effect of allopurinol. There was no trend observed; we used multiple independent measurements of HRV, i.e. four 24 h tapes during placebo and two 24 h tapes on allopurinol the order of which was randomized; the absolute size of the confidence limits interval for the effect of allopurinol on the HRV parameter results were in general small in relation to the standard deviation of the baseline values of the HRV parameters; and the study had 95% power to detect a 10% difference at the 5% level of significance, i.e. difference in mean of 24 h (SDNN, mean heart rate and ventricular ectopic counts) (13 ms, 5 beats min−1 and 5, respectively). Clearly this study does not totally exclude a small effect of allopurinol which might be detected if much larger numbers of patients were studied. However, any effect seen only by using huge numbers of patients may be so small as to be clinically insignificant even if it is statistically significant.
Another minor limitation in our study was that it was performed before β-adrenoceptor blockers were more widely used in CHF patients. However β-adrenoceptor blockers are unlikely to uncover any beneficial effect of allopurinol on HRV as they along with most other effective CHF therapies restore HRV and by implication autonomic tone towards normal. Therefore allopurinol is likely to have less rather than more room for improving HRV in the presence of β-adrenoceptor blockade.
In conclusion, this study does not support the hypothesis that allopurinol induced increases in NO bioactivity are accompanied by favourable effects on resting HRV or on spontaneous dysrhythmia counts in patients with chronic heart failure. It remains possible that modifying oxidant stress with allopurinol during special circumstances, such as acute ischaemic episodes might still be beneficial. Although allopurinol had a neutral effect on HRV and dysrhythmia counts in this study, further research is required before the complete role of allopurinol or other modifiers of oxidant stress can be fully delineated.
Acknowledgments
We thank the patients who took part in the study and both Nurse Amanda Duncan and Sister Jessamine Robson for help with data collection. The study was supported in part by both the British Heart Foundation and the Scottish Office Department of Health.
References
- 1.Wijbenga JAM, Balk AHM, Meij SH, Simoons ML, Malik M. Heart rate variability index in congestive heart failure relation to clinical variables and prognosis. Eur Heart J. 1998;19:329–334. doi: 10.1053/euhj.1998.1148. [DOI] [PubMed] [Google Scholar]
- 2.Nolan J, Batin PD, Andrews R, et al. prospective study of heart rate variability and mortality in chronic heart failure. Results of United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart) Circulation. 1998;98:1501–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 3.Quintana M, Storck N, Lindblad LE, Lindvall K, Ericson M. Heart rate variability as a means of assessing prognosis after acute myocardial infarction. Eur Heart J. 1997;18:789–797. doi: 10.1093/oxfordjournals.eurheartj.a015344. [DOI] [PubMed] [Google Scholar]
- 4.Kleiger RE, Miller P, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 5.Panina G, Khot UN, Nunziata E, Cody RJ. Role of spectral measures of heart rate variability as markers of disease progression in patients with chronic congestive heart failure not treated with angiotensin converting enzyme inhibitors. Am Heart J. 1996;131:153–157. doi: 10.1016/s0002-8703(96)90064-2. [DOI] [PubMed] [Google Scholar]
- 6.Jung J, Heisel A, Tscholl D, Fries R, Schieffer H, Ozbek C. Assessment of heart rate variability by using different commercially available systems. Am J Cardiol. 1996;78:120–123. doi: 10.1016/s0002-9149(96)00242-1. 10.1016/s0002-9149(96)00243-3. [DOI] [PubMed] [Google Scholar]
- 7.Tuininga YS, van Veldhuisen DJ, Brouwer J, et al. Heart rate variability in left ventricular dysfunction and heart failure: effects and implications of drug treatment. Br Heart J. 1994;72:509–513. doi: 10.1136/hrt.72.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yee KM, Struthers AD. Can drug effects on mortality in heart failure be predicted by any surrogate measure. Eur Heart J. 1997;18:1860–1864. doi: 10.1093/oxfordjournals.eurheartj.a015193. [DOI] [PubMed] [Google Scholar]
- 9.Adamopoulos S, Piepoli M, McCance A, et al. Comparison of different methods for assessing sympathovagal balance in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1992;70:1576–1582. doi: 10.1016/0002-9149(92)90460-g. [DOI] [PubMed] [Google Scholar]
- 10.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 11.Bell DM, Johns TE, Lopez LM. Endothelial dysfunction: implications for therapy of cardiovascular diseases. Ann Pharmacother. 1998;32:459–470. doi: 10.1345/aph.17084. [DOI] [PubMed] [Google Scholar]
- 12.Manning AS, Coltart DJ, Hearse DJ. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984;55:545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- 13.Tabayahi K, Suzuki Y, Nagamine S, Ito Y, Sekino Y, Mohri H. A clinical trial allopurinol (Zyloric) for myocardial protection. J Thoracic Cardiovasc Surg. 1991;101:713–8. [PubMed] [Google Scholar]
- 14.Chowdhary S, Townend JN. Role of nitric oxide in regulation of cardiovascular autonomic control. Clin Sci. 1999;97:5–17. [PubMed] [Google Scholar]
- 15.Chowdhary S, Vaile JC, Farmer MR, Coote JH, Townend JN. Cardiac vagal modulation by endogenous nitric oxide in human. Eur Heart J. 1999;20(Suppl):132. [Google Scholar]
- 16.Giverts MM, Colucci WS. New targets for heart failure therapy: endothelin, inflammatory cytokines and oxidative stress. Lancet. 1998;352(Suppl):34–38. doi: 10.1016/s0140-6736(98)90017-4. [DOI] [PubMed] [Google Scholar]
- 17.Drexler H, Zzeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcicullation of hypercholesterolaemic patients by L-arginine. Lancet. 1991;338:1456–1450. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 18.Butler R, Morris AD, Struthers AD. Allopurinol improves endothelial function in patients with type 2 diabetes. Eur Heart J. 1997;18(Suppl):1902. [Google Scholar]
- 19.Farquharson C, Butler RA, Hill A, Belch JJF, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. JACC. 1999;32(SupplA):216. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 20.Cardillo C, Kilcoyne CM, Cannon RO, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolaemic but not in hypertensive patients. Hypertension. 1997;30:1957–1963. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Malik M. for the Task Force of the ESC and NASPE. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 22.Armitage P, Berry G. Statistical methods in medical research. Oxford, England: Blackwell Scientific Publications; 1987. pp. 222–226. [Google Scholar]
- 23.Anker SD, Leyva SD, Poole Wilson PA, Coats AJS. Uric acid is a an independent predictor of impaired prognosis in patients with chronic heart failure. J Am Coll Cardiol. 1998;31:154A. [Google Scholar]
- 24.Coghlan JG, Flitter WD, Clutton SM, et al. Allopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;107:248–256. [PubMed] [Google Scholar]
- 25.Rashid MA, William-Olsson G. Influence of allopurinol on cardiac complications in open heart operations. Ann Thorac Surg. 1991;52:127–130. doi: 10.1016/0003-4975(91)91433-v. [DOI] [PubMed] [Google Scholar]