Abstract
Aims
To examine whether systemic β2-adrenoceptor responses, such as tachycardia, tremor and hypokalaemia, can be used as a surrogate for the 20 min pharmacokinetic profile of inhaled salbutamol.
Methods
A retrospective analysis of eight separate published studies in healthy volunteers was performed, each with an identical protocol evaluating the early lung absorption profile of a nominal 1200 µg dose of salbutamol given by different inhaler devices. Peak postural finger tremor, plasma potassium and heart rate were assesssed.
Results
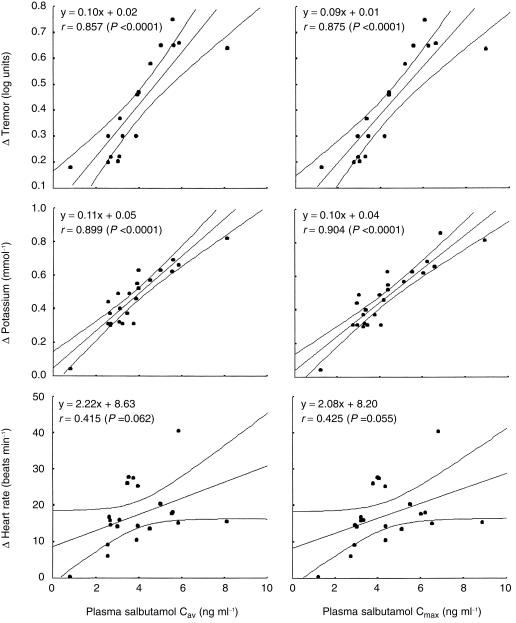
We found the maximum (Cmax) and average (Cav) plasma concentrations of salbutamol to be correlated (P < 0.0001) to change in plasma potassium (Cmax r = 0.904; Cav r = 0.899) and tremor (Cmax r = 0.875; Cav r = 0.857). No significant correlations existed between change in heart rate and Cmax (r = 0.425) or Cav (r = 0.415).
Conclusions
Systemic β2-adrenoceptor responses, in particular hypokalaemia and tremor, but not heart rate, appear to be good surrogates for evaluating the lung delivery of inhaled salbutamol. Consequently it is suggested that potassium or tremor responses may be used to evaluate the relative lung delivery of salbutamol from different inhaler devices.
Keywords: β2-adrenoceptor, pharmacokinetics, salbutamol
Introduction
The relative lung delivery of inhaled salbutamol from different devices may be reliably quantified by comparing the lung bioavailability of salbutamol from the early lung absorption profile [1]. It is known that inhaled salbutamol exerts dose-related systemic effects such as tachycardia, hypokalaemia and tremor [2]. The question arises whether systemic β2-adrenoceptor responses can be used as a surrogate for inhaled pharmacokinetics. In this respect we have performed eight separate studies evaluating the early lung absorption profile of salbutamol given by different inhaler devices [3–10]. Each study has used identical methodology in healthy volunteers and measured the 20 min pharmacokinetic profile for the same nominal 1200 µg dose of salbutamol, as well as assessing postural finger tremor, plasma potassium and heart rate.
Methods
Subjects
Eighty-two patients were included in the studies, and their demographic data are shown in Table 1. All gave written, informed consent, and were randomized into single (investigator) or double-blind crossover studies, which were approved by the Tayside Committee for Medical Research Ethics.
Table 1.
Demographic data for patients in the eight studies.
| Reference | Devices studied | n | Mean (s.d.) age (years) | Mean (s.d.) % predicted FEV1 |
|---|---|---|---|---|
| 3 | Three CFCpMDI formulations | 10 | 20.5 (0.9) | 112 (8.9) |
| 4 | CFCpMDI + Volumatic – three methods | 10 | 20.5 (0.9) | 112 (8.9) |
| 5 | Turbohaler/HFApMDI + NebuChamber | 10 | 21 (2.2) | 105 (9.5) |
| 6 | Diskhaler/Accuhaler/Easibreathe | 10 | 24 (5.4) | 103.2 (7.9) |
| 7 | CFCpMDI/HFApMDI/Diskhaler | 12 | 20.6 (0.6) | 111.4 (7.9) |
| 8 | Sidestream nebuliser/HFApMDI + Volumatic/NebuChamber | 12 | 22 (1.4) | 102 (7.4) |
| 9 | HFApMDI ± AeroChamber/Nebuhaler/Volumatic | 10 | 22 (0.7) | 103.8 (6.6) |
| 10 | Turbohaler/Accuhaler | 8 | 21 (2.5) | 106.4 (11.7) |
| Pooled studies | 82 | 21.5 (2.2) | 107.0 (8.2) |
CFC = chlorofluorocarbon, HFA = hydrofluoroalkane, pMDI = pressurized metered dose inhaler.
Protocols
The study visits were separated by at least 3 days. At each visit patients inhaled the same nominal 1200 µg dose of salbutamol from the appropriate device. For pressurized metered dose inhalers, Turbohaler and Easibreathe this was given as 12 sequential 100 µg inhalations. For the Diskhaler and Accuhaler six 200 µg inhalations were taken. For the Sidestream nebuliser the salbutamol was given as a single 1200 µg dose. Where spacer devices were used each inhalation of 100 µg was taken separately, except in one study [4] where the objective was to test different methods of inhalation via a spacer device. Inhaler technique in each case was closely monitored and as per the manufacturer's instructions.
Measurements
At each visit heart rate, tremor and potassium were measured at baseline and 20 min post inhalation. Plasma salbutamol levels were measured at 5, 10 and 20 min post inhalation.
Heart rate was measured from the standard lead II of an electrocardiogram monitor, finger tremor with an accelerometer transducer (Entran, Ealing, UK) [11], and plasma potassium analysed by flame photometry using an IL943 analyser (Instrumentation Laboratory Ltd, Warrington, UK). Plasma salbutamol was assayed by high performance liquid chromatography with solid phase extraction and fluorescence detection [12].
Statistical analysis
The maximum (Cmax) and average (Cav) plasma concentrations of salbutamol were calculated as well as peak systemic β2-adrenoceptor responses (as change from baseline). Least squares regression analysis was used to devise correlation coefficients for Cmax and Cav vs change in heart rate, tremor and plasma potassium using SPSS for Windows (Statistical Products and Service Solutions Inc., Chicago, IL, USA).
Results
In the eight papers, a total of 24 pharmacokinetic profiles were measured. Change in plasma potassium and tremor were highly correlated to Cmax and Cav (Figure 1). No significant correlations existed between change in heart rate and Cmax or Cav.
Figure 1.
Mean and 95% CI from linear regression least square analysis for the average (Cav) and maximum (Cmax) plasma salbutamol concentrations vs change from baseline in tremor (n = 17), plasma potassium (n = 24) and heart rate (n = 21). Each point represents a different device from a given study. Also shown are the corresponding regression equations and Pearson's regression coefficient (r).
Discussion
We have demonstrated that systemic β2-adrenoceptor responses, in particular hypokalaemia and tremor, but not heart rate, appear to be a good surrogate for evaluating the early pharmacokinetic absorption profile of inhaled salbutamol. The lack of significant correlation between plasma salbutamol and heart rate is difficult to explain, but may be due to the different mechanisms involved in this β2-response vs tremor or hypokalaemia. Salbutamol-induced tachycardia is due to the direct stimulation of cardiac β2-adrenoceptors [13] as well as indirect activation of peripheral receptors [14], inducing vasodilatation and consequent reflex vagal withdrawal. In contrast, tremor and hypokalaemia are solely due to direct stimulation of skeletal muscle β2-adrenoceptors. Presumably this explains the greater variability in heart rate response to salbutamol because of its direct and indirect actions.
We compared the lung deposition from the devices using the early pharmacokinetic profile of salbutamol in the first 20 min post-inhalation, which represents bioavailability from the lung [1]. In this situation there is no need to administer oral charcoal to block gut absorption, as the fraction absorbed from the gastrointestinal tract contributes 0.3% to the overall bioavailability over the first 30 min post inhalation [15].
All the patients were healthy volunteers, and while we appreciate that absolute drug absorption from the lung decreases with airway calibre, the relative lung bioavailability in patients with severe asthma will still be the same [16].
Consequently it is suggested that potassium or tremor responses may be used to evaluate the relative lung delivery of salbutamol from different inhaler devices.
Acknowledgments
This study was funded by a University of Dundee Research Grant.
References
- 1.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark DJ, Lipworth BJ. Dose–response of inhaled drugs in asthma. Clin Pharmacokin. 1997;32:58–74. doi: 10.2165/00003088-199732010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Clark DJ, Gordon-Smith J, McPhate G, Clark G, Lipworth BJ. Lung bioavailability of generic and innovator salbutamol metered dose inhalers. Thorax. 1996;51:325–326. doi: 10.1136/thx.51.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark DJ, Lipworth BJ. Effect of multiple actuations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer device. Thorax. 1996;51:981–984. doi: 10.1136/thx.51.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipworth BJ, Clark DJ. Lung delivery of salbutamol by dry powder inhaler (Turbuhaler) and small Volume antistatic metal spacer (Airomir CFC-free MDI plus Nebuchamber) Eur Respir J. 1997;10:1820–1823. doi: 10.1183/09031936.97.10081820. [DOI] [PubMed] [Google Scholar]
- 6.Lipworth BJ, Clark DJ. Lung delivery of salbutamol given by breath activated pressurised aerosol and dry powder inhaler devices. Pulm Pharmacol. 1997;10:211–214. doi: 10.1006/pupt.1997.0093. [DOI] [PubMed] [Google Scholar]
- 7.Clark DJ, Lipworth BJ. Lung bioavailability of chlorofluorocarbon free, dry powder and chlorofluorocarbon containing formulations of salbutamol. Br J Clin Pharmacol. 1996;41:247–249. doi: 10.1111/j.1365-2125.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Lipworth BJ, Clark DJ. Lung delivery of non-CFC salbutamol via small Volume metal spacer and large Volume plastic spacer devices compared with an open vent jet nebuliser. Br J Clin Pharmacol. 1998;45:160–163. doi: 10.1046/j.1365-2125.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipworth BJ, Clark DJ. Early lung absorption profile of non-CFC salbutamol via small and large Volume plastic spacer devices. Br J Clin Pharmacol. 1998;46:45–48. doi: 10.1046/j.1365-2125.1998.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipworth BJ, Clark DJ. Comparative lung delivery of salbutamol given via Turbuhaler and Diskus dry powder inhaler devices. Eur J Clin Pharmacol. 1997;53:47–49. doi: 10.1007/s002280050335. 10.1007/s002280050335. [DOI] [PubMed] [Google Scholar]
- 11.Lipworth BJ, McDevitt DG. Beta-adrenoceptor responses to inhaled salbutamol in normal subjects. Eur J Clin Pharmacol. 1989;36:239–245. doi: 10.1007/BF00558154. [DOI] [PubMed] [Google Scholar]
- 12.Lipworth BJ, Clark RA, Dhillon DP, et al. Pharmacokinetics, efficacy and adverse effects of sublingual salbutamol in patients with asthma. Eur J Clin Pharmacol. 1989;37:567–571. doi: 10.1007/BF00562546. [DOI] [PubMed] [Google Scholar]
- 13.Hall JA, Petch ML, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional β2-receptors in the human heart. Circulation Res. 1989;65:546–553. doi: 10.1161/01.res.65.3.546. [DOI] [PubMed] [Google Scholar]
- 14.Arnold JM, McDevitt DG. Heart rate and blood pressure responses to intravenous boluses of isoprenaline in the presence of propanolol, practolol and atropine. Br J Clin Pharmacol. 1983;16:175–184. doi: 10.1111/j.1365-2125.1983.tb04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrystyn H, Corlett SA, Silkstone V. Lung bioavailability of generic and innovator salbutamol MDIs. Thorax. 1996;51:658. doi: 10.1136/thx.51.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipworth BJ, Clark DJ. Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax. 1997;52:1036–1039. doi: 10.1136/thx.52.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]