Abstract
Aims
Itraconazole is a potent inhibitor of CYP3A4 activity and is often used in combination with corticosteroids. Since the latter are partly metabolized by CYP3A4, we studied the interaction between itraconazole, prednisone and methylprednisolone in healthy male subjects.
Methods
The effects of 4 days administration of oral itraconazole (400 mg on the first day then 200 mg day−1 for 3 days) on the pharmacokinetics of prednisolone after a single oral dose of prednisone (60 mg) and the pharmacokinetics of methylprednisolone after single oral dose of methylprednisolone (48 mg) were studied in 14 healthy male subjects in a two-period cross-over trial. Plasma cortisol concentrations were determined as a pharmacodynamic index.
Results
Itraconazole increased the mean area under the methylprednisolone concentration-time curve from 2773 ng ml−1 h to 7011 ng ml−1 h (P < 0.001) and the elimination half-life from 3.2 h to 5.5 h (P < 0.001). The pharmacokinetics of prednisolone were unchanged. Cortisol concentrations at 24 h were lower after administration of methylprednisolone with itraconazole than after methylprednisolone alone (24 ng ml−1 vs 109 ng ml−1, P < 0.001).
Conclusions
Itraconazole increased methylprednisolone concentrations markedly with enhanced suppression of endogenous cortisol secretion, but had no effect on prednisolone pharmacokinetics. The pharmacokinetic interaction between methylprednisolone and itraconazole is probably related to inhibition of hepatic CYP3A4 activity by itraconazole.
Keywords: corticosteroids, drug–drug interaction, itraconazole, pharmacokinetics
Introduction
Itraconazole is a triazole antifungal agent with a spectrum of activity broader than other azole antifungals including Aspergillus fumigatus [1]. Azole antifungals inhibit lanosterol 14-demethylase, a fungal enzyme belonging to the superfamily of cytochromes P450 (CYP450). Poor selectivity of enzyme inhibition means that CYP enzymes of the human liver (in particular, CYP3A4) [2] may be inhibited by azoles, leading to competition with compounds which are metabolized by these enzymes. This inhibition is responsible for drug–drug interactions between itraconazole and cyclosporin [3–5], terfenadine [6–8], warfarin [9], digoxin [10, 11], midazolam [12, 13], triazolam [14], lovastatin [15], buspirone [16], and to a much lesser extent zolpidem [17]. Such interactions may be clinically relevant. For example, it is recommended that the dose of cyclosporin be reduced by 50% when administered concurrently with itraconazole in order to maintain non-toxic plasma cyclosporin concentrations [5].
Itraconazole is frequently administered concurrently with immunosuppressive drugs including glucocorticoids, the metabolism of which is partly mediated by CYP3A4. Among azole derivatives, ketoconazole has been shown to modify the pharmacokinetics of methylprednisolone and to increase the adrenal suppressive effects of the corticosteroid [18]. These changes were smaller when the methylprednisolone dose was reduced by 57% during ketoconazole therapy [19]. Studies investigating the interaction between ketoconazole and prednisolone report either an increased exposure to the steroid both in healthy subjects [20] and mice [21] or a lack of interaction in healthy subjects [22] between the two drugs.
In two recent studies, itraconazole was found to increase plasma concentrations of methylprednisolone with incremental adrenal suppression in healthy subjects [23, 24]. Along the same lines, grapefruit juice, known to increase the bioavailability of many CYP3A4 substrates, was shown to increase the total AUC of methylprednisolone by 75% and the elimination half-life of methylprednisolone by 35% [25]. In another recent study, itraconazole given for 4 days only slightly increased the AUC(0,∞) and t½ of oral prednisolone [26].
The purpose of the present study was to investigate the influence of itraconazole on pharmacokinetics of both prednisone and methylprednisolone in healthy subjects, and to assess its effect on the plasma concentrations of cortisol.
Methods
Subjects
Fourteen healthy male volunteers aged between 20 and 30 years (mean 23.7 years) participated in the study. The subjects gave written consent after full explanation of its objectives. Their weight ranged from 56 to 83 kg (mean 72.2 kg) and their height from 169 to 192 cm (mean 181 cm). All subjects were moderate consumers of alcohol (< 20 g day−1), coffee and tobacco (0–5 cigarettes/days). They were selected after a physical evaluation, laboratory tests including biochemical and haematological profiles, HIV 1–2 and HCV antibodies, hepatitis B surface antigen screening, and a 12 lead-electrocardiogram. None of the subjects reported a history of drug abuse and urinanalysis for cannabinoids, opioids, amphetamines, cocaine and benzodiazepines was negative. Concurrent medication and alcohol were prohibited throughout the study period. The study was approved by the Ethics Committee of Pitié-Salpêtrière Hospital (Comité Consultatif des Personnes Participant à la Recherche Biomédicale, Pitié-Salpêtrière, Paris, France).
Study design
The study was a randomized, open, cross-over design comprising two phases separated by an interval of 9 days. In each phase, the subjects were given orally at 08.00 h, 400 mg itraconazole (Sporanox®, Janssen) on the first day (day 1) then 200 mg for 3 days (days 2, 3, 4). Itraconazole was ingested after a standardized breakfast to optimize bioavailability. The day before the first dose of itraconazole (day 0) and the last day of repeated itraconazole administration (day 4), subjects were given at 08.00 h either 60 mg prednisone (Cortancyl®, Roussel) or 48 mg methylprednisolone (Medrol®, Upjohn).
Blood sampling
On day 0 and day 4, blood samples were drawn 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18 and 24 h after dosing to assess prednisolone or methylprednisolone pharmacokinetics and cortisol concentrations. On day 4, blood samples were obtained according the same schedule (0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24 h post dosing) and 72 and 96 h post dosing to assess itraconazole and hydroxy-itraconazole pharmacokinetics. Blood samples were collected in heparinized tubes and centrifuged immediately (2000 g; 15 min). Plasma was separated and stored at −20 °C in plastic tubes until assayed.
Adverse events
Clinical adverse events spontaneously reported were recorded during the study. Biochemical and haematological profiles were also recorded.
Determination of drugs and cortisol concentrations
Prednisolone, methylprednisolone and cortisol levels were quantified by high-performance liquid chromatography using a modification of the method of Prasad et al. [26]. Steroids, including the internal standard (dexamethasone, Sigma-Aldrich, France) were extracted with dichloromethane (10 ml) from 1 ml of plasma after adding NaOH (0.01 m, 1.0 ml). Prednisolone, methylprednisolone and cortisol were separated isocratically on a normal-phase silica column (Resolve spherical silica 5 µm; 4.6 mm internal diameter; 150 mm length, Waters Millipore, Milwaukee). The degassed mobile phase comprised methanol, acetic acid, distilled water, tetrahydrofuran, n-hexane and dichloromethane (1.85; 0.2; 0.1; 1.5; 8; 88.4:v/v). At a flow rate of 1.2 ml min−1, the retention times for prednisolone, dexamethasone, cortisol and methylprednisolone were 7.2, 10.3, 12.3 and 13.2 min, respectively. Calibration curves were linear over the range 20–1000 ng ml−1 (r2 = 0.999). The limit of quantification for all corticosteroids was 20 ng ml−1. The overall precision was 4.4% and 3.2% for prednisolone and methylprednisolone, respectively.
Itraconazole and hydroxy-itraconazole were assayed by h.p.l.c. [27]. Calibration curves were linear over the range 10–1000 ng ml−1; The limit of quantification was 10 ng ml−1 for both itraconazole and hydroxy-itraconazole. Accuracy and precision of the assay was derived from analyses of quality control samples independently prepared in human plasma. For itraconazole, the overall precision (interassay coefficient of variation) was 3.7% and accuracy 96.0% (n = 54 replicates). For hydroxy-itraconazole, the overall precision was 3.7% and accuracy 95.5% (n = 54 replicates).
Pharmacokinetics
Pharmacokinetic analysis was performed using SIPHAR software (Simed, Créteil, France). The pharmacokinetics of both corticosteroids, itraconazole and its active metabolite, were characterized by peak concentration in plasma (Cmax), time to peak concentration (tmax), elimination half-life (t1/2), and areas under the drug concentration-time curves. The latter was calculated by the linear trapezoidal rule up to 24 h (AUC(0,24 h)) for both corticoids and up to 96 h for itraconazole and hydroxy-itraconazole. t1/2 and AUC were estimated using a model-independent approach.
The pharmacokinetics of cortisol were characterized by AUC over 24 h, concentrations 24 h after corticosteroid ingestion (C24 h), time to reach the lowest concentration (tmin) and elimination rate constant.
Statistical analysis
All data are expressed as mean values and differences between means and their 95% confidence intervals (CI). tmax and tmin are expressed as median values and range. Continuous variables were analysed by t-test for paired values (methylprednisolone and prednisolone) or anova including treatment and subject effects (cortisol). tmax and tmin were compared using the nonparametric Kruskal–Wallis test. Differences were regarded as statistically significant when P values were < 0.05. If anova showed a significant treatment effect, treatment levels were compared by means of t-test taking into account a global error risk related to the number of simultaneous tests performed (Bonferroni). If the Kruskal–Wallis test found a global difference, nonparametric Tukey-type multiple comparisons were applied.
Results
Methylprednisolone
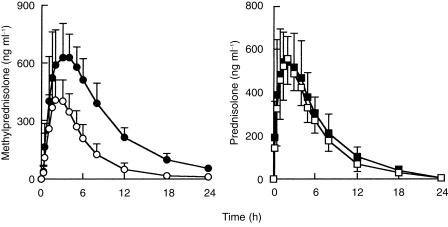
Itraconazole markedly increased the plasma concentrations of methylprednisolone (Table 1; Figure 1). The mean Cmax (95% confidence interval) was increased from 453 ng ml−1 before itraconazole to 713 ng ml−1 (P < 0.001). The mean AUC(0,24 h) after a single oral dose of 48 mg methylprednisolone was 2773 ng ml−1 h. Itraconazole increased the AUC (0–24) of methylprednisolone to 7011 ng ml−1 h P < 0.001). Itraconazole increased the t½ of methylprednisolone from 3.2 h to 5.5 (P < 0.001). tmax was not affected by itraconazole.
Table 1.
Pharmacokinetic parameters of methylprednisolone after a single 48 mg oral dose before and after repeated administration of itraconazole for 4 days to 14 subjects.
| Cmax (ng ml−1) | tmax (h) | t1/2 (h) | AUC(0,24 h) (ng ml−1 h) | |
|---|---|---|---|---|
| Methylprednisolone | 453 [401,505] | 2 (1–4) | 3.2 [2.5–3.9] | 2773 [2223,3323] |
| Methylprednisolone+itraconazole | 713 [637,788] | 3 (1–5) | 5.5 [5.2,5.9] | 7011 [6275,7747] |
| Differences between means | 260* [177,343] | – | 2.25* [1.5,3] | 4238* [3421,5055] |
Data are mean [95% CI]; tmax is given as median (range). Cmax peak plasma concentration, tmax time to Cmax, t1/2 elimination half-life, AUC area under the plasma concentration-time curve.
paired t-test : P < 0.001.
Figure 1.
Mean (± s.d.) plasma concentration-time profiles for methylprednisolone and prednisolone in 14 healthy subjects after a single oral dose of methylprednisolone (48 mg) or prednisone (60 mg) before (○) and during (•) dosing with oral itraconazole (400 mg on day 1 then 200 mg day−1 for 3 days).
Prednisolone
The pharmacokinetics of prednisolone after prednisone alone and following itraconazole administration did not differ significantly (Table 2; Figure 1).
Table 2.
Pharmacokinetic parameters of prednisolone after a single 60 mg oral dose before and after repeated administration of itraconazole for 4 days to 14 subjects.
| Cmax (ng ml−1) | tmax (h) | t1/2 (h) | AUC(0,24 h) (ng ml−1 h) | |
|---|---|---|---|---|
| Prednisolone | 587 [532,642] | 2 (1–4) | 3.7 [3.3,4.1] | 3860 [3365,4356] |
| Prednisolone + itraconazole | 668 [592,744] | 3 (1–5) | 4.2 [3.7,4.7] | 4571 [3997,5145] |
| Differences between means | 81 [−5,167] | – | 0.5 [−0.2,1.2] | 710 [−69,1489] |
Data are mean [95% CI]; tmax is given as median (range). Cmax peak plasma concentration, tmax time to Cmax, t1/2 elimination half-life, AUC area under the plasma concentration-time curve.
Cortisol
Overall analysis of the four phases showed a significant treatment effect on C24 h (P < 0.001), tmin (P < 0.01) and elimination rate constant (P < 0.01).
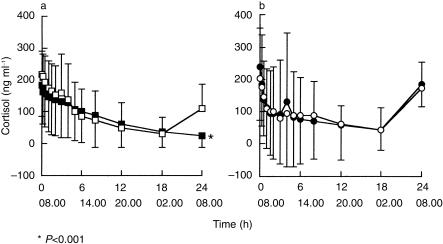
Simple contrasts demonstrated that mean cortisol C24 h after methylprednisolone ingestion during the itraconazole phase (24 ng ml−1) was significantly lower than after methylprednisolone alone (109 ng ml−1), prednisone alone (173 ng ml−1) or prednisone during the itraconazole phase (185 ng ml−1) (Table 3; Figure 2). In addition, cortisol C24 h after methylprednisolone alone was lower than after prednisone administration during the itraconazole phase. Among the 14 subjects, two had undetectable cortisol C24 h after methylprednisolone ingestion and six after methylprednisolone during itraconazole phase, while no subject had a cortisol C24 h-value under 95 ng ml−1 after prednisone before and during itraconazole administration.
Table 3.
Pharmacokinetics parameters of cortisol after methylprednisolone (MP) 48 mg or prednisone (PN) 60 mg with or without itraconazole treatment (itra).
| C24 h (ng ml−1) | AUC(0,24 h) (ng ml−1 h) | tmin (h) | Elimination rate constant (h−1) | |
|---|---|---|---|---|
| MP | 109* [68,149] | 1933 [1073,2793] | 15 (8–24) | 5.6 [3.2,8] |
| MP+itra | 24* [5,43] | 1790 [896,2684] | 24 (0–24) | 7.0 [4.9,9.1] |
| Difference between means | −85 [−123,−46] | −143 [−499,211] | – | 1.4 [−0.4,3] |
| PN | 173* [142,203] | 2010 [727,3293] | 4 (1–18) | 3.2 [0.1,6.3] |
| PN+itra | 185* [149,221] | 2031 [913,3149] | 4.5 (1.5–18) | 3.7 [0.8,6.6] |
| Difference between means | 12 [−24,49] | 21 [−921,964] | – | 0.5 [−0.4,1.4] |
| Simple contrasts | MP+itra = MP | NS | MP = PN | NS |
| MP+itra = PN | MP+itra = PN | |||
| MP+itra = PN+itra | MP+itra = PN+itra | |||
| MP = PN+itra | MP = MP+itra | |||
| MP = PN | PN = PN+itra | |||
| PN = PN+itra | MP = PN+itra |
Data are mean [95% CI], tmin is given as median (range). C24 h cortisol concentrations at 24 h, AUC area under the cortisol concentration-time curve, tmin time to reach the lowest concentrations of cortisol.
anova : P < 0.001, °anova : P < 0.01.
Figure 2.
Mean ± (s.d.) plasma cortisol concentration-time profiles in 14 healthy subjects after a single oral dose of a) methylprednisolone (48 mg) or b) prednisone (60 mg) before (□) and during (▪) daily dosing with oral itraconazole (400 mg on day 1 then 200 mg day−1 for 3 days). *P <0.001.
Simple contrasts between tmin showed that cortisol reached its lowest concentration after prednisone administration (median of tmin = 4 h, range from 7.5 to 7.1 h) earlier than after administration of methylprednisolone and independently of itraconazole ingestion (median of tmin = 15 h, range from 8 to 24 h before itraconazole, median of tmin = 24 h, range from 0 to 24 h during itraconazole). anova of the elimination rate constant demonstrated a global difference between the four phases, without significant difference on simple contrast when a multiple comparison test was applied. anova showed no significant treatment effect on cortisol AUC(0,24 h) between the four phases.
Itraconazole and hydroxyitraconazole
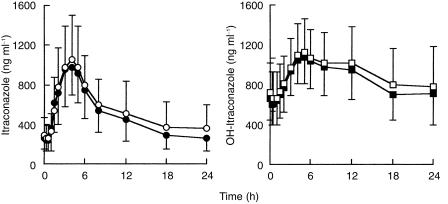
Pharmacokinetic parameters of itraconazole and hydroxy-itraconazole were similar during administration of methylprednisolone and prednisone (Table 4; Figure 3).
Table 4.
Pharmacokinetics parameters of itraconazole and hydroxyitraconazole when coadministered with prednisone or methylprednisolone dosing (day 4) in 14 subjects.
| Cmax (ng.ml−1) | tmax.(h) | t1/2.(h) | AUC(0,96 h) (ng.ml−1 h) | |
|---|---|---|---|---|
| Itraconazole+prednisone | 1066 [885 −1247] | 4 (3–5) | 35.1 [25.5 −44.7] | 28008 [18952 −37064] |
| Itraconazole+methylprednisolone | 1097 [875 −1319] | 4 (2–5) | 31.8 [26.4 −37.2] | 31656 [19843 −43469] |
| Differences between means | 31 [−89 −151] | – | −3.3 [−9.6 −2.8] | 3648 [−1133 −8429] |
| Hydroxyitraconazole+prednisone | 1037 [906 −1168] | 5 (4–12) | 28.5 [17.9 −39.1] | 55274 [33096 −77452] |
| Hydroxyitraconazole+methylprednisolone | 1103 [933 −1273] | 5 4–12 | 27.6 [17.7 −37.5] | 58875 [33922 −83828] |
| Differences between means | 67 [−38 −172] | – | −0.9 [−3.7 −1.9] | 3602 [−4748 −11952] |
Data are mean [95% CI]; tmax is given as median (range).
Figure 3.
Mean±(s.d.) plasma concentration-time profiles of itraconazole and hydroxy-itraconazole in 14 healthy subjects after daily dosing with oral itraconazole (400 mg on day 1 then 200 mg day−1 for 3 days). ○ ITRA+MP, • ITRA+PN; □ ITRA+MP, ▪ ITRA+PN.
Side-effects
No relevant side-effect or changes in biochemical and haematological profiles was noted during any phase of the study.
Discussion
The results of this study indicate that itraconazole markedly increased the plasma concentrations of methylprednisolone, but did not affect the concentrations of prednisolone after prednisone administration. This interaction is probably related to inhibition of CYP3A4 activity by itraconazole. Although itraconazole was found to be 10 times less potent in vitro than ketoconazole as an inhibitor of cyclosporin A metabolism by human liver microsomes, several reports in the literature mention interactions between itraconazole and some substrates of CYP3A4. For instance, a study in healthy subjects showed that concomitant ingestion of itraconazole increases the concentration of lovastatin acid (the active metabolite of lovastatin) about 10–30 fold [15]. Such a mechanism was proposed to explain the increased muscular toxicity of lovastatin used in combination with other inhibitors of CYP3A4. Itraconazole was also found to affect markedly the pharmacokinetics of both triazolam [14] and midazolam [12, 13], two CYP3A4 substrates, in healthy subjects. This pharmacokinetic interaction was associated with increased sedative effects of both hypnotics.
Cortisol and corticosteroids are partly metabolized to 6β-hydroxyderivatives. One previous study has demonstrated that 6β-hydroxylation of cortisol is a CYP3A4 dependent metabolic pathway [28]. A significant pharmacokinetic interaction between ketoconazole, a very potent inhibitor of CYP3A4, and methylprednisolone has been demonstrated in healthy subjects [18, 19]. Recent studies in healthy subjects showed a significant interaction between itraconazole and methylprednisolone orally or intravenously administered, with an increase of plasma concentrations of methylprednisolone and an incremental effect on endogenous cortisol secretion [23, 24]. The results of the present investigation show an even larger effect on methylprednisolone pharmacokinetics.
In another recent study, a minor interaction between itraconazole and prednisolone was found in 10 healthy volunteers [26]. Similarly, interaction between itraconazole and prednisolone was found in our study in 15 healthy subjects. Hence the two corticosteroids appear to be very different with regard to sensitivity to inhibitors of steroid metabolism. Other work supporting this has shown that troleandomycin and erythromycin inhibit methylprednisolone metabolism but have no effect on prednisolone metabolism [30, 31]. In addition, inducers of the hepatic microsomal enzyme system such as phenobarbitone, carbamazepine and phenytoin are associated with a more pronounced acceleration of methylprednisolone metabolism compared with prednisolone metabolism in children [32]. Interactions between ketoconazole and prednisolone seem to be less consistent than with methylprednisolone [20–22]. CYP3A4 could be the main metabolic pathway for methylprednisolone whereas it may be a subsidiary one for prednisolone. This hypothesis was also suggested by Harris and colleagues [33] who reported a lack of correlation between the erythromycin breath test, used as marker of in vivo CYP3A4 activity, and prednisolone clearance in pre and postmenopausal women. However it remains possible that the interaction does not occur after a single dose of prednisone but only after chronic dosing.
The clinical relevance of an interaction between itraconazole and corticosteroids remains questionable. Nevertheless, in patients with allergic bronchopulmonary aspergillosis, the introduction of itraconazole therapy was followed by an improvement in clinical status leading to a decrease in prednisone and methylprednisolone dosage [34].
In our study, both corticosteroids and itraconazole were given orally. As CYP3A4 is found extensively in the liver and in the gut [35], it is possible that inhibition of CYP3A4 plays an important role in the interaction between itraconazole and oral methylprednisolone. However, itraconazole can also inhibit P-glycoprotein, a transmembrane efflux pump, and methylprednisolone is a P-glycoprotein substrate [36]. Under this assumption, the pharmacokinetics of intravenous methylprednisolone should be less affected during itraconazole treatment, as reported previously in healthy volunteers for the interaction between fluconazole and midazolam [37]. However, this does not seem to be the case because in healthy subjects, the clearance of intravenous methylprednisolone was shown to be dramatically decreased when given concomitantly with itraconazole [24].
Plasma cortisol was determined as a pharmacodynamic index of glucocorticoid activity. When methylprednisolone was administered with itraconazole, suppression of endogenous cortisol was prolonged. In addition, 6 of the 14 subjects had no detectable cortisol in the plasma when methylprednisolone and itraconazole were taken together. No difference could be demonstrated between the four phases on the cortisol AUC(0,24 h) which may be related to the inter and intrasubject variability. Similar results have been found for ketoconazole and methylprednisolone, but in addition to prolonged adrenal suppression, the cortisol AUC(0,24 h) was lower during ketoconazole therapy [18]. However, it cannot be ruled out that part of the enhanced suppression of endogenous cortisol is related to a pharmacodynamic property of ketoconazole. As far as itraconazole is concerned, no impairment of cortisol synthesis could be demonstrated during chronic itraconazole treatment in patients [38, 39]. Reversible adrenal insufficiency occurred in one among eight patients receiving high-dose itraconazole (600 mg day−1) for a mean duration of 5.5 months [39]. In our study, the baseline profile of cortisol during the itraconazole phase was not determined. However, the cortisol concentrations at 08.00 h t0 did not differ during the itraconazole phase compared with drug free values. Thus we postulate that the secretion of cortisol was maintained after 4 days of itraconazole administration. It seems reasonable to consider that suppression of endogenous cortisol is related only to the effect of the corticosteroid.
Our results show differences for the cortisol suppression between the two corticosteroids. Prednisolone inhibited cortisol secretion more rapidly than did methylprednisolone. As previously suggested, we think this finding is related to the differences in the pharmacokinetic profile between the two corticosteroids [41].
The pharmacokinetics of itraconazole and hydroxy-itraconazole are similar to those determined in other studies in healthy subjects performed with multiple doses [17, 42]. A regimen of 400 mg on the first day followed by 200 mg day−1 for 3 consecutive days leads to plasma concentrations similar to those obtained after 200 mg day−1 over 15 days. Cmax values of 1292 ± 355 ng ml−1 and 697 ± 391 ng ml−1 were reported at day 15 after treatment with an oral solution of itraconazole in two studies performed in bone marrow transplant recipients (5 mg kg−1 day−1) [43] and in HIV-infected patients receiving 200 mg day−1 [44].
Corticosteroid therapy is an important risk factor for systemic mycoses including invasive aspergillosis [45]. Itraconazole is frequently administered in combination with corticosteroids. The aim of our study was to examine to what extent itraconazole, as a potent inhibitor of CYP3A4, is able to affect the pharmacokinetics and pharmacodynamics of two widely used corticosteroids. Our results suggest a preference for the use of prednisone in combination with itraconazole because of the lack of a pharmacokinetic interaction. When methylprednisolone is used in association with itraconazole, exposure to the corticosteroid is markedly increased and may lead to an enhancement of side-effects. It is therefore reasonable to reduce the dose of methylprednisolone (dosed chronically or as pulse therapy) during concomitant treatment with itraconazole.
Acknowledgments
AP-HP, Direction de la Recherche Clinique supported the study.
References
- 1.Grant SM, Clissold SP. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37:310–344. doi: 10.2165/00003495-198937030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Back DJ, Tjia JF, Abel SM. Azoles, allylamines and drug metabolism. Br J Dermatol. 1992;126:14–18. doi: 10.1111/j.1365-2133.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 3.Trenk D, Brett W, Jähnchen E, Birnbaum D. Time course of cyclosporine/itraconazole interaction. Lancet. 1987;i:1335–1336. doi: 10.1016/s0140-6736(87)91232-3. [DOI] [PubMed] [Google Scholar]
- 4.Shaw MA, Gumbleton M, Nicholls PJ. Interaction of cyclosporine and itraconazole. Lancet. 1987;i:637. doi: 10.1016/s0140-6736(87)93038-8. [DOI] [PubMed] [Google Scholar]
- 5.Kramer MR, Marshall SE, Denning DW, et al. Cyclosporine and itraconazole interaction in heart and lung transplant recipients. Ann Intern Med. 1990;113:327–329. doi: 10.7326/0003-4819-113-4-327. [DOI] [PubMed] [Google Scholar]
- 6.Pohjola-Sintonen S, Viitasalo M, Toivonene L, Neuvonen P. Torsades de pointes ventricular tachycardia caused by terfenadine–itraconazole interaction. Br Med J. 1993;306:186. doi: 10.1136/bmj.306.6871.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane JK, Hue-Teh S. Syncope and cardiac arrhythmia due to an interaction between itraconazole and terfenadine. Am J Med. 1993;95:445–446. doi: 10.1016/0002-9343(93)90317-i. [DOI] [PubMed] [Google Scholar]
- 8.Pohjola-Sintonen S, Viitasalo M, Toivonen L, Neuvonen P. Itraconazole prevents terfenadine metabolism and increases risk of torsades de pointes ventricular tachycardia. Eur J Clin Pharmacol. 1993;45:191–193. doi: 10.1007/BF00315505. [DOI] [PubMed] [Google Scholar]
- 9.Yeh J, Shiu-Ching S, Summerton C, Richardson C. Potentiation of action of warfarin by itraconazole. Br Med J. 1990;301:669. doi: 10.1136/bmj.301.6753.669-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachs MK, Blanchard LM, Green PJ. Interaction of itraconazole and digoxin. Clin Infect Dis. 1993;16:400–403. doi: 10.1093/clind/16.3.400. [DOI] [PubMed] [Google Scholar]
- 11.Rex J. Itraconazole–digoxin interaction. Ann Intern Med. 1992;116:525. doi: 10.7326/0003-4819-116-6-525. [DOI] [PubMed] [Google Scholar]
- 12.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:81–85. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 13.Ahonen J, Olkkola KT, Neuvonen PJ. Effect of itraconazole and terbinafine on the pharmacokinetics and pharmacodynamics of midazolam in healthy volunteers. Br J Clin Pharmacol. 1995;40:270–272. [PMC free article] [PubMed] [Google Scholar]
- 14.Varhe A, Olkkola KT, Neuvonen JJ. Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;56:601–607. doi: 10.1038/clpt.1994.184. [DOI] [PubMed] [Google Scholar]
- 15.Neuvonen PJ, Jalava KM. Itraconazole drastically increases plasma concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1996;60:54–61. doi: 10.1016/S0009-9236(96)90167-8. [DOI] [PubMed] [Google Scholar]
- 16.Kivistö KT, Lambert TS, Kantola T, Neuvonen PJ. Plasma buspirone concentrations are greatly increased by erythromycin and itraconazole. Clin Pharmacol Ther. 1997;62:348–354. doi: 10.1016/S0009-9236(97)90038-2. [DOI] [PubMed] [Google Scholar]
- 17.Luurila H, Kivistö KT, Neuvonen PJ. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of zolpidem. Eur J Clin Pharmacol. 1998;54:163–166. doi: 10.1007/s002280050439. [DOI] [PubMed] [Google Scholar]
- 18.Glynn AM, Slaughter RL, Brass C, D'Ambrosio R, Jusko WJ. Effect of ketoconazole on methylprednisolone pharmacokinetics and cortisol secretion. Clin Pharmacol Ther. 1986;39:654–659. doi: 10.1038/clpt.1986.114. [DOI] [PubMed] [Google Scholar]
- 19.Kandrotas RJ, Slaughter RL, Brass C, Jusko WJ. Ketoconazole effects on methylprednisolone disposition and their joint suppression of endogenous cortisol. Clin Pharmacol Ther. 1987;42:465–470. doi: 10.1038/clpt.1987.179. [DOI] [PubMed] [Google Scholar]
- 20.Zürcher RM, Frey BM, Frey FJ. Impact of ketoconazole on the metabolism of prednisolone. Clin Pharmacol Ther. 1989;45:366–372. doi: 10.1038/clpt.1989.42. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich B, Frey FJ, Speck RF, Frey BM. Pharmacokinetics/ pharmacodynamics of ketoconazole–prednisolone interaction. J Pharmacol Exp Ther. 1992;260:487–490. [PubMed] [Google Scholar]
- 22.Yamashita SK, Ludwig EA, Middleton E, Jusko WJ. Lack of pharmacokinetic and pharmacodynamic interactions between ketoconazole and prednisolone. Clin Pharmacol Ther. 1991;49:558–570. doi: 10.1038/clpt.1991.66. [DOI] [PubMed] [Google Scholar]
- 23.Varis T, Kaukonen KM, Kivistö KT, Neuvonen PJ. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther. 1998;64:363–368. doi: 10.1016/S0009-9236(98)90066-2. [DOI] [PubMed] [Google Scholar]
- 24.Varis T, Kivisto KT, Backman JT, Neuvonen PJ. Itraconazole decreases the clearance and enhances the effects of intravenously administered methylprednisolone in healthy volunteers. Pharmacol Toxicol. 1999;85:29–32. doi: 10.1111/j.1600-0773.1999.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 25.Varis T, Kivistö KT, Neuvonen PJ. Grapefruit juice can increase the plasma concentrations of oral methylprednisolone. Eur J Clin Pharmacol. 2000;56:489–493. doi: 10.1007/s002280000171. [DOI] [PubMed] [Google Scholar]
- 26.Varis T, Kivistö KT, Neuvonen PJ. The effects of itraconazole on the pharmacokinetics and pharmacodynamics of oral prednisolone. Eur J Clin Pharmacol. 2000;56:57–60. doi: 10.1007/s002280050720. [DOI] [PubMed] [Google Scholar]
- 27.Prasad VK, Ho B, Haneke C. Simultaneous determination of prednisolone acetate, prednisolone, prednisone, cortisone and hydrocortisone in swine plasma using solid-phase and liquid-liquid extraction techniques. J Chromatogr. 1986;378:305–316. doi: 10.1016/s0378-4347(00)80727-6. [DOI] [PubMed] [Google Scholar]
- 28.Woestenborghs R, Lorreyne W, Heykants J. Determination of itraconazole in plasma and animal tissues by HPLC. J Chromatogr. 1987;413:332–327. doi: 10.1016/0378-4347(87)80249-9. [DOI] [PubMed] [Google Scholar]
- 29.Ged C, Rouillon JM, Pichard L, et al. The increase in urinary excretion of 6β-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989;28:373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szefler SJ, Brenner M, Jusko WJ, Spector SL, Flesher KA, Ellis EF. Dose- and time-related effect of troleandomycin on methylprednisolone elimination. Clin Pharmacol Ther. 1982;32:166–171. doi: 10.1038/clpt.1982.143. [DOI] [PubMed] [Google Scholar]
- 31.La Force CF, Szefler SJ, Miller MF, et al. Inhibition of methylprednisolone elimination in the presence of erythromycin therapy. J Allergy Clin Immunol. 1983;72:34–39. doi: 10.1016/0091-6749(83)90049-0. [DOI] [PubMed] [Google Scholar]
- 32.Bartoszek M, Brenner AM, Szefler SJ. Prednisolone and methylprednisolone kinetics in children receiving anticonvulsivant therapy. Clin Pharmacol Ther. 1987;42:424–432. doi: 10.1038/clpt.1987.173. [DOI] [PubMed] [Google Scholar]
- 33.Harris RZ, Tsunoda SM, Mroczkowski P, Wong H, Benet LZ. The effect of menopause and hormone replacement therapies on prednisolone and erythromycin pharmacokinetics. Clin Pharmacol Ther. 1996;59:429–435. doi: 10.1016/S0009-9236(96)90112-5. [DOI] [PubMed] [Google Scholar]
- 34.Denning DW, Van Wye JE, Lewiston NJ, Stevens DA. Adjunctive therapy of allergic bronchopulmonary aspergillosis with itraconazole. Chest. 1991;100:813–819. doi: 10.1378/chest.100.3.813. [DOI] [PubMed] [Google Scholar]
- 35.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitoh HM, Hatakeyama O, Eguchi O, Oda M, Takada M. Involvement of intestinal P-glycoprotein in the restricted absorption of methylprednisolone from rat small intestine. J Pharm Sci. 1998;87:73–75. doi: 10.1021/js970163u. [DOI] [PubMed] [Google Scholar]
- 37.Ahonen J, Olkkola KT, Neuvonen PJ. Effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. Eur J Clin Pharmacol. 1997;51:415–419. doi: 10.1007/s002280050223. [DOI] [PubMed] [Google Scholar]
- 38.Queiroz-Telles F, Purim KS, Boguszewski CL, Afonso FC, Graf H. Adrenal response to corticotrophin and testosterone during long-term therapy with itraconazole in patients with chromoblastomycosis. J Antimicrob Chemother. 1997;40:899–902. doi: 10.1093/jac/40.6.899. [DOI] [PubMed] [Google Scholar]
- 39.Phillips P, Graybill JR, Fetchick R, Dunn JF. Adrenal response to corticotropin during therapy with itraconazole. Antimicrob Agents Chemother. 1987;31:647–649. doi: 10.1128/aac.31.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharkey PK, Rinaldi MG, Dunn JF, Hardin TC, Fetchick RJ, Graybill JR. High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;34:707–713. doi: 10.1128/aac.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szefler SJ, Ebling WF, Georgitis JW, Jusko WJ. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol. 1986;30:323–329. doi: 10.1007/BF00541537. [DOI] [PubMed] [Google Scholar]
- 42.Hardin TC, Graybill R, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JC. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32:310–1313. doi: 10.1128/aac.32.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prentice AG, Warnock DW, Johnson SAN, Phillips MJ, Oliver DA. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 44.Reynes J, Bazin C, Ajana F, et al. Pharmacokinetics of itraconazole (oral solution) in two groups of human immunodeficiency virus-infected adults with oral candidiasis. Antimicrob Agents Chemother. 1997;41:2554–2558. doi: 10.1128/aac.41.11.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denning DW. Aspergillosis: diagnosis and treatment. Int J Antimicrob Agents. 1996;6:161–168. doi: 10.1016/0924-8579(95)00042-9. [DOI] [PubMed] [Google Scholar]