Abstract
Aims
To further evaluate mephenytoin as a probe for CYP2C19 phenotyping.
Methods
Healthy subjects (n = 2638) were phenotyped using the urinary (S)-mephenytoin to (R)-mephenytoin ratio. This method was evaluated for (a) the stability of the S/R-ratio following sample storage, (b) the intraindividual reproducibility of the ratio, and (c) the occurrence of adverse events.
Results
After prolonged storage, the S/R-ratio of samples from extensive metabolisers (EM) increased up to 85%. In 1.5% of the cases (1 out 66), this led to incorrect classification of phenotype. In EMs, but not in poor metabolisers (PMs), the S/R-ratio increased after acid treatment. The intraindividual reproducibility of the mephenytoin phenotyping procedure was 28%. No major side-effects were observed and there was no relationship between the incidence of side-effects and the phenotype of the subject.
Conclusions
After prolonged storage the S/R-ratio significantly increased in EMs and, although low, the risk of incorrect classification should not be ignored. Our data support the use of mephenytoin as a safe drug for CYP2C19 phenotyping.
Keywords: CYP2C19, mephenytoin, phenotyping
Introduction
Mephenytoin is widely used as an index drug for the assessment of CYP2C19 phenotype. In extensive metabolisers (EMs) for CYP2C19, the pathways of (S) and (R)-mephenytoin metabolism differ considerably, whereas in poor metabolisers (PMs), the metabolic pathways are similar. Extensive and poor metabolisers can be characterized using racemic mephenytoin by measuring the S/R-ratio [2] or the 4′-hydroxylation index in urine [3]. Some disadvantages in using mephenytoin as a probe drug have been reported, including the difficulty in obtaining mephenytoin tablets and analytical reference materials [4], as well as stability problems with respect to the S/R-ratio in urine samples of extensive metabolisers [5] and the possibility of a sedative effect of the drug [6]. The instability of the S/R-drug ratio due to storage is caused by a labile metabolite of S-mephenytoin, which is excreted in urine of EMs but not of PMs [7]. This labile metabolite has shown to be sensitive to both low pH and storage conditions. It has been identified as a S-mephenytoin cysteine conjugate, which is easily hydrolysed back to S-mephenytoin, resulting in an increased S/R-ratio [8]. CYP2C19 phenotype can be confirmed by treating urine samples with acid, resulting in a significant increase in the S/R-ratio in EMs, but not in PMs [9]. In this study we have evaluated the performance of mephenytoin as a probe for CYP2C19 phenotyping by analysing samples from a large population phenotyped with a drug cocktail which included mephenytoin. This phenotyping method was evaluated for (a) the stability of the S/R-ratio following sample storage (b) the intraindividual reproducibility of the ratio, and (c) the occurrence of adverse events.
Parts of this paper have been presented at the ‘European symposium on the Prediction of Drug Metabolism in Man: progress and problems’ Liège, Belgium 19–20 May 1998 [1].
Methods
Urine samples were analysed from 2638 unrelated subjects (1830 males and 808 females) who participated in a study using mephenytoin (100 mg) in a cocktail with dextromethorphan (22 mg) and caffeine (100 mg) as described elsewhere [10]. All subjects gave their written informed consent and an independent Ethics Committee approved the protocol. All urine was collected for 8 h post dose and a 20 ml aliquot was stored at −20 °C until analysis. (S)-mephenytoin and (R)-mephenytoin concentrations were analysed by gas chromatography [10]. Subjects with S/R-ratios of ≥ 0.8 were classified as PM and those with ratios of < 0.8 were classified as EM [11]. In addition, samples from subjects assigned initially as PMs were re-analysed after acid treatment, to confirm the phenotype [9]. Short-term sample storage stability at −20 °C was investigated using a random selection of samples from 30 EMs, initially analysed within 30 days of collection and then re-analysed within 6 months (maximum storage: 7 months). Long-term storage stability at −20 °C was investigated using a selection of samples from 66 EMs and all available PM samples (n = 51). Re-analysis after acid treatment (addition of 0.1 ml of 12 m HCl to 0.5 ml sample and shaking for 10 min at room temperature) was performed on a random selection of samples from 47 EMs and on all available PM samples (n = 51). Only samples with S/R-ratios above 0.1 were selected, since below this value (S)-mephenytoin concentrations might be close to or below the limit of detection. The intraindividual variability of the mephenytoin S/R-ratio was assessed in 129 volunteers who had been phenotyped twice and reproducibility was defined as the mean of all individual coefficients of variation (CV%) between the first and second result. Adverse events in 797subjects were classified according to the WHO dictionary for adverse events [12]. A paired t-test and linear regression (Microsoft Excel®) and a Chi-square test (SAS® version 6.12) were used, to analyse the data. P values less than 0.05 were considered statistically significant.
Results
The effects of storage and acid treatment of samples on the S/R-ratio are given in Table 1. After treatment with acid, samples from six PMs (11.8%) showed a significant increase in S/R-ratio (S/R-ratio ≥ 1.40) and therefore these subjects were characterized as false PMs. Samples from EMs that were re-analysed after short-term storage showed a 22% increase in the mean S/R-ratio (P < 0.05). Long-term storage resulted in a much larger increase of 85% (P < 0.05). In one individual sample the ratio shifted from EM (0.122) to PM (1.016) after storage for 1086 days. Long-term storage of samples from PMs did not affect the mean metabolic ratio (P > 0.07). Pre-treating the samples with acid caused a significant increase in the S/R-ratio for both the EMs (2497%; P < 0.01) and false PMs (137%; P = 0.02). In contrast no significant changes were observed in samples from the remaining PMs(P = 0.15).
Table 1.
Changes in the S/R-ratio of mephenytoin in urine samples of volunteers characterized by in-vivo phenotyping.
| Factor | Extensive metabolisers % change# | n | True poor metabolisers % change# | n | False poor metabolisers* % change# | n |
|---|---|---|---|---|---|---|
| Storage < 7 months | + 21.6% | 30‡ | NA | – | NA | – |
| 95% CI for differences | 5.6%–35% | – | – | – | ||
| Storage > 7 months | + 84.9% | 66‡ | −0.9% | 45 | −3.2 | 6 |
| 95% CI for differences | 62.0%–108% | −2.0%–0.1% | −9.8%–3.5% | |||
| Acid treatment | + 2497% | 47‡ | 1.0% | 45 | 137% | 6‡ |
| 95% CI for differences | 2163%–2830% | −0.4%–2.4% | 35%–239% |
Subjects who are assumed to be false positive are indicated by an increase in the S/R-ratio of 1.4 or greater after acid treatment
comparison between initial analysis and reanalysis.
P value < 0.05.
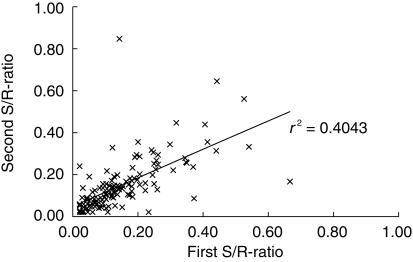
The reproducibility of the phenotyping procedure in 129 EM subjects is shown in Figure 1. No significant differences in the mean S/R-ratios were found between the first (0.151) and the second phenotyping (0.150; 95% CI for difference = −0.017–0.019; P = 0.92). However in some subjects, large intraindividual differences were apparent in 21 out of the 129 repeat analyses that showed a CV% of > 50% and in one case this led to a false classification as PM (S/R-ratio: 0.143 (first analysis), 0.846 (second analysis) and 4.65 (after acidification)). The intraindividual reproducibility of the mephenytoin CYP2C19 phenotyping test was estimated to be 28%.
Figure 1.
Intra-individual reproducibility of the CYP2C19 phenotyping procedure following administration of mephenytoin (100 mg) on two occasions. Linear regression between first and second S/R-ratio (slope ± 95% interval: 0.6748 ± 0.1439 (P < 0.05); intercept ± 95% interval: 0.0482 ± 0.0276 (P < 0.05)).
A total of 22% of the subjects studied experienced an adverse event (AE). The majority of the recorded AEs were of mild intensity (98.9%) and no interventions were necessary. Of these 73% had a possible relationship to treatment. The following AEs were recorded: insomnia (50%), abdominal discomfort and nausea (23%), drowsiness and vertigo (20%) and headache (9%). Application of the chi-square test showed no significant relationship between phenotype and the number of adverse events (P = 0.358).
Discussion
Mephenytoin is the most common probe used for the assessment of CYP2C19 phenotype in both epidemiological studies and in clinical pharmacological research. Although for practical and scientific reasons, genotyping is rapidly replacing phenotyping, we still recommend the latter for the ultimate confirmation of functional metabolic capacity. In the current study we evaluated the performance of mephenytoin as a probe for CYP2C19 phenotype. Previously the risk of false phenotype classification due to reproducibility errors in analysing mephenytoin using gas chromatography was estimated at 0.3% [10]. In the present study we have shown that due to poor analyte stability in urine samples from EMs the additional risk of false classification increased with 1.5% (1 out of 66 subjects) to 1.8%. However, false positive EMs will be detected by the acidification procedure and therefore this additional risk can easily be eliminated by application of this procedure.
Acid treatment resulted in a significant increase in the S/R-ratios of EMs and false PMs, but not of true PMs and therefore proved necessary for a correct classification of the metaboliser status. Omitting this procedure increases the risk of incorrect classification in up to 12% of all PMs. If quantitative information on the S/R ratio is considered important, e.g. for correlation with CYP2C19 activity, samples should be analysed as soon as possible since prolonged storage significantly increases the S/R-ratio in EMs.
The intraindividual reproducibility of the mephenytoin S/R-ratio showed that it is a reasonably reproducible measure for the CYP2C19 phenotype within an individual, although PMs should be confirmed by acidification of urine. However, the S/R-ratio showed large variation within an individual which may be due to variation in CYP2C19 activity, or in the activity of nonpolymorphic pathways which also contribute to this parameter. Therfore, the S/R-ratio cannot be regarded as a reliable quantitative index of the CYP2C19 activity without further research. Mephenytoin administered at the 100 mg dose level in combination with dextromethorphan and caffeine has been shown to be a safe drug for phenotyping with respect to CYP2C19 and no relationship was found between adverse events and CYP2C19 phenotype.
Acknowledgments
This project was supported by grants of The Dutch Ministry of Economic Affairs (‘Senter voor technologie, energie en milieu’; BIO96052) and ‘Stichting voor Technische Wetenschappen (STW)’ (GPR66.4084).
References
- 1.Oosterhuis B, Tamminga WJ, Wemer J, et al. Mephenytoin as a marker for CYP219 enzyme activity: influence of analytical factors on the outcome of phenotyping. Proceedings of ‘European Symposium on the Prediction of Drug Metabolism in Man: Progress and Problems’; 19–20 May; COST B1 Liège, Belgium. 1998. pp. 197–200. [Google Scholar]
- 2.Wedlund PJ, Aslanian WS, Jacqz E, McAllister CB, Branch RA, Wilkinson GR. Phenotypic differences in mephenytoin pharmacokinetics in normal subjects. J Pharmacol Exp Ther. 1985;243:662–669. [PubMed] [Google Scholar]
- 3.Küpfer A, Preisig R. Pharmacogentics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26:753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- 4.Partovian C, Jacqz-Aigrain E, Keundjian A, Jaillon P, Funck-Bretano C. Comparison of chloroguanide and mephenytoin for the in-vivo assessment of genetically determined CYP2C19 activity in humans. Clin Pharmacol Ther. 1995;58:257–263. doi: 10.1016/0009-9236(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Blouin RA, McNamara PJ, Steinmetz J, Wedlund PJ. Limitation to the use of the urinary S/R-mephenytoin ratio in pharmacogenetic studies. Br J Clin Pharmacol. 1991;31:350–352. doi: 10.1111/j.1365-2125.1991.tb05542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setiabudy R, Chiba K, Kusaka M, Ishizaki T. Caution in the use of a 100mg dose of racemic mephenytoin for phenotyping Southeastern Oriental subjects. Br J Clin Pharmacol. 1992;33:665–666. [PMC free article] [PubMed] [Google Scholar]
- 7.Wedlund PJ, Sweetman BJ, Wilkinson GR, Branch RA. Pharmacogenetic association between the formation of 4-hydroxymephenytoin and a new metabolite of S-mephenytoin in man. Drug Metab Dispos. 1987;5:277–279. [PubMed] [Google Scholar]
- 8.Tybring G, Nordin J, Bergman T, Bertilsson L. An S-mephenytoin cysteine conjugate identified in urine of extensive but not of poor metabolizers of S-mephenytoin. Pharmacogenetics. 1997;7:355–360. doi: 10.1097/00008571-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tybring G, Bertilsson L. A methodological investigation on the estimation of the S-mephenytoin hydroxylation phenotype using the urinary S/R ratio. Pharmacogenetics. 1992;2:241–243. doi: 10.1097/00008571-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55:177–184. doi: 10.1007/s002280050615. [DOI] [PubMed] [Google Scholar]
- 11.Sanz EJ, Villén T, Alm C, Bertilsson L. S-Mephenytoin hydroxylation phenotypes in a Swedish population determined after coadministration with debrisoquin. Clin Pharmacol Ther. 1989;45:495–499. doi: 10.1038/clpt.1989.63. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Uppsala: WHO Collaborating Centre for International Drug Monitoring; 1995. WHO adverse reaction dictionary. [Google Scholar]