Abstract
Aims
To assess the absolute bioavailability, pharmacokinetics and metabolism of beclomethasone dipropionate (BDP) in man following intravenous, oral, intranasal and inhaled administration.
Methods
Twelve healthy subjects participated in this seven-way cross-over study where BDP was administered via the following routes: intravenous infusion (1000 µg), oral (4000 µg, aqueous suspension), intranasal (1344 µg, aqueous nasal spray) and inhaled (1000 µg ex-valve, metered dose inhaler). The contribution of the lung, nose and gut to the systemic exposure was assessed by repeating the inhaled, intranasal and oral dosing arms together with activated charcoal, to block oral absorption. Blood samples were collected for 24 h postdose for the measurement of BDP, beclomethasone-17-monopropionate (B-17-MP) and beclomethasone (BOH) in plasma by liquid chromatography tandem mass spectrometry.
Results
Intravenous administration of BDP (mean CL 150 l h−1, Vss 20 l, t½ 0.5 h) was associated with rapid conversion to B-17-MP which was eliminated more slowly (t1/2 2.7 h). In estimating the parameters for B-17-MP (mean CL 120 l h−1, Vss 424 l) complete conversion of BDP to B-17-MP was assumed. The resultant plasma concentrations of BOH were low and transient. BDP was not detected in plasma following oral or intranasal dosing. The mean absolute bioavailability (%F, 90% CI; nominal doses) of inhaled BDP was 2% (1–4%) and not reduced by coadministration of charcoal. The mean percentage F of the active metabolite B-17-MP was 41% (31–54%), 44% (34–58%) and 62% (47–82%) for oral, intranasal and inhaled dosing without charcoal, respectively. The corresponding estimates of nasal and lung absorption, based on the coadministration of charcoal, were < 1% and 36% (27–47%), respectively.
Conclusions
Unchanged BDP has negligible oral and intranasal bioavailability with limited absorption following inhaled dosing due to extensive (95%) presystemic conversion of BDP to B-17-MP in the lung. The oral and intranasal bioavailabilities of the active metabolite B-17-MP were high and similar, but direct absorption in the nose was insignificant. The total inhaled bioavailability of B-17-MP (lung + oral) was also high (62%) and approximately 36% of this was due to pulmonary absorption. Estimates of oral bioavailability and pulmonary deposition based on total BOH were approximately half those found for B-17-MP.
Keywords: absolute bioavailability, beclomethasone dipropionate, beclomethasone-17-monopropionate, inhaled, intranasal, intravenous, lung deposition, oral, pharmacokinetics
Introduction
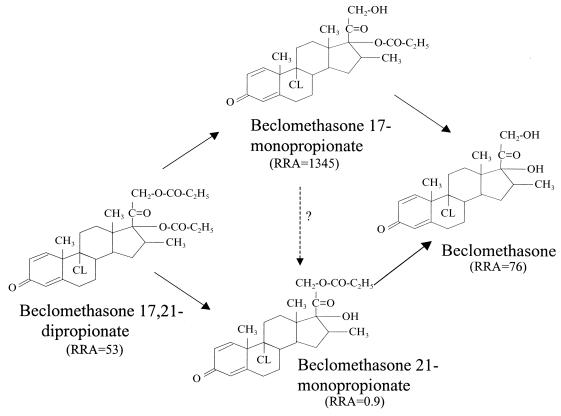
Beclomethasone 17,21-dipropionate (BDP) is a topically active corticosteroid used in the treatment of asthma and rhinitis. It was first available in 1972 in a pressurized metered-dose inhaler (MDI) and later in a dry powder inhaler and an aqueous nasal spray. BDP is actually a pro-drug with weak glucocorticoid receptor binding affinity, that is hydrolysed via esterase enzymes to an active metabolite beclomethasone 17-monopropionate (B-17-MP). Minor inactive metabolites, beclomethasone 21-monopropionate (B-21-MP) and beclomethasone (BOH), are also formed [1–4]. The chemical structures and relative receptor affinities for BDP and its main metabolites are shown in Figure 1.
Figure 1.
Chemical structures, major in vivo degradation pathway and glucocorticoid receptor affinities relative to dexamethasone (RAA = 100) [28] for BDP and its main metabolites.
The systemic exposure that occurs following inhaled and intranasal dosing with BDP is assumed to come from absorption via the lungs and nasal cavity, respectively, and the swallowed portion of the dose. However, in both cases, the relative contribution from oral absorption is not known. In addition, the contributions to systemic exposure made by unchanged BDP and its active metabolite B-17-MP have not been documented for different routes of administration.
The pharmacokinetic characteristics of the other currently available inhaled corticosteroids have been well documented [5]. In contrast, the absolute bioavailability of BDP has not been reported for the inhaled, intranasal or oral routes of administration, and the intravenous pharmacokinetics for the parent drug and its metabolites have not been defined in man. However, the recent development of the sensitive liquid chromatography tandem mass spectrometry (LC-MS-MS) assay and the availability of a well tolerated intravenous formulation of BDP have enabled this information to be obtained from the present study. In addition to the administration of BDP by four different routes (intranasal, inhaled, oral and intravenous), the use of activated charcoal to block oral absorption was also investigated. This approach was included in an attempt to estimate the contribution of the swallowed portion of the dose to the systemic exposure, following intranasal and inhaled dosing. Although the effect of oral charcoal on the systemic exposure to inhaled BDP has been investigated [6] its efficiency in blocking oral absorption was not estimated. The use of activated charcoal to bind drugs in the gut has been successful in reducing the oral absorption of other inhaled drugs with an efficiency of 92–98% [7–9].
The aims of the present study were to determine the intravenous pharmacokinetic parameters for BDP, B-17-MP and BOH in man. This information was then used to assess the absolute bioavailability of BDP and B-17-MP, when BDP is administered by the oral, intranasal and inhaled routes. An additional aim was to determine the efficiency of the charcoal block in preventing the oral absorption of BDP.
Methods
Materials
The following BDP formulations were supplied by GlaxoWellcome Clinical Trial Supplies: BDP aqueous nasal spray, 42 µg/spray (Beconase AQ® [beclomethasone dipropionate, monohydrate] Nasal Spray, 0.042%); BDP MDI 50 µg/actuation, ex-valve dose (Beclovent® [beclomethasone dipropionate, USP] Inhalation Aerosol, Becotide®); BDP for oral dosage (Beconase AQ® [beclomethasone dipropionate, monohydrate] BDP aqueous suspension, 0.42 mg ml−1); BDP for intravenous injection supplied as 5 ml ampoules containing 1 mg BDP in 1 ml vehicle (97.5% propylene glycol, 2.5% ethanol). Placebo MDIs and aqueous nasal sprays were also supplied for demonstration and training purposes. Activated charcoal (Medicoal®) was administered orally for the charcoal block procedure.
Subjects
Twelve healthy male subjects, age range 22–39 years and body mass index within 19–29 kg m−2, participated in this study. Subjects were nonsmokers and were allowed no medication during the study period. Subjects with a history of rhinitis including seasonal rhinitis were excluded. The protocol was approved by an Independent Ethics Review Committee (Northwick Park Hospital, Clinical Pharmacology Unit) and informed consent was obtained from each subject. The study was conducted according to the Declaration of Helsinki. A prestudy medical screen was performed which included training in the correct use of nasal spray and metered dose inhaler devices. Between 7 to 10 days after the final dosing occasion a poststudy medical screen was also performed.
Dose selection
Published pharmacokinetic data for intranasal and intravenous BDP were not available prior to this study. An intranasal dose of 1344 µg (32 sprays) was selected based on the results of a pilot study. The latter was a dose-escalation study in six healthy subjects and comprised three doses: 336 µg (8 sprays) the usual daily clinical dose, 672 µg (16 sprays) and 1344 µg (32 sprays). Blood samples were collected for 12 h post dose for the measurement of BDP and B-17-MP. The optimum dose for the current study was one closest to that used clinically and which produced high enough plasma concentrations to allow an adequate estimation of the AUC for B-17-MP. An inhaled dose of 1000 µg was chosen based on pharmacokinetic data from a previous study [10]. This dose is clinically relevant and produces plasma concentrations which allow a good estimate of the AUC. An oral dose of 4000 µg was chosen in an attempt to obtain similar systemic exposure to the other routes of administration. The elimination kinetics of BDP and B-17-MP have been shown to be linear over a wide dose range [10]. An intravenous dose of 1000 µg was chosen to allow estimation of pharmacokinetic parameters for BDP, B-17-MP and BOH. Previous attempts to quantify the inactive metabolite BOH following inhaled dosing resulted in only a few values above the assay detection limit [10, 11]. In addition, plasma was not assayed for the minor and inactive metabolite B-21-MP, since it could not be detected in most samples following inhaled dosing [12].
Study design
This was a single dose, open label, seven-way cross-over study with a 72 h washout period between treatments. Subjects attended the clinic on the evening before each study day at approximately 20.00 h and their breath was tested for alcohol and carbon monoxide. A snack was provided at about 22.00 h and following this the subjects fasted until 2 h post dose (10.00 h). The first treatment for each subject was intravenous BDP with the other six treatments administered in a randomized sequence. The treatments were: (i) a single intravenous dose of BDP (1000 µg, administered as a 10 minute infusion via a syringe pump), (ii) a single intranasal dose of BDP via the aqueous nasal spray (1344 µg, administered as 16 sprays to each nostril, with a 2 min interval between each spray), (iii) a single inhaled dose of BDP (1000 µg via a metered dose inhaler administered as 20 actuations, with a 2 min interval between each), and (iv) a single oral dose of BDP (4000 µg dispensed as 9.7 ml of the aqueous nasal spray suspension and administered into the mouth via a syringe). The intranasal, inhaled and oral treatment periods (treatments v, vi and vii) were repeated with the co-administration of activated charcoal, to block the oral absorption of swallowed drug. A suspension of 5 g activated charcoal in 50 ml water was given 2 min before and 2, 62, 122 and 242 min after each BDP treatment. In the case of the inhaled and the oral treatments, the subjects rinsed their mouths immediately after dosing and prior to the second charcoal administration.
Blood sampling
Blood samples (5.5 ml) were obtained following cannulation of a forearm vein at the following times: predose and 10, 20, 30, 45 min and 1, 1.5, 2, 4, 6, 8, 12, 18, and 24 h postdose (relative to the start of the drug administration). In the case of the intravenous dosing period, the 10 min blood sample was taken just before the end of the infusion. Blood was drawn into potassium oxalate/sodium fluoride tubes in order to inhibit further esterase hydrolysis, mixed immediately and placed on ice. Within 15 min, the sample was centrifuged at 1000 g for 15 min in a refrigerated centrifuge. The plasma samples were stored at −70 °C prior to determination of BDP, B-17-MP and BOH. The sample handling and storage procedures were shown to prevent the hydrolysis of BDP and its metabolites.
Bioanalysis
The analysis of plasma samples for BDP, B-17-MP and BOH was performed using LC-MS-MS. The plasma samples were mixed thoroughly and centrifuged prior to solid phase extraction (SPE) on C18 50 mg Microlute II 96 well blocks. The procedure was fully automated using a custom built Zymark robotic 96 well solid phase extraction system. The resulting extracts were evaporated to dryness under a stream of heated nitrogen (nominally 40 °C) and reconstituted with 75 µl of 40/60 (v/v) acetonitrile/25 mm ammonium formate pH 5 Buffer. The method employed a 150 × 2.1 mm i.d. RPC8 5 µm Symmetry Shield (Waters Corps.) analytical column and a Perkin Elmer API-3000 Mass Spectrometer. To provide adequate separation and short run times, a 6 min gradient elution profile was used with a flow rate of 0.3 ml min−1 and no splitting (Solvent A: 25 mm ammonium formate pH 5.0 and B:100% acetonitrile – start 55% A and 35% B, end 10% A and 90% B). Protonated molecules, MH+, were used as precursor ions with selected reaction monitoring of the following transitions for BDP, B-MP-17, BOH, respectively: m/z 521→ 319, m/z 465→279 and m/z 409→279. The calibration range for this method was 50–3000 pg ml−1 from 0.6 ml plasma for BDP and B-17-MP and 50–1000 pg ml−1 for BOH. The coefficients of variation for the three analytes were as follows: BDP 7.9%, B-17-MP 5.9%, BOH 8.1%. The limit of quantification for each analyte was 50 pg ml−1.
Data analysis
Standard model independent methods were used to calculate pharmacokinetic parameters for each treatment and analyte using WinNonlin version 1.5. Where possible the following pharmacokinetic parameters were calculated: the area under the plasma concentration-time curve extrapolated to infinity (AUC(0,∞)) where the extrapolated AUC was estimated from Clast(predicted)/terminal elimination rate constant, the mean residence time (MRT), elimination half-life (t½) based on log-linear regression, nonextrapolated area under the plasma concentration-time curve (AUC(0,t)), maximum plasma concentration (Cmax) and the time to Cmax (tmax). Following intravenous treatment the plasma clearance (CL) and steady-state volume of distribution (Vss) were also estimated. Where indicated, the nominal dose was used to normalize AUC values for the intravenous and extravascular routes prior to estimation of the absolute bioavailability of BDP and B-17-MP. In performing this calculation the extravascular AUC values obtained following charcoal administration were corrected using the median efficiency of the charcoal block. Where an observed MRT was available for both the extravascular and intravenous dosing the mean absorption time (MAT) was estimated as MAT = MRT extravascular – MRT intravenous. MRTs were first corrected for the input time by subtraction of t/2 where (t = duration of dosing or infusion time).
Results
Intravenous pharmacokinetics of BDP,B-17-MP and BOH
The mean plasma concentration time data for BDP, B-17-MP and BOH following intravenous dosing are shown in Figure 2 and the derived pharmacokinetic parameters in Table 1. BDP was not extensively distributed to the tissues (Vss, 20 l) but was eliminated rapidly with a high clearance (150 l h−1) and short half-life (0.5 h). The disappearance of BDP was accompanied by the appearance of B-17-MP and after a delay of approximately 1.5 h transient BOH concentrations were detected in plasma. When plotting the mean BOH concentration data (Figure 2) values below the assay detection limit were set to zero. BOH was only detected at a few time points in most subjects with peak concentrations occurring approximately 4 h postdose, and therefore the pharmacokinetics of BOH (CL, Vss and t1/2) could not be reliably characterized. However, based on the mean concentration-time data, the elimination half-life of BOH appeared to be similar to that of B-17-MP. The most significant component in plasma was B-17-MP. Its elimination half-life (2.7 h) was longer than that found for BDP but its clearance (120 l h−1) was similar. In comparison with BDP, the tissue distribution of B-17-MP was extensive (Vss, 424 l). In calculating the clearance and Vss of B-17-MP, the assumption was made that all BDP was converted to B-17-MP. There is a small error associated with this, as some BDP is known to be converted to B-21-MP. However, this is a very minor pathway [12]. The analysis of B-21-MP in the plasma samples was attempted (detection limit 50 pg ml−1) but it could not be quantified even following intravenous dosing. To correct for the molecular weight difference between BDP (mol. wt. 521) and B-17-MP (mol. wt. 465) a dose of 875 µg was assumed for the latter. In agreement with the larger Vss for B-17-MP, its MRTiv (3.5 h) was longer than that of BDP (0.1 h). The MRTiv values were also used, where indicated, to calculate the MAT for the other routes of administration (Tables 2 and 3).
Figure 2.
The mean ± s.e. mean log plasma concentration time profiles for BDP (□), B-17-MP (○) and BOH (▵) following intravenous administration of 1000 µg of BDP as 10 min infusion.
Table 1.
Pharmacokinetic parameters for BDP, B-17-MP and BOH following intravenous administration of BDP.
| Analyte | AUC(0,∞) (pg ml−1 h) | Cmax (pg ml−1) | tmax (h) | CL (l h−1) | Vss (l) | MRT (h) | t½ (h) |
|---|---|---|---|---|---|---|---|
| BDP | 6660 | 35356 | 0.2* | 150 | 20 | 0.1 | 0.5* |
| (4385–10117) | (20244–61748) | (0.2–0.3) | (98.8–228) | (10.6–39.4) | (0.1–0.2) | (0.3–0.7) | |
| B-17-MP | 6185 | 2633 | 0.2* | 120 | 424 | 3.5 | 2.7* |
| (5361–7135) | (2158–3211) | (0.2–0.8) | (104–139) | (362–496) | (3.0–4.1) | (1.3–5.3) | |
| BOH | 126*# | 57.2* | – | – | – | – | |
| (0–557) | (0–113) |
Values are geometric mean (95% CI) except values denoted with
which are median (range).
Denotes AUC(0,t).
Table 2.
Pharmacokinetic parameters for B-17-MP following administration of BDP orally and intranasally with and without activated charcoal.
| Group | AUC (pg ml−1 h) | Cmax (pg ml−1) | tmax (h) | t1/2 (h) | MRT (h) | MAT (h) |
|---|---|---|---|---|---|---|
| Oral | 10158 | 703 | 4.0* | 8.8* | 15.7 | 11.7 |
| (6757–15270) | (563–879) | (1.5–6.0) | (4.9–47.7) | (11.1–22.0) | (7.8–17.5) | |
| Oral with charcoal | 231*# | 99.9* | 1.5* | – | – | – |
| (0–2159) | (0–361) | (0.3–6.0) | ||||
| Intranasal | 3660 | 310 | 4.0* | 5.7* | 12.4 | 7.6 |
| (2220–6036) | (256–375) | (4.0–8.0) | (2.6–109.5) | (7.1–21.8) | (3.7–15.8) | |
| Intranasal with charcoal | 89.4# | 66.8* | 1.5* | – | – | – |
| (0–1799) | (0–251) | (1.0–6.0) |
Values are geometric mean (95% CI) except values denoted with
which are median (range).
Denotes AUC(0,t).
Table 3.
Pharmacokinetic parameters for BDP and B-17-MP following administration of BDP via the inhaled route with and without activated charcoal.
| Group | Analyte | AUC (pg ml−1 h) | Cmax (pg ml−1) | tmax (h) | t1/2 (h) | MRT (h) | MAT (h) |
|---|---|---|---|---|---|---|---|
| Inhaled | BDP | 151 | 319 | 0.3* | – | – | – |
| (84.6–269) | (184–553) | (0.2–0.5) | |||||
| B-17-MP | 3851 | 944 | 1.0* | 2.7* | 4.1 | 0.6 | |
| (2831–5238) | (671–1327) | (0.8–6.0) | (2.1–3.6) | (3.5–4.6) | (−2.1–1.8) | ||
| Inhaled | BDP | 217# | 459 | 0.5* | – | – | – |
| with charcoal | (134–353) | (294–716) | (0.2–0.5) | ||||
| B-17-MP | 2383 | 705 | 0.8* | 2.3* | 3.5 | −0.2 | |
| (1541–3686) | (436–1141) | (0.8–1.0) | (1.7–5.8) | (3.0–4.0) | (−2.2–2.3) |
Values are geometric mean (95% CI) except values denoted with
which are median (range).
Denotes AUC(0,t).
Oral bioavailability of BDP and B-17-MP
Following oral administration of BDP, either with or without activated charcoal, no unchanged BDP was detected in the plasma. The mean plasma concentration-time data for B-17-MP following oral dosing are shown in Figure 3 and the derived pharmacokinetic parameters in Table 2. The rate of appearance of B-17-MP in plasma was slow (tmax, 4 h) as was its elimination (t1/2, 8.8 h) compared with intravenous dosing. The MRToral for B-17-MP was also longer (15.7 h) than the MRTiv which resulted in an oral MAT of 11.7 h. The total oral bioavailability of B-17-MP, estimated as the geometric mean ratio for the dose normalized (AUCoral/AUCiv).100 and (90% confidence intervals), was 41% (31–54%) (Table 4). The use of the charcoal block procedure resulted in a very low plasma AUC for B-17-MP (Table 2). By comparing this value with the plasma AUC for oral dosing without charcoal for each subject, the median (range) efficiency of the charcoal block method was estimated as 96% (82–100%).
Figure 3.
The mean ± s.e. mean plasma concentration-time profiles for B-17-MP following oral administration of 4000 µg of BDP as an aqueous suspension, with (□) and without (○) the oral co-administration of activated charcoal. Unchanged BDP was not detected.
Table 4.
Relative systemic exposure and absolute bioavailability of BDP, B-17-MP and calculated total BOH following administration of BDP intravenously and via the oral, intranasal and inhaled routes with and without activated charcoal.
| AUC normalized to a 1000 µg dose | Bioavailability relative to intravenous2 | ||||||
|---|---|---|---|---|---|---|---|
| Route | Analyte1 | No charcoal AUC (pg ml−1 h) | With charcoal AUC (pg ml−1 h) | Total (%F) | Oral (%F) | Lung (%F) | Nose (%F) |
| Intravenous | BDP | 6660 | – | 100 | – | – | – |
| Inhaled | BDP | 151 | 217 | 2 | 0 | 2 | – |
| Intravenous | B-17-MP | 6185 | – | 100 | – | – | – |
| Oral | B-17-MP | 2540 | 58 | 41 | 41 | – | – |
| Intranasal | B-17-MP | 2723 | 67 | 44 | 43 | – | < 1 |
| Inhaled | B-17-MP | 3851 | 2383 | 62 | 26 | 36 | – |
| Intravenous | Total BOH | 10138 | – | 100 | – | – | – |
| Oral | Total BOH | 2132 | 49 | 21 | 21 | – | – |
| Intranasal | Total BOH | 2287 | 56 | 23 | 22 | < 1 | |
| Inhaled | Total BOH | 3343 | 2158 | 33 | 13 | 20 | |
Total BOH is the sum of the measured BDP, B-17-MP and BOH AUC values expressed as BOH equivalents.
Values corrected for charcoal block efficiency.
Nasal bioavailability of BDP and B-17-MP
In the pilot study only 2/6 subjects had detectable plasma concentrations of B-17-MP for the 336 µg dose whereas all subjects had detectable plasma concentrations at both the 672 µg and 1344 µg dose levels. However, only the 1344 µg dose allowed an adequate estimation of the plasma AUC. Intranasal administration of BDP did not result in detectable concentrations of unchanged BDP in the plasma. The mean plasma concentration-time data for B-17-MP following intranasal dosing, with and without activated charcoal, are shown in Figure 4 and the derived pharmacokinetic parameters in Table 2. The plasma profile following intranasal dosing was similar to that seen following oral dosing (Figure 3). The rate of appearance of B-17-MP in plasma was slow (tmax, 4 h) and B-17MP was eliminated more slowly (t1/2, 5.7 h) than following intravenous dosing. The MRTnasal for B-17-MP was 12.4 h, which resulted in an apparent intranasal MAT of 7.6 h. The total intranasal bioavailability of B-17-MP, estimated as the geometric mean ratio for the dose normalized (AUCnasal/AUCiv).100 and (90% confidence intervals), was 44% (34–58%) (Table 4). This value included both the absorption directly from the nose and the absorbed portion of the swallowed fraction. Blocking the oral component with the charcoal block procedure resulted in very low plasma concentrations of B-17-MP (Table 2). On correcting these data for the efficiency of the charcoal block, the nasal bioavailability was estimated to be < 1% (Table 4).
Figure 4.
The mean ± s.e. mean plasma concentration time profiles for B-17-MP following intranasal administration of 1344 µg of BDP as an aqueous nasal spray, with (□) and without (○) the oral co-administration of activated charcoal. Unchanged BDP was not detected.
Inhaled bioavailability of BDP and B-17-MP
Administration of BDP by inhalation produced detectable concentrations of both unchanged BDP and B-17-MP in the plasma. The mean plasma concentration-time data for BDP and B-17-MP following inhaled dosing, with and without activated charcoal, are shown in Figures 5 and 6, respectively, and the corresponding pharmacokinetic parameters are reported in Table 3. BDP was absorbed and eliminated rapidly. However, it was not possible to conclude whether the elimination half-life was different from that found following intravenous dosing as insufficient data were available for its estimation (Figure 5). B-17-MP did not appear as rapidly in the plasma compared to intravenous dosing. The inhaled bioavailability of BDP, estimated as the geometric mean ratio for the dose normalized (AUCinhaled/AUCiv).100 and (90% confidence intervals), was only 2% (1–4%) (Table 4). The charcoal block procedure did not significantly reduce the bioavailability of BDP which confirmed the lack of oral absorption of unchanged BDP (Tables 3 and 4). The total inhaled bioavailability of B-17-MP, estimated as the geometric mean ratio for the dose normalized (AUCinhaled/AUCiv).100 and (90% confidence intervals), was only 62% (47–82%) (Table 4). This value included both the absorption directly from the lungs and the absorbed portion of the swallowed fraction. Following correction for the efficiency of the charcoal block the absolute bioavailability of inhaled B-17-MP was 36% (27–47%) (Table 4). Therefore, the lung and gut were assumed to contribute 36% and 26% to systemic exposure, respectively.
Figure 5.
The mean ± s.e. mean plasma concentration-time profiles for BDP following inhaled administration of 1000 µg nominal dose of BDP via a metered-dose inhaler, with (□) and without (○) the oral coadministration of activated charcoal.
Figure 6.
The mean ± s.e. mean plasma concentration-time profiles for B-17-MP following inhaled administration of 1000 µg nominal dose of BDP via a metered-dose inhaler, with (□) and without (○) the oral coadministration of activated charcoal.
Discussion
The high clearances of BDP and B-17-MP indicate extensive extra-hepatic metabolism, which is in agreement with their wide tissue distribution the major route of elimination being mediated by high capacity esterases [2–4]. It is also apparent that B-17-MP clearance is elimination rate limited whereas BOH demonstrated formation rate limited kinetics. Since both BDP and B-17-MP are lipophilic molecules, a large Vss was expected. Although this was found for B-17-MP, the value for BDP was considerably smaller. The plasma protein binding (87%) is not high enough to limit tissue the distribution of BDP [5], but one factor that may have influenced Vss is rapid metabolism in the blood and well perfused tissues, resulting in the near complete elimination of BDP before maximal tissue distribution is achieved. Therefore, the apparent Vss for BDP is dominated by the initial rapid elimination phase, leading to smaller value than expected.
The absence of detectable concentrations of BDP in the plasma following oral administration is predictable from its very high clearance (Table 1), which would be expected to result in a high first pass metabolism. However, despite B-17-MP having a similarly high clearance (Table 1), its oral bioavailability was high (Table 4). It is possible that systemic rather than first pass metabolism predominated for B-17-MP elimination, whereas gut and hepatic metabolism was greater for BDP. An alternative explanation for this finding is that the oral bioavailability of BDP was also limited by dissolution or absorption rate limited kinetics. Evidence for this comes from the longer elimination half-life and greater MRT for B-17-MP after oral dosing (Table 2) compared with intravenous dosing (Table 1). This was taken as evidence of dissolution or absorption rate limited kinetics. Previous attempts to measure the oral bioavailability of BDP did not use either an intravenous reference dose or a specific assay method, but relied on the conversion of BDP and B-17-MP to BOH prior to measurement of total BOH. This approach, which is discussed further below, appeared to underestimate the bioavailability of the oral route [13].
The pilot intranasal study demonstrated that although B-17-MP was detectable following the standard daily dose, the assay method was not sensitive enough to detect systemic absorption in all subjects. The charcoal block method showed that essentially all the systemic exposure came from the swallowed fraction resulting in a similar total bioavailability to that found after oral dosing with the same formulation. Furthermore, since charcoal administration blocked most of the systemic exposure via this route it is clear that the systemic exposure is not reflective of nasal absorption but oral absorption. The estimate of < 1% absorption directly from the nose was similar to estimates for intranasal fluticasone propionate, a corticosteroid with negligible oral bioavailability [14]. In both cases it is likely that nasal absorption from an aqueous suspension is limited by the short time available for dissolution of the poorly soluble compounds in the nose prior to clearance by the nasal cilia [15].
In contrast to the other extravascular routes of administration, unchanged BDP was detected in the plasma after inhalation. Although BOH was not assayed in plasma in this part of the study, previous studies have not been able to quantify BOH in plasma following an inhaled dose [9–11]. Prior to the present work we had hypothesized that if BDP lacked significant oral absorption, then the measurement of unchanged BDP in the plasma may provide a method of assessing the lung deposition of inhaled BDP. However, it is apparent that the low bioavailability of inhaled BDP resulted from extensive (95%) presystemic lung metabolism of BDP prior to absorption, thereby invalidating this approach.
In contrast to the data from oral dosing, there was no evidence of absorption limited kinetics following inhalation. For B-17-MP the elimination half-life agreed with that found following intravenous dosing and the MATs of BDP and B-17-MP were short suggesting that pulmonary absorption was not a rate limiting factor. A desirable property for a topically acting inhaled steroid is prolonged residence at the site of action [5]. However, BDP appears to be similar to budesonide [5] and does not possess this feature. This is in contrast to fluticasone propionate, whose pulmonary MAT is approximately 5 h [5].
In the present study the pulmonary bioavailability of B-17-MP was estimated as 36%. Assuming the remaining dose is swallowed the 26% exposure via the gut is consistent with an oral bioavailability of approximately 40% for B-17-MP. These estimates are based on nominal doses and assume negligible loss of drug on the actuator with no exhalation of the dose. The 36% bioavailability of B-17-MP is considered to be a consequence of the lung deposition of BDP itself since its elimination from the lung requires conversion to B-17-MP. Therefore, following inhaled dosing with activated charcoal, the plasma AUC of B-17-MP is considered to be a reasonable measure of total lung deposition, assuming conversion to B-21-MP and BOH is negligible [12]. The pulmonary bioavailability of BDP is at the high end of the range of values reported for other MDIs [5] However, interindividual variability in the pharmacokinetics after inhalation is generally large, which the present findings confirmed. Therefore, considering the other factors that can contribute to the variability in a particular study, such as type of study subject (patients or healthy subjects), inhaler technique, dose regimen and assay methodology, the value obtained seems to be a reasonable estimate. Previous attempts to assess the lung deposition of BDP from an MDI used a combination of gamma scintigraphy with 99mTc-labelled BDP formulations and pharmacokinetic data derived from a nonspecific assay method that measured total BOH in plasma after the hydrolysis of the plasma samples [16–18]. A nonuniform 99mTc labelling of the BDP particles and presystemic metabolism of BDP probably account for the low estimate (5%) of BDP lung deposition by this method [17].
Previous studies have assessed the pharmacokinetics of BDP in terms of total BOH. This was done to simplify the methodology, and was achieved by the hydrolysis of BDP and its metabolites to BOH in the plasma samples prior to analysis [13, 16, 18]. For comparative purposes, we calculated the total BOH AUC for our data by converting the BDP, B-17-MP and BOH AUC values to BOH equivalents and summing them. This approach produced a lower estimate for oral (21%) and lung bioavailability (20%) compared with calculations based on B-17-MP. However, in calculating oral and pulmonary bioavailability from total BOH, a major assumption is made, that the clearance of total BOH is independent of the route of administration. It is unlikely that BDP, B-17-MP and BOH have the same clearance. Therefore the observed clearance of total BOH depends on the relative proportions of the BDP, B-17-MP and BOH in plasma which differ for intravenous, oral and inhaled administration. Moreover, the use of total BOH data for pharmacokinetic and mass balance calculations is also flawed, as some BDP-related material is counted more than once (as BDP and again after conversion to B-17-MP and BOH). Pharmacokinetic calculations based on total BOH are therefore not reliable.
There are concerns about the potential of corticosteroids to produce unwanted systemic effects on the hypothalamic-pituitary-adrenal axis, on bone metabolism and on growth rates in children. Although, there is some evidence of significant systemic effects with inhaled BDP when used at higher doses [19], its rapid systemic clearance probably contributes to its safety profile. Drug delivery via inhalation has significant advantages over systemic delivery for the treatment of lung diseases. Despite this, inhaled delivery is known to produce significant systemic exposure to corticosteroids via pulmonary absorption [5]. However, the extent to which oral absorption contributes to unwanted systemic exposure varies from < 1% for fluticasone propionate [20] to 23% for triamcinolone acetonide [21]. The present study confirmed that inhaled BDP has a high systemic contribution from the swallowed fraction.
A high degree of pulmonary presystemic metabolism for BDP is essential for its topical activity in the lung [2]. This factor needs to be taken into consideration in the development of new chlorofluorocarbon-free (CFC-free) BDP MDIs, containing solutions of drug in propellant which generate ultrafine aerosols [10, 16]. The reported increase in peripheral lung deposition [17] and the expected increase in absorption rate, results in a fourfold to fivefold greater absorption of unchanged BDP after inhalation of CFC-free BDP [10]. This may partially offset the greater bioavailablity claimed for CFC-free BDP formulations [17], as the exposure of the lung to the active metabolite B-17-MP may not increase in line with the greater lung deposition of BDP. Therefore, in comparing the CFC and CFC-free formulations of BDP, it may not be possible to relate the differences in B-17-MP systemic exposure to potential changes in clinical efficacy.
This study has also generated the first pharmacokinetic data following intranasal administration of BDP. Negligible systemic exposure following intranasal dosing has been demonstrated for fluticasone propionate and mometasone furoate [22]. However, other intranasal corticosteroids such as budesonide [23] and triamcinolone acetonide [24] produce high systemic exposure. The finding that relatively low doses of intranasal corticosteroids such as BDP can be associated with detectable effects on the HPA axis [25] is explained by the high intranasal bioavailability of B-17-MP found in the present study. In contrast, intranasal corticosteroids, such as fluticasone propionate and mometasone furoate, with low systemic bioavailability do not produce detectable effects on cortisol even when administered at high doses [22, 26, 27].
In conclusion, we have shown that BDP and B-17-MP are rapidly eliminated following intravenous administration. Unchanged BDP has negligible oral and intranasal bioavailability with limited absorption, following inhaled dosing, which is due to extensive (95%) presystemic conversion of BDP to B-17-MP in the lung. The oral and intranasal bioavailabilities of the active metabolite B-17-MP were high and similar (≈40%), but direct absorption in the nose was insignificant. The total inhaled bioavailability of B-17-MP (lung + oral) was also high (62%) of which approximately 36% was due to pulmonary absorption. However, estimates of oral bioavailability and pulmonary deposition based on total BOH were approximately half those found for B-17-MP.
Acknowledgments
We would like to thank the staff of the Northwick Park Hospital Clinical Pharmacology Unit for their dedication during the clinical phase of the study and Rodger Barnett, Pharmaceutical Sciences, GlaxoWellcome for co-ordinating the development of the intravenous formulation of BDP.
References
- 1.Rohdewald P, Mollmann HW, Hochhaus G. Affinities of glucocorticoids for glucocorticoid receptors in human lung. Agents Actions. 1985;17:3–4. doi: 10.1007/BF01982622. [DOI] [PubMed] [Google Scholar]
- 2.Wurthwein G, Rohdewald P. Activation of beclomethasone dipropionate by hydrolysis to beclomethasone-17-monopropionate. Biopharm Drug Dispos. 1990;11:381–394. doi: 10.1002/bdd.2510110503. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P, Ryrfeldt A. Biotransformation of topical glucocorticoids, budesonide and beclomethasone 17, 21-dipropionate in human liver and lung homogenate. J Pharm Pharmacol. 1984;36:763–765. doi: 10.1111/j.2042-7158.1984.tb04868.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin LE, Tanner RJN, Clark TJH, Cochrane GM. Absorption and metabolism of orally administered beclomethasone dipropionate. Clin Pharmacol Ther. 1974;15:267–275. doi: 10.1002/cpt1974153267. [DOI] [PubMed] [Google Scholar]
- 5.Derendorf H. Pharmacokinetic and pharmacodynamic properties of inhaled corticosteroids in relation to efficacy and safety. Resp Med. 1997;91:22–28. doi: 10.1016/s0954-6111(97)90102-5. [DOI] [PubMed] [Google Scholar]
- 6.Trescoli-Serrano C, Ward MJ, Rajput R, Garcia-Zorcaco M. Does swallowing charcoal affect the gastrointestinal absorption of inhaled beclomethasone. Eur Respir J. 1995;8:S304. [Google Scholar]
- 7.Borgstrom L, Nillsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–1070. doi: 10.1023/a:1015951402799. [DOI] [PubMed] [Google Scholar]
- 8.Ward JK, Dow J, Dallow N, Eynott P, Melleri S, Ventresca GP. Enantiomeric disposition of inhaled, intravenous and oral racemic-salbutamol in man – no evidence of enantioselective lung metabolism. Br J Clin Pharmacol. 2000;49:15–22. doi: 10.1046/j.1365-2125.2000.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorsson L, Edsbacker S, Conradson TB. Lung deposition of busesonide from the turbuhaler is twice that from a pressurised metered dose inhaler. Eur Resp J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 10.Daley-Yates PT, Baggen S, Tournant J, Pereira A. Beclomethasone dipropionate chlorofluorocarbon and hydrofluoroalkane metered dose inhalers: relationship between systemic exposure, dose, fine particle mass and particle size in healthy volunteers. Eur Respir J. 1999;14:P1358. [Google Scholar]
- 11.Rohdewald P, Rehder S. Plasma levels of beclomethasone dipropionate (BDP) and its metabolite (17-BMP) following BDP inhalation. Eur Respir J. 1994;7:328S. [Google Scholar]
- 12.Falcoz C, Kirby SM, Smith J, Olsson P, Ventresca P. Pharmacokinetics and systemic exposure of inhaled beclomethasone dipropionate. Eur Respir J. 1996;9:162S. [Google Scholar]
- 13.Soria I, Harrison LI, Machacek JH, Cline AC, Stampone PA. Beclomethasone relative availability of oral versus inhaled beclomethasone dipropionate from an HFA-134A metered dose inhaler. Biopharm Drug Dispos. 1998;19:297–302. doi: 10.1002/(sici)1099-081x(199807)19:5<297::aid-bdd105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Daley-Yates PT, McAllister A. Systemic bioavailability of fluticasone propionate administered as nasal drops and aqueous nasal spray formulations. Br J Clin Pharmacol. 2001;51:103–105. doi: 10.1046/j.0306-5251.2001.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant ML, Brown P, Gurevich N, McDougall IR. Comparison of the clearance of radiolabelled nose drops and nasal spray as mucosally delivered vaccine. Nuclear Med Commun. 1999;20:171–174. doi: 10.1097/00006231-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Harrison LI, Soria I, Cline AC, Ekholm BP. Pharmacokinetic differences between chlorofluorocarbon and chlorofluorocarbon-free metered dose inhalers of beclomethasone dipropionate in adult asthmatics. J Pharm Pharmacol. 1999;51:1235–1240. doi: 10.1211/0022357991776967. [DOI] [PubMed] [Google Scholar]
- 17.Leach C. Enhanced drug delivery through reformulating MDIs with HFA propellants- drug deposition and its effect on preclinical and clinical programs. In: Dalby RN, Byron PR, Farr SJ, editors. Respiratory Drug Delivery V, Proceedings. Inter Pharm Press; 1996. pp. 133–144. ISBN 1-57491-018-3. [Google Scholar]
- 18.Harrison LI, Dahl DR, Cline AC, et al. Pharmacokinetics and dose proportionality of beclomethasone from three strengths of a CFC-free beclomethasone dipropionate metered-dose inhaler. Biopharm Drug Dispos. 1997;18:635–643. doi: 10.1002/(sici)1099-081x(199710)18:7<635::aid-bdd53>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Dahl R. Beclomethasone dipropionate and budesonide: the clinical evidence reviewed. Resp Med. 1989;92(Suppl B):1–45. [Google Scholar]
- 20.Falcoz C, Mackie A, McDowall J. Oral bioavailability of fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:459–460. doi: 10.1046/j.1365-2125.1996.36110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derendorf H, Hochhaus G, Rohatagi S. Pharmacokinetics of triamcinolone acetonide after intravenous, oral and inhaled administration. J Clin Pharmacol. 1995;35:302–305. doi: 10.1002/j.1552-4604.1995.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 22.Daley-Yates PT, Kunka RL, Shen YY, Andrews SM, Callejas S, Ng C. The relative systemic exposure to fluticasone propionate and mometasone furoate administered as aqueous nasal sprays in healthy subjects. J Allergy Clin Immunol. 2000;105:S201. [Google Scholar]
- 23.Thorsson L, Borga O, Edsbacker S. Systemic availability of budesonide after nasal administration of three different formulations; pressurized aerosol, aqueous spray pump and powder. Br J Clin Pharmacol. 1999;47:619–624. doi: 10.1046/j.1365-2125.1999.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeal W, Faulds D. Triamcinolone acetonide a review of its pharmacological properties and therapeutic efficacy in the management of allergic rhinitis. Drugs. 1997;53:257–280. doi: 10.2165/00003495-199753020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Wihl JA, Anderson KE, Johansson SA. Systemic effects of two intranasally administered glucocorticoids. Allergy. 1997;52:620–626. doi: 10.1111/j.1398-9995.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 26.McDowell JE, Mackie AE, Ventresca GP, Bye A. Pharmacokinetics and bioavailability of intranasal fluticasone in humans. Clin Drug Invest. 1997;1:44–52. [Google Scholar]
- 27.Price AC, Daley-Yates PT, Wright AM, Callejas S. Negligible absolute bioavailability and no HPA-axis effects after multiple 200µg daily doses of fluticasone propionate (FP) administered from the aqueous nasal spray (FPANS) Eur Resp J. 2000;16:279s. [Google Scholar]
- 28.Wurthwein G, Rehder S, Rohdewald P. Lipophilicity and receptor affinity of glucocorticoids. Pharm Zgt Wiss. 1992;137:161–167. [Google Scholar]