Abstract
Aims
To investigate the effects of age and disease states on the expression and activity of intestinal CYP3A4 in a paediatric population.
Methods
Duodenal biopsies and surgical sections were collected from 104 paediatric patients (age range 2 weeks to 17 years) and from 11 foetuses. An S9 fraction was prepared in each case. CYP3A4 expression was assessed by Western blotting and by immunohistochemistry; activity was measured by the rate of formation of 6β-hydroxytestosterone from testosterone. Villin expression was used as a marker of enterocyte harvest to normalize CYP3A4 expression and activity data.
Results
In the 74 histologically normal paediatric biopsies there were statistically significant increases in CYP3A4 expression (r2 = 0.19, P = 0.001) and activity (r2 = 0.17, P = 0.02) with age. CYP3A4 was practically absent in fetal duodenum and was expressed at relatively low levels in neonates (P < 0.05 between neonates and children > 5 years). Active coeliac disease resulted in significant (P < 0.001) decreases in CYP3A4 expression and activity.
Conclusions
Duodenal CYP3A4 is present at significantly lower levels in neonates and in patients with active coeliac disease. This may have clinical significance with respect to the oral bioavailability of CYP3A4 substrates.
Keywords: children, coeliac disease, CYP3A4, cystic fibrosis, duodenum, fetus, intestine, paediatrics
Introduction
Enzymes of the cytochrome P450 3A subfamily are abundant in liver and gut and contribute to the first-pass metabolism of many orally administered drugs [1, 2]. In humans CYP3A4 is the predominant form of cytochrome P450 both in the liver and the small bowel. Within the intestine it is expressed in the villous tip of the enterocyte, and exhibits a gradient from the duodenum to the colon [3, 4]. Together with the efflux transporter, P-glycoprotein, intestinal CYP3A4 is considered to provide a protective barrier to the ingestion of dietary xenobiotics and to modulate the oral bioavailability of many drugs [5, 6]. In consequence, intestinal CYP3A4 is also an important site of drug-drug [7, 8] and drug-food [9] interactions. Apart from the metabolism of drug substrates, intestinal CYP3A is also involved in the breakdown of dietary compounds of toxicological significance, such as aflatoxin B1 [10] and 2-amino-3,4-dimethylimidazole[4,5-F]-quinoline (MeIQ) a mutagen found especially in cooked red meat [11]. Thus, the enzyme helps to modulate the systemic exposure to these substances and their active metabolites as well as their transit to the colon. An increased incidence of lymphomas and adenocarcinomas associated with coeliac disease [12] may be mediated in part by the activity of intestinal cytochromes including CYP3A4 and CYP1A1 [13, 14].
Previously, we have documented significant differences in the expression and activity of enterocytic and hepatic forms of the rat orthologues of CYP3A4 as a function of age and sex [15]. Enterocytic, but not hepatic, CYP3A shows a marked surge at weaning in the rat and, unlike hepatic CYP3A, there is no marked sex difference in its developmental expression and activity. We now extend these studies to the development of enterocytic CYP3A4 in neonates and children. While the ontogeny of hepatic CYP3A4 has been studied, relatively little is known about the development of the enzyme in the human intestine [16]. However, of note is the observation that mRNA for CYP3A7, the main fetal hepatic form of cytochrome P450 [17], has been detected in low abundance relative to CYP3A4 in adult human liver [18] but has not been found in adult small intestine [19, 20].
A secondary aim of this study was to investigate the effects of disease of the small bowel on the expression and activity of CYP3A4 in paediatric patients. The enteropathy associated with coelic disease would be expected to be associated with a significant decrease in the presence of CYP3A4, while an increase might be anticipated in cystic fibrosis as a consequence of villous elongation.
The study was carried out using samples of small bowel obtained by biopsy or during surgery. CYP3A4 content was determined by immunostaining of sections and Western blotting of S9 fractions. The activity of the enzyme was assessed by the formation of 6β–hydroxy testosterone from testosterone in S9 fractions.
Methods
Patient samples
Two duodenal biopsies from adjacent areas were collected after informed written consent (South Sheffield Ethics Committee, Study Number 96/144) from each of 103 paediatric patients undergoing endoscopy or surgery for diagnostic or therapeutic purposes (male/female = 60/43; age range = 2 weeks to 17 years). The biopsies were collected and processed blind to the clinical diagnosis and other factors, such as concurrent drug therapy, likely to influence CYP3A4 expression. This information was obtained subsequently from the patient notes. The small intestinal tissue was found to be histologically normal in 74 patients, 14 had active coeliac disease, and 15 were treated coeliac patients with histologically normal biopsies.
Tissue samples were obtained at surgery from six neonates (0–3 months) from the margins of repair to the duodenum and proximal jejunum.
Two further proximal small bowel S9 fractions were prepared, one from a 50-year-old adult male patient undergoing surgery for intestinal obstruction and another from a 4-year-old female patient undergoing surgery for closure of a stoma. Both sections were confirmed as duodenal/proximal jejunal sections and were histologically normal. Both of these S9 fractions were used as standards in the villin Western blot assay and the adult S9 fraction was used in the enzyme kinetic studies.
Proximal small bowel sections from 11 fetuses (mean gestational age 13 weeks, range 9–15 weeks) were a generous gift from Dr Sylvia Pender (Department of Paediatric Gastroenterology, St Bartholomew's Hospital, London). These had been snap-frozen in liquid nitrogen and stored at −80° C.
A further set of duodenal and proximal jejunal biopsy samples were obtained for diagnostic purposes from the following paediatric patients and used for the immunostaining experiments:
nine patients with coeliac disease (male/female = 3/6; aged 1–16 years, mean 5.5. years). Biopsies were obtained from all patients on diagnosis and after a gluten-free diet, and in 6 patients on gluten re-challenge.
18 patients with cystic fibrosis (male/female = 8/10; aged 1–16 years, mean 6.5 years).
18 patients with histologically normal biopsies and no significant medical history (male/female = 9/9; aged 1–15 years, mean 6 years).
10 patients with a histological assessment of partial villous atrophy (male/female = 7/11; aged 0.5–13 years, mean 4 years). This group comprised of infants with milk protein intolerance and older children with a diagnosis of coeliac disease who were either only partially compliant with a gluten-free diet or whose biopsies were judged to have been taken too soon on re-challenge.
None of the patients included in this study was taking significant CYP3A inhibitors or inducers.
Materials
Goat antirat polyclonal cytochrome P450 3A2 antibody and microsomes from an AHH-1 TK ± human lymphoblastoid cell line expressing CYP3A4 were purchased from Cambridge Biosciences (Cambridge, UK). The goat antibody recognizes human CYPs 3A4, 3A5 and 3A7, and has been validated for assessing CYP3A content of human tissues (Gentest Corporation, Woburn, MA, USA). Normal rabbit serum, rabbit antigoat biotinylated antibody and streptavidin were obtained from Dako (Patts, Denmark), villin mouse monoclonal antibody from The Binding Site (Birmingham, UK), trypsin from ICN Biochemicals (Ohio,USA), alkaline phosphatase conjugated goat antimouse antibody from PE Applied Biosystems (Warrington, UK), bovine serum albumin and BCA protein assay reagent from Pierce (Chester, UK), ethyl acetate, methanol and ethanol from Rathburn Chemicals (Walkerburn,UK), acrylamide solution (40% v/v) from Bio-Rad Micromeasurements (York, UK), Immobilon-P from Millipore (Watford, UK), and enhanced chemiluminescent reagents (Tropix) from Applied Biosystems (Warrington, UK). Other antibodies were purchased from Sigma (Poole, UK), as were other chemicals, which were of analytical or electrophoresis grade.
Preparation of S9 fractions
The biopsy and neonatal surgical samples were placed immediately into ice-cold buffered saline containing 5 mm EDTA, 0.5 mm dithiothreitol and 40 µg ml−1 phenylmethylsulphonyl fluoride (PMSF). Each sample was transferred to an homogenizer tube together with 100 µl extraction buffer containing 5 mm histidine, 0.25 m sucrose, 0.5 mm EDTA and 40 µg ml−1 PMSF. The fetal tissue samples were defrosted in a small volume of extraction buffer. The volume of extraction buffer added to fetal and neonatal tissue samples was adjusted according to the size of the gut section. In the case of larger adult duodenal sections used for enzyme kinetic studies, the enterocytes were harvested by scraping on ice using a glass microscope slide as described previously [21].
Tissue sections/enterocytes were homogenized using 10 up and 10 down strokes of a Potter Elvehjem homogenizer, transferred to 0.5 ml Eppendorf tubes and centrifuged at 10 000 g for 10 min at 4 °C. The supernatant (S9 fraction) was removed, 5 µl was taken for protein assay, and both supernatant and pellet stored at −80 °C.
Protein assay
S9 fraction (5 µl) was diluted 40-fold with water and protein was determined in triplicate using the Pierce BCA kit (Pierce, Chester, UK) which utilizes the biuret reaction and the formation of a purple complex between bicinchoninic acid and cuprous ions. The reaction was carried out in 96-well plates with a Titre Tek colourimetric platereader set at a wavelength of 550 nm and with output through Excel (Microsoft, Seattle, USA).
Western blotting
Western blots from S9 fractions were obtained after denaturation and reduction by boiling for 2 min in treatment buffer (62.5 mm Tris-HCl, 2% w/v SDS, 20% w/v glycerol and 10% v/v mercaptoethanol). A Flowgen G31010 electrophoresis unit (Flowgen, Lichfield, UK) was used with 10% SDS PAGE resolving and 2.7% stacking gels. Alternate lanes were loaded with 10 µl control buffer and others with 10 µg S9 protein or standard. End lanes were loaded with Novex Mark 12™ and Seeblue™ molecular weight markers. The unit was attached to a Biorad 500/200 power supply (Biorad, CA,USA), adjusted to 150 mV and run for 2 h. Transfer onto Immobilon-P membranes was performed using a CBS semidry blotter (EU-4000) (CBS Scientific Co, CA, USA) at 80 mA for 50 min. Blotting of all patient samples with the antirat CYP3A2 antibody revealed a single band at 50 KDa.
Quantification of CYP3A4
On each membrane three lanes were loaded with S9 fraction and three with dilutions of microsomes from a human lymphoblastoid cell line expressing CYP3A4 as standard. The membranes were probed with goat antirat CYP3A2 polyclonal antibody (1 : 20 000). Antibody binding was detected using an alkaline phosphatase conjugated rabbit antigoat immunoglobulin type G (1 : 20 000). The Tropix Western Light ™ chemiluminescent detection system was used for visualization, and the blots were recorded using Kodak X-OMAT film. Densitometric analysis was performed using a DC120 digital camera (Kodak) and the KDS 1d image analysis software. All blots were obtained in triplicate for each patient sample. A linear densitometric response to CYP3A4 standards was obtained up to 50 pmol mg−1 protein (r2 = 0.95–0.98; n = 4).
Quantification of villin
Villin is a constitutively expressed cytoskeletal protein that is enterocyte specific and which can be used to normalize the content of other proteins with respect to factors such as depth of biopsy and proteolysis [22]. The expression of villin in the tissue samples was determined relative to the average signal from the two standard S9 preparations loaded onto each membrane. The membranes were probed with a mouse monoclonal villin antibody (1 : 5000) which also recognizes human villin. Antibody binding was detected using an alkaline phosphatase conjugated goat antimouse immunoglobulin G (1 : 20 000). Densitometric analysis was performed as described for CYP3A4. Western blotting of the S9 fractions for villin revealed two distinct bands at 50 and 95 KDa. Because the sum of the intensities of the two bands in the standards was relatively constant across blots while individual band intensities varied relative to each other, the combined densitometric values for the two bands was used to quantify villin.
Determination of CYP3A4 activity
The 6β-hydroxylation of testosterone was used as a primary measure of CYP3A4 activity [23]. Selectivity for CYP3A4 was confirmed using the N-demethylation of dextromethorphan as a further probe for the enzyme [24], using S9 fractions from two normal biopsy samples.
Testosterone incubation procedure
The incubation mixture comprised 200 µl 1.15% w/v potassium chloride, 50 µl testosterone in 50% methanol/water to give a final concentration of 100 µm, 200 µl of a NADPH generating system (0.5 mm NADP, 5 mm glucose-6-phosphate, 5 mm MgCl2, 1 unit glucose 6-phosphate dehydrogenase ml−1), 100 µl S9 fraction (1 mg protein ml−1) and 450 µl 0.2 m potassium phosphate buffer, pH 7.4. Incubations were carried out in 15 ml uncapped glass tubes at 37 °C in a shaker water bath for 30 min. Reactions were started by the addition of S9 fraction and stopped by addition of ethyl acetate (2.0 ml) containing 12.5 µl of a 10 µg ml−1 solution of 11β-hydroxytestosterone as assay internal standard. For enzyme kinetic studies, using the S9 fraction from a sample of adult duodenum, the substrate concentration range was 0–250 µm.
Assay of 6β-OH testosterone
The incubate/ethyl acetate mixture was shaken for 20 min, centrifuged at 2000 g for 10 min and the organic phase removed and evaporated to dryness using a vortex evaporator (Buchler, USA). The residue was reconstituted in 200 µl mobile phase, of which 150 µl was injected on column. Chromatography (h.p.l.c.) was carried out as described previously (15). The limit of quantification of the assay was 30 pmol and the intra-and interassay coefficients of variation were 9.5 and 9.8%, respectively.
Dextromethorphan incubation procedure
The incubation mixture comprised 50 µl 1.15% w/v potassium chloride, 50 µl dextromethorphan solution 1.0 m (final concentration 200 µm), 50 µl NADPH generating system, 50 µl S9 fraction (5 mg protein ml−1) and 50 µl 0.2 m phosphate buffer, pH 7.4. Incubations were carried out in Eppendorf tubes at 37 °C in a shaker water bath for 20 min. Reactions were started by the addition of S9 fraction and stopped by addition of 25 µl 60% w/v perchloric acid. Laudenosine (25 µl of a 5 µg ml−1 solution) was added as assay internal standard.
Assay of 3-methoxymorphinan
After centrifugation, 70 µl of the supernatant from the incubation mixture was assayed by h.p.l.c. using a Spectroflow 400 solvent delivery system (Kratos Analytical Instruments, USA), a Waters manual injector (Waters Corp, USA), a Waters radial compression unit containing a Novapak CN column (4 µ) with a Novapak HP-CN guard column, a Schoeffel fluorimetric detector (Schoeffel Instrument Corp, USA) and a RE571.2 chart recorder (Venture Smith Industries, USA). Excitation and emission wavelengths of 280 nm and 310 nm, respectively, were used. The mobile phase was 90 : 10 water:acetonitrile with 0.03% triethylamine adjusted to pH 3 with orthophosphoric acid at a flow rate of 3 ml min−1. The retention times of laudenosine, 3-methoxymorphinan and dextromethorphan were 7, 12.7 and 14 min, respectively. The limit of quantification of the assay was 10 pmol mg−1 protein min−1 and the intra- and interassay coefficients of variation were 5.6%. and 8.9%, respectively.
Immunostaining
Sections of formalin-fixed, paraffin-embedded tissue were cut onto glass slides using a microtome (Leica, Germany) and dried at 37 °C. The paraffin wax was removed with xylene and decreasing concentrations of ethanol in water. The sections were then incubated for 30 min in methanol containing 1.7% v/v (100 vol) hydrogen peroxide solution to block any endogenous peroxidase activity. All subsequent operations were carried out in a humidified chamber, washing the sections with Tris buffer (pH 7.6) between each incubation. Incubation with 20% normal rabbit serum for 15 min was followed by 30 min with an optimal dilution of primary antibody (1 : 500 goat antirat CYP3A2 polyclonal), 30 min with an optimal dilution of secondary antibody (1 : 400 biotinylated rabbit antigoat) and 30 min with a 1 : 600 dilution of streptavidin-biotin-peroxidase complex. Peroxidase activity was visualized using a 0.035% w/v solution of 3,3-diaminobenzidine tetra-hydrochloride containing 0.3% v/v 100 volume hydrogen peroxide. The sections were then counter-stained with haematoxylin. Immunostaining was identified as a brown colour. Negative controls were prepared without adding the primary antibody, and liver sections were used as positive controls; these showed no and strong staining for CYP3A, respectively. Sections were viewed using a Leica DMIRB microscope (Leica, Germany), and images were generated using a Spot digital imaging system (Diagnostic Instruments Inc, USA). Three sections from each tissue sample were stained.
The amount of staining related to CYP3A4 was scored on a 0–4 scale by three independent observers. The observers disagreed by one full scale point for only three of the slides examined.
Data analysis
Statistical analysis was performed using SPSS version 6.0 (SPSS inc, USA).
Equation 1 was fitted to the data for 6β-hydroxy testostosterone formation at varying testosterone concentrations incubated with adult duodenal S9 fraction by nonlinear least squares regression using Grafit version 3.0.
| (1) |
where: Vmax = maximum velocity; [S] = substrate concentration; Km = apparent Michaelis–Menten constant; n = Hill exponent.
Results
Effects of age
Protein expression
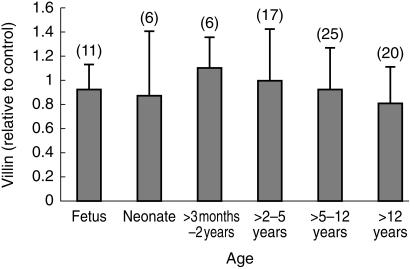
No significant change in villin expression in the small bowel was detected in normal samples from fetuses up to those from 16 year olds (r2 = 0.03; n = 74; P = 0.13) (Figure 1). Likewise no significant difference (Mann–Whitney U-test) was found in villin expression between males and females (0.93 ± 0.40 s.d. and 0.92 ± 0.32 s.d., respectively; 95% confidence interval for difference = −0.1272–0.147).
Figure 1.
Age related changes in relative duodenal villin expression in histologically normal sections. The numbers in each group are give in brackets and error bars are ± s.d. No statistical differences were detected.
A significant correlation was observed between villin and CYP3A4 expression in the normal biopsy samples (r2 = 0.28; n = 74; P < 0.001).
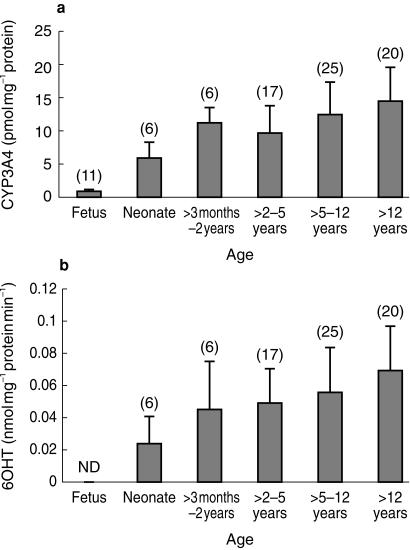
CYP3A expression in the S9 fraction from normal biopsies, normalized to villin, increased significantly with age (r2 = 0.19; n = 74; P = 0.001). When the fetal results were included and the data analysed in discrete age bands (to reflect dosage bands in paediatric drug treatment) a clearer ontogenic pattern was observed (Figure 2a). One-way anova followed by Scheffe's post hoc test indicated significant differences (P < 0.05) between data for foetuses and all other age groups and between data for neonates and children over 5 years of age.
Figure 2.
a) Age related changes in the villin corrected expression of CYP3A4 in histologically normal duodenal sections. The numbers in each group are given in brackets and error bars are ± s.d. Statistical significance differences (P < 0.05) were achieved between foetus and all other groups and between neonate and children > 5 years. b) Age related changes in villin corrected CYP3A4 activity measured by the rate of 6OHT formation in histologically normal duodenal sections. The numbers in each group are given in brackets and error bars are ± s.d. A statistically significant difference (P < 0.05) was observed only between neonates and children > 12 years.
No significant differences were detected (Mann–Whitney U-test) in CYP3A expression between normal samples from male and female subjects (11.4 ± 5 and 12.2 ± 5 pmol mg−1 protein, respectively; 95% confidence interval for difference = −2.76–1.16). anova detected no sex by age effect.
CYP3A4 activity
The increase in CYP3A expression with age was mirrored by a corresponding change in enzyme activity (r2 = 0.17; n = 74; P = 0.02). Activity was not detected in foetal samples, and one-way anova with Scheffe's post hoc test indicated a significant difference between the data for neonates and children over 5 years of age (Figure 2b). Significant differences in activity between samples from males and females were not detected (0.052 ± 0.028 and 0.059 ± 0.03 nmol mg−1 protein min−1, respectively; 95% confidence interval for difference = −0.0184–4.4 × 10−3).
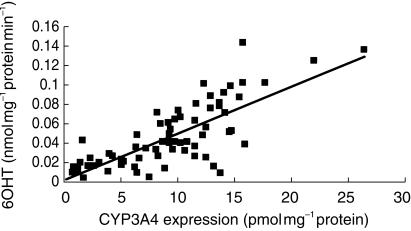
A clear correlation was found between villin-corrected CYP3A expression and the rate of 6β-hydroxytestosterone formation by small bowel S9 fraction (r2 = 0.60; n = 89; P < 0.001) (Figure 3).
Figure 3.
Correlation between CYP3A4 expression and the rate of 6OHT formation in S9 fractions from human paediatric duodenal biopsies (r2 = 0.6, n = 89, P < 0.001).
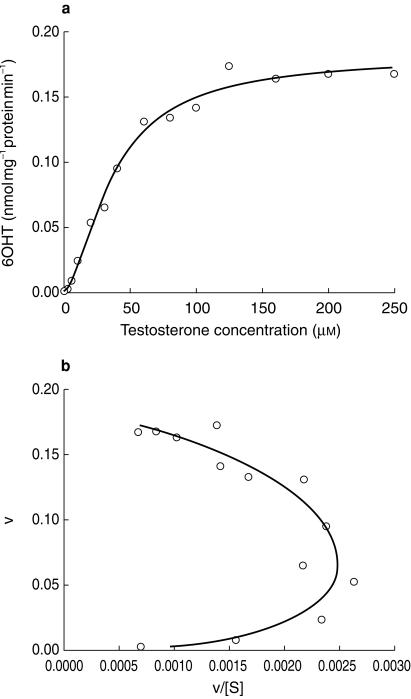
Evaluation of the kinetics of 6β-hydroxytestosterone formation in the S9 fraction from adult duodenum indicated a sigmoidal relationship between rate of metabolism and substrate concentration, clearly confirmed by a convex Eadie–Hofstee plot (Figure 4). Fitting of equation 1 to the data gave a Vmax value of 0.18 nmol min−1.mg protein−1, a Km value of 37 µm and an n of 1.6.
Figure 4.
Velocity (v) vs substrate concentration (S) and Eadie–Hofstee plots for the formation of 6β-hydroxytestosterone (6OHT) by human duodenal S9 fraction. Lines are best fits of a single Hill function. Data are means from two experiments performed in triplicate.
Data summarizing the relative ability of S9 fractions from two biopsy samples to hydroxylate testosterone and to N-demethylate dextromethorphan are shown in Table 1. A similar ratio of activity was shown for the two reactions in the two samples.
Table 1.
Comparison of the capacity of two duodenal S9 fractions to metabolize dextromethorphan and testosterone.
| 3 mm (nmol mg−1 protein min−1) | 6OHT (nmol mg−1 protein min−1) | |
|---|---|---|
| S9 fraction 1 (S1) | 1.98 ± 0.17 | 0.13 ± 0.013 |
| S9 fraction 2 (S2) | 0.74 ± 0.014 | 0.043 ± 0.007 |
| S2/S1 (%) | 37% | 33% |
Each result is the mean of two experiments (±s.d.) run in triplicate.
Effects of disease
Coeliac disease
Protein expression
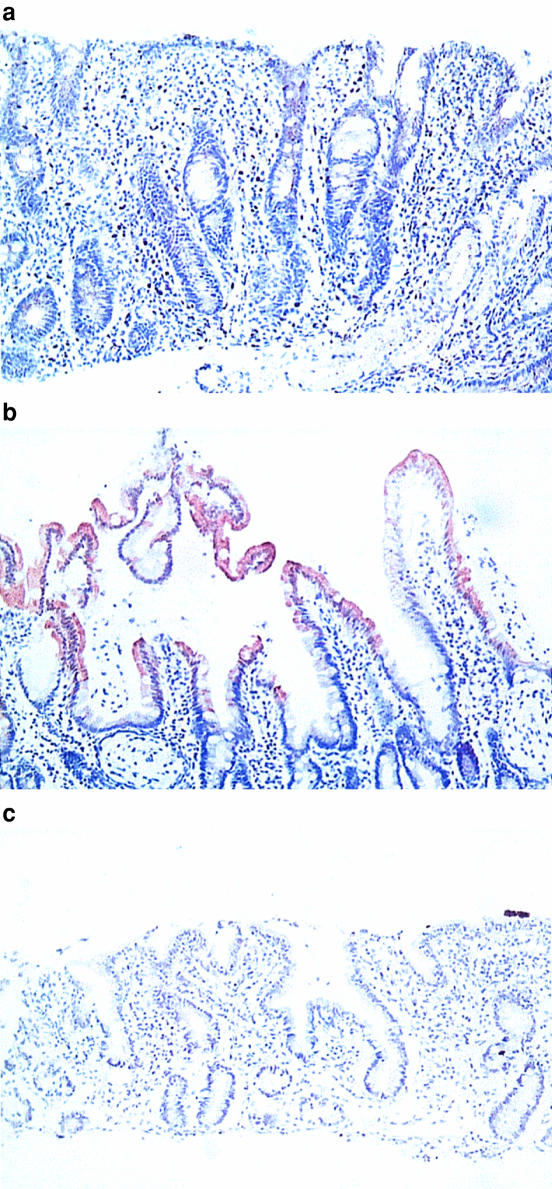
Representative histological sections obtained at diagnosis, after a gluten-free diet and on gluten re-challenge are shown in Figure 5. Villous atrophy in the diagnostic biopsy was accompanied by an absence of CYP3A stain. A gluten-free diet restored normal villous architecture and a typical CYP3A staining pattern decreasing from the villous tips to the crypts can be seen. Gluten re-challenge resulted in a return to disrupted villous architecture and an absence of CYP3A staining.
Figure 5.
Cross sections (×200) of duodenum immunostained using a polyclonal goat anti-rat CYP3A2 antibody which fully cross reacts with human CYP3A. a = on diagnosis of coeliac disease; b = after gluten free diet; c = after gluten rechallenge. The CYP3A enzyme is indicated by the brown stain.
The mean staining scores were 0.89 ± 0.74 s.d., 2.97 ± 0.56 s.d. and 0.60 ± 0.44 s.d., respectively, for sections obtained at diagnosis, after a gluten-free diet and on re-challenge. The differences between the first and second and between the second and the third scores were statistically significant (P < 0.01 and 0.03, respectively; Wilcoxon matched pairs signed rank test). The staining score for the group of patients with partial villous atrophy was 1.80 ± 0.79 s.d. This was significantly different from the diagnosis and gluten-free results (P < 0.02 and 0.002, respectively; Mann–Whitney U-test).
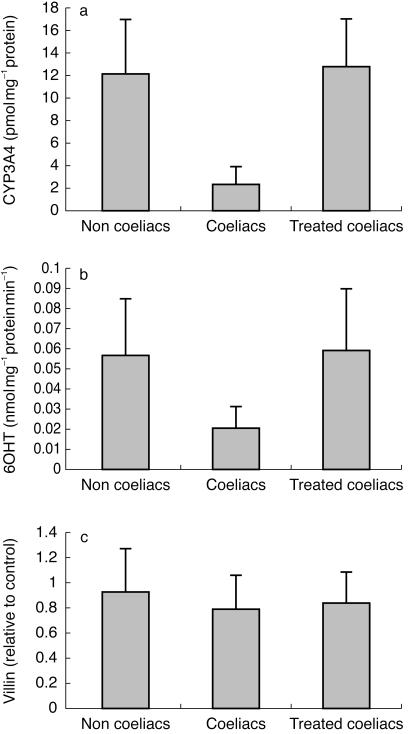
A significant difference was observed in the expression of CYP3A, normalized for villin, in S9 fractions from patients with active coeliac disease compared with noncoeliacs and treated coeliac patients (P < 0.001; Mann–Whitney U-test) (Figure 6a). However, no difference was detected between any of these groups with respect to the expression of villin (Figure 6c).
Figure 6.
a) Villin-corrected expression of CYP3A4 in duodenal biopsies from histologically confirmed coeliac patients with active disease (n = 14) compared with normals (n = 74) and treated coeliacs (n = 15). Error bars are ± s.d. Statistical differences were detected between coeliacs and other groups (P < 0.001). b) Villin-corrected activity of CYP3A4 measured by the rate of 6OHT formation in duodenal biopsies from histologically confirmed coeliac patients with active disease (n = 14) compared with normals (n = 74) and treated coeliacs (n = 15). Error bars are ± s.d. Statistical differences were detected between coeliacs and the other groups (P < 0.001). c) The expression of villin in duodenal biopsies from histologically confirmed coeliac patients with active disease (n = 14) compared with normals (n = 74) and treated coeliacs (n = 15). Error bars are ± s.d. No statistically significant differences were detected.
CYP3A4 activity
Mirroring the effects of coeliac disease and its treatment on CYP3A expression, 6β-hydroxytestosterone formation was significantly lower in samples from patients with active disease compared to noncoeliacs and treated patients (P < 0.001; Mann–Whitney U-test) (Figure 6b).
Cystic fibrosis
Protein expression
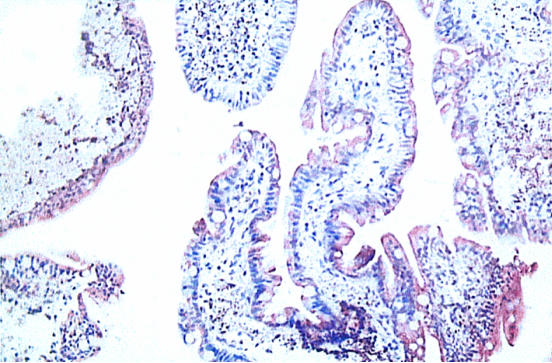
The staining pattern in a sample from a representative patient is shown in Figure 7. There is typical elongation of villous structure with strong staining for CYP3A. The mean staining score for samples from patients with cystic fibrosis (2.89 ± 0.56 s.d.) was similar to that for those from control subjects without disease (2.78 ± 1.08 s.d.). In view of this lack of difference, the patient samples were not studied further by Western blotting.
Figure 7.
Cross-section (x200) of duodenum from a patient with cystic fibrosis. The CYP3A enzyme is indicated by the brown stain.
Discussion
The detection of a single band in immunoblots of the S9 intestinal fraction with the antirat CYP3A2 antibody indicates the presence of CYP3A4 and the absence of CYP3A5. The latter is reported to migrate slightly more slowly than CYP3A4 [25], and others have either failed to detect it in adult small bowel [26] or have observed it using a selective CYP3A5 antibody only after prolonged exposure [22].
Villin has been validated as a marker for the enterocyte content of intestinal sections [22, 27]. The two distinct bands observed on immunoblotting were presumed to represent intact villin and a fragment arising from cleavage of the upper domain structure of the protein. There was a significant correlation between the signal for the combined bands and CYP3A4 expression but not when the bands were analysed separately. Villin expression was found to be invariant with age in our samples, including those from fetuses. This is consistent with the demonstration of early developmental expression of the protein in animals [28].
The steady increases in CYP3A4 expression and activity in human small bowel from the fetal through to the end of the neonatal stage contrasts with the marked surge at weaning (20 days) in these attributes observed in the rat intestine [15]. By the time of weaning in the human (4–6 months), the enzyme system is well established. This species difference presumably reflects the relatively later maturation of the intestine in rodents in the early postnatal period [29]. Thus the rat appears to be a poor animal model for assessing developmental changes in intestinal drug metabolism in humans.
The strong correlation between the rate of 6β-hydroxy-testosterone formation and CYP3A4 expression in the small bowel samples is consistent with the known selectivity of this reaction for CYP3A4, as is the association with the N-demethylation of dextromethorphan [23, 24]. A sigmoidal relationship between the rate of 6β-hydroxy testosterone formation and the concentration of testosterone (diagnosed more clearly by an Eadie–Hofstee plot; Figure 4) is a characteristic feature of the enzyme kinetics of hepatic CYP3A4 and is indicative of marked positive co-operativity [30]. The observation of similar behaviour with the intestinal enzyme is not unexpected and suggests that, like hepatic CYP3A4, it will be subject to variable activation by drug and nutrient substrates, thereby amplifying its efficiency.
The finding of decreased expression of CYP3A in untreated coeliac disease and its return to normal on a gluten-free diet is in agreement with that of a previous study in adults [31]. In addition, we have shown a further decrease in expression on gluten re-challenge. The intermediate level of expression in the patients with partial villous atrophy indicates that the level of CYP3A is related directly to the degree of enteropathy. A similar level of villin expression in coeliac and noncoeliac patients may reflect the facts that some of the former had only partial villous atrophy, and that villin is expressed in crypt cells as well as at the villous tip and these cells are produced at a faster rate in active disease [32]. Elongation of villi did not appear to be associated with any net change in the level of CYP3A expression in patients with cystic fibrosis. Possible differences in enzyme activity were not assessed.
Our findings of increasing intestinal CYP3A4 activity with developmental age and the marked decrease in activity in untreated celiac disease have implications for the oral bioavailability of drugs used in paediatric practice that are significant substrates of the enzyme. These include midazolam, cyclosporin and carbamazepine.
Oral midazolam is often used for premedication in children, some of whom may be under investigation for coeliac disease. In adults up to 50% of the dose is metabolized by CYP3A4 in the small bowel [33]. However, while our data suggest that the oral bioavailability of midazolam might be slightly greater in healthy children compared with adults, the reverse appears to be true. Whereas on average 36% of an oral dose is bioavailable in adults this decreases to 15–27% in children, suggesting that factors in addition to differences in intestinal enzyme activity are in operation [34, 35]. No data are available on the oral bioavailability of midazolam in neonates, in whom we have shown that CYP3A4 activity is significantly lower than in older children. About 30% of an oral dose of cyclosporin is metabolized by intestinal CYP3A4 [36]. It appears to have a similar bioavailability in paediatric heart and kidney transplant patients compared with adults, and in paediatric liver transplant patients less than 5 years of age oral bioavailability is reported to be less than that in adult patients [37]. No studies appear to have been done exclusively in neonates. CYP3A4, and to a lesser extent CYP2C8 catalyse the biotransformation of carbamazepine to its main metabolite carbamazepine-10,11-epoxide. The oral clearance of carbamazepine decreases with age [38] and the ratio of the epoxide metabolite to carbamazepine has also been shown to decrease with age [39].
The limited number of pharmacokinetic studies carried out in patients with coeliac disease have been reviewed, indicating a complex interplay of factors and the importance of adequate study design [40]. For example, conflicting data are available in adults for propranolol, which is metabolized by several cytochromes P450 and by direct glucuronidation. Two studies found an increase in the oral absorption rate of propranolol in active disease [41, 42], while two other studies found no difference in either rate or extent of absorption compared to healthy controls [43, 44]. Systematic studies specifically with drugs that are significant substrates of intestinal CYP3A4 are lacking. In patients with cystic fibrosis the extent of drug absorption varies widely; rate of absorption appears to be slower than normal but the extent of absorption is not altered significantly [45].
While intestinal CYP3A4 plays a significant role in the oral absorption of many drugs, the impact of changes in its developmental expression are likely to be modulated by several factors. Of particular relevance is the role of intestinal transporters. Parallel studies of the developmental expression of these systems, particularly of the efflux transporter P-glycoprotein, which is present together with CYP3A4 in the villous tip, are indicated. Thus, knowledge of the relative development of CYP3A4 and P-glycoprotein in neonates and children and the impact of gastrointestinal disease will allow better understanding of the oral bioavailability of cosubstrates, such as cyclosporin, in paediatric patients.
Acknowledgments
The assistance of Miss Jenny Walker, Paediatric Surgeon, Sheffield Children's Hospital in collecting surgical specimens is gratefully acknowledged. This work was supported by grant S/F/0763 from Action Research.
References
- 1.Guengerich FP. Comparisons of catalytic selectivity of cytochrome P450 subfamily enzymes from different species. Chem-Biol Interact. 1997;106:161–182. doi: 10.1016/s0009-2797(97)00068-9. [DOI] [PubMed] [Google Scholar]
- 2.Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokin. 1994;26:144–160. doi: 10.2165/00003088-199426020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochrome P450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paine MF, Khalighi M, Fisher JM, et al. Characterisation of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–1562. [PubMed] [Google Scholar]
- 5.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P4503A and P-glycoprotein: Implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 6.Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Advanced Drug Delivery Rev. 1997;27:161–170. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Gomez DY, Wacher VJ, Tomlanovich SJ, Hebert MF, Benet LZ. The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporin. Clin Pharmacol Ther. 1995;58:15–19. doi: 10.1016/0009-9236(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 8.Gorski JC, Jones DR, Haehner-Daniels BD, et al. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 9.Kupferschmidt HHT, Ha HR, Ziegler WH, Meier PJ, Krahenbuhl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 10.Kolars JC, Benedict P, Schmiedlin-Ren P, Watkins PB. Aflatoxin B1-adduct formation in rat and human small bowel enterocytes. Gastroenterology. 1994;106:433–439. doi: 10.1016/0016-5085(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 11.McKinnon RA, Burgess WMM, Hall P, Abdul-Aziz Z, McManus ME. Metabolism of food-derived heterocyclic amines in human and rabbit tissues by P4503A proteins in the presence of flavenoids. Cancer Res. 1992;52:2108s–2113s. [PubMed] [Google Scholar]
- 12.Holmes GK. Celiac disease and malignancy. J Pediatr Gastroenterol Nutr. 1997;28:S20–S23. doi: 10.1097/00005176-199700001-00007. [DOI] [PubMed] [Google Scholar]
- 13.Fontana RJ, Lown KS, Paine MF, et al. Effects of chargrilled meat diet on expression of CYP3A, CYP1A, and P-glycoprotein levels in healthy volunteers. Gastroenterology. 1999;117:89–98. doi: 10.1016/s0016-5085(99)70554-8. [DOI] [PubMed] [Google Scholar]
- 14.Hoensch HP, Hartmann F. The intestinal enzymatic biotransformation system: Potential role in protection from colon cancer. Hepatogastroenterology. 1981;28:221–228. [PubMed] [Google Scholar]
- 15.Johnson TN, Tanner MS, Tucker GT. Ontogeny of enterocytic and hepatic cytochrome P4503A in the rat. Biochem Pharmacol. doi: 10.1016/s0006-2952(00)00485-8. in press. [DOI] [PubMed] [Google Scholar]
- 16.de Wildt SN, Kearns GL, Leeder JS, van der Anker JN. Cytochrome P4503A ontogeny and drug disposition. Clin Pharmacokin. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Raucy JL, Carpenter SL. The expression of xenobiotic metabolizing cytochrome P450 in fetal tissues. J Pharmacol Toxicol Methods. 1993;29:121–128. doi: 10.1016/1056-8719(93)90062-j. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver. Evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 19.Kolars JC, Lown KS, Schmiedlin-Ren P, et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics. 1994;4:247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kivisto KT, Bookjans G, Fromm MF, et al. Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue. Br J Clin Pharmacol. 1996;42:387–389. doi: 10.1046/j.1365-2125.1996.42615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters WH, Kremers PG. Cytochromes P-450 in the intestinal mucosa of man. Biochem Pharmacol. 1989;38:1535–1538. doi: 10.1016/0006-2952(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 22.Lown KS, Kolars JC, Thummel KE, et al. Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel: Lack of prediction by the erythromycin breath test. Drug Metab Dispos. 1994;22:947–955. [PubMed] [Google Scholar]
- 23.Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: Identification of the major microsomal steroid hormone 6β-hydroxylase cytochrome P450 enzyme. Arch Biochem Biophys. 1988;263:424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- 24.Gorski JC, Jones DR, Wrighton SA, Hall SD. Characterisation of dextromethorphan N-demethylation by human liver microsomes: Contribution of the cytochrome P450, 3A subfamily. Biochem Pharmacol. 1994;48:173–182. doi: 10.1016/0006-2952(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 25.Wrighton SA, Ring BJ, Watkins PB, Watkins PB, Vanderbranden M. Identification of a polymorphically expressed member of the human cytochrome P450 lll family. Mol Pharmacol. 1989;36:97–105. [PubMed] [Google Scholar]
- 26.Zhang QY, Dunbar D, Ostrowska A, et al. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–809. [PubMed] [Google Scholar]
- 27.Robine S, Huet C, Moll R, Sahuquillo-Merino C, et al. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzell RM, Chafel MM, Matsudaira PT. Differential localization of villin and fibrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 1989;106:407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- 29.Calvert R, Malka D, Menard D. Developmental pattern of glucose-6-phosphatase activity in the small intestine of the mouse fetus. Histochemistry. 1979;63:209–220. doi: 10.1007/BF00644543. [DOI] [PubMed] [Google Scholar]
- 30.Houston JB, Kenworthy KE. In vitro-in vivo scaling of CYP kinetic data not consistent with the classical Michaelis Menton model. Drug Metab Dispos. 2000;28:246–254. [PubMed] [Google Scholar]
- 31.Lang CC, Brown RM, Kinirons MT, et al. Decreased intestinal CYP3A in celiac disease: Reversal after successful gluten-free diet: a potential source of interindividual variability in first pass drug metabolism. Clin Pharmacol Ther. 1996;59:41–46. doi: 10.1016/S0009-9236(96)90022-3. [DOI] [PubMed] [Google Scholar]
- 32.Bisset WM. Disorders of the alimentary tract and liver. In: Campbell AGM, McIntosh N, editors. Forfar and Arneils Textbook of Paediatrics. 5. New York: Churchill Livingston; 1998. pp. 439–441. [Google Scholar]
- 33.Thummel KE, O'Shea D, Paine MF, et al. Oral first pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 34.Smith MT, Eadie MJ, O'Rouke Brophy T. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981;19:271–278. doi: 10.1007/BF00562804. [DOI] [PubMed] [Google Scholar]
- 35.Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–272. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- 36.Wu CY, Benet LZ, Hebert MF, et al. Differentiation of absorption and first pass gut and hepatic metabolism in humans: Studies with cyclosporin. Clin Pharmacol Ther. 1995;58:492–497. doi: 10.1016/0009-9236(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 37.Cooney GF, Dunn SP, KaiSeries B, et al. Oral cyclosporin A pharmacokinetics in paediatric renal and liver transplant recipients. Transplant Proc. 1994;26:2779–2780. [PubMed] [Google Scholar]
- 38.Summers B, Summers RS. Carbamazepine clearance in paediatric epilepsy patients. Clin Pharmacokin. 1989;17:208–216. doi: 10.2165/00003088-198917030-00006. [DOI] [PubMed] [Google Scholar]
- 39.Korinthenberg R, Haug C, Hannak D. The metabolization of carbamazepine to CBZ-10,11-epoxide in children from the newborn age to adolescence. Neuropediatrics. 1994;25:214–216. doi: 10.1055/s-2008-1073024. [DOI] [PubMed] [Google Scholar]
- 40.Gubbins PO, Bertch KE. Drug absorption in gastrointestinal disease and surgery. Clin Pharmacokinet. 1991;21:431–447. doi: 10.2165/00003088-199121060-00004. [DOI] [PubMed] [Google Scholar]
- 41.Parsons RL, Kaye CM, Raymond K, Trounce JR, Turner P. Absorption of propranolol and practolol in coeliac disease. Gut. 1976;17:139–143. doi: 10.1136/gut.17.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitis G, Lucas ML, Bishop H, et al. Altered jejunal surface pH in coeliac disease: its effects on propranolol and folic acid absorption. Clin Sci. 1982;63:373–380. doi: 10.1042/cs0630373. [DOI] [PubMed] [Google Scholar]
- 43.Schneider RE, Babb J, Bishop H, et al. Plasma levels of propranolol in treated patients with coeliac disease and patients with Crohn's disease. Br Med J. 1976;2:794–795. doi: 10.1136/bmj.2.6039.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandle GI, Ward A, Rawlins MD, Record CO. Propranolol absorption in untreated coeliac disease. Clin Sci. 1982;63:81–85. doi: 10.1042/cs0630081. [DOI] [PubMed] [Google Scholar]
- 45.Rey E, Treluyer J, Pons G. Drug disposition in cystic fibrosis. Clin Pharmacokin. 1998;35:313–329. doi: 10.2165/00003088-199835040-00004. [DOI] [PubMed] [Google Scholar]