Abstract
Aims
To measure the milk to plasma ratio (M/P) of quinapril and its active metabolite quinaprilat in lactating mothers and to assess likely infant exposure.
Methods
A single dose of quinapril 20 mg was administered to six healthy mothers who had been breastfeeding their infants for at least 2 weeks. Blood was sampled for the measurement of quinapril and quinaprilat at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h. Milk was collected for measurement of quinapril and quinaprilat concentrations over the periods −4–0, 0–4, 4–8, 8–12, 12–18, 18–24 h. The areas under the plasma and milk concentration-time curves were estimated and an M/P ratio derived for both quinapril and quinaprilat.
Results
The M/P ratio for quinapril was 0.12 (95% CI 0.09,0.14). No quinapril was detected in milk after 4 h. No quinaprilat was detected in any of the milk samples. The estimated ‘dose’ of quinapril that would be received by the infant was 1.6% (95% CI 1.0,2.2) of the maternal dose, adjusted for respective weights.
Conclusions
Quinapril appears to be ‘safe’ during breastfeeding according to conventional criteria, although as always, the risk:benefit ratio should be considered when it is to be given to a nursing mother.
Keywords: milk, pharmacokinetics, quinapril, quinaprilat
Introduction
Quinapril is a nonsulphydryl angiotensin converting enzyme (ACE) inhibitor which is de-esterified in vivo to the active diacid metabolite quinaprilat [1]. It is used in the treatment of hypertension and heart failure. As with the other ACE inhibitors, quinapril might be used in mothers who are breastfeeding their children. Therefore, it is important to know the amounts of quinapril and quinaprilat that pass into human milk so that some assessment can be made of the risk to the suckling infant. The best index of the amount of drug that passes into human milk is the milk to plasma ratio (M/P) based on the area under the concentration-time curves (AUC) of the drug in milk and plasma. From this, the potential dose received by the infant can be calculated, and compared with the maternal dose, correcting for respective body weights.
The aim of this study was to assess the M/PAUC for quinapril and quinaprilat in women who are breastfeeding, and to assess the likely infant exposure.
Methods
Subjects
Subjects were recruited if they were actively lactating for more than 2 weeks postpartum, aged 18–45 years, in good health as determined by medical history, physical examination, electrocardiogram and standard laboratory measurements, and using reliable contraception. The mothers had volunteered for the study on the basis that they were to stop breastfeeding at the time of the study, and that breast milk during and following the study would not be fed to their infants. Exclusion criteria included history of significant chronic disease, angioedema, alcoholism, drug abuse, use of medication other than contraception during the 2 weeks prior to the start of the study, pregnancy, blood donation within 1 month prior to the start of the study or history of sensitivity to quinapril or other ACE inhibitors. The study was approved by the Canterbury Area Health Board ethics committee and written informed consent was obtained from each participant.
Drug dosage and administration
Each woman received one 20 mg tablet of quinapril orally at around 09.00 h on the day of the study. The exact time was noted.
Blood and milk collection
Blood was sampled via an indwelling catheter until 12 h and by venepuncture thereafter for measurement of quinapril and quinaprilat concentrations. Samples were taken just prior to the dose administration, and again at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h after tablet intake. All the milk from both breasts was expressed by electric or manual breast pump over the periods −4–0, 0–4, 4–8, 8–12, 12–18, and 18–24 h after drug dosing. A sample of milk was retained from each expression for measurement of quinapril and quinaprilat concentrations and the remaining milk was discarded. For both blood and milk samples, exact times were recorded if they were different from those in the protocol. The samples were stored at −80 °C then sent in bulk under dry ice to Ann Arbor, Michigan for analysis.
Analytical methods
Quinapril and quinaprilat were assayed in plasma using gas chromatography with electron capture detection. Details of the method and its precision have been given previously [2].
The method for determining quinapril and quinaprilat concentrations in human breast milk was validated for a range of 5–1000 ng ml−1 using a 1.0 ml sample. Three eight-point standard curves were generated on each of 3 separate days. Quality control samples were prepared at three levels from spiked pools of human breast milk and assayed in duplicate with each standard curve. A 1.0 ml aliquot of each unknown, standard and quality control sample was taken and the pH was adjusted. After extraction, the sample was analysed on a gas-liquid chromatographic system with electron-capture detection. Method specifics were considered confidential by the analytical contract laboratory.
The percent error for the mean of the standard values at the lower limit of quantification (5 ng ml−1) was 2.0% for quinapril and 4.0% for quinaprilat, while the percent coefficient of variation (CV%) was 3.2% for quinapril and 5.1% for quinaprilat. The CV% across all levels of the standard curve was 3.7% for quinapril and 4.3% for quinaprilat. Quality control sample CV% did not exceed 5.2% and averaged 2.7% and 5.0% for quinapril and quinaprilat, respectively.
Stability of quinapril and quinaprilat was established through three freeze/thaw cycles and for 8 days at room temperature.
The recovery of quinapril, quinaprilat and the internal standard extracted for breast milk could not be done because of the nature of the assay.
Pharmacokinetic analysis
The area under the plasma concentration vs time curve from zero to infinity (AUC(0,∞)) was calculated for both quinapril and quinaprilat using the linear trapezoidal rule for the ascending part of the curve and the log-linear trapezoidal rule for the descending part of the curve. An estimation of the terminal elimination rate constant (λz) was made using linear regression of the visually determined postabsorptive elimination phase, to enable extrapolation of the AUCs from the last measurable point (Clast) to infinity (i.e. Clast λz−1) The AUC for milk was calculated using rectangular areas (sum of concentrations × collection times).
Results
Six healthy women volunteers, aged 25–32 years (mean 29), and weighing 57–105 kg (mean 76) participated in the study. Four women (subjects 1–4) were taking norethisterone 0.35 mg daily at the time of the study. Subject 4 was also using clobetasol 17-propionate topically and subject 6 required two 1 g doses of paracetamol within the 24 h study period. The breastfed infants were aged from 16 weeks to 9 months.
The mean results for quinapril and quinaprilat in plasma and milk are shown in Table 1.
Table 1.
Milk and plasma concentrations, and M/P ratios for quinapril and quinaprilat
| Subject | Weight (kg) | Quinapril Dose weight adjusted (mg kg−1) | Plasma AUC(0,∝) (µg l−1 h) | Milk AUC (µg l−1 h) | M/P | Baby's dose weight adjusted (% maternal dose) | Quinaprilat Plasma AUC(0,∝) (µg l−1 h) | Milk* AUC (µg l−1 h) | M/P* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 0.313 | 240 | 21.0 | 0.09 | 1.01 | 2824 | < 60 | < 0.02 |
| 2 | 87 | 0.230 | 215 | 27.8 | 0.13 | 1.81 | 2101 | < 60 | < 0.03 |
| 3 | 65 | 0.308 | 180 | < 20.0 | < 0.11 | 0.97 | 1955 | < 60 | < 0.03 |
| 4 | 57 | 0.351 | 241 | 35.5 | 0.15 | 1.52 | 1379 | < 60 | < 0.04 |
| 5 | 76 | 0.263 | 350 | 30.1 | 0.09 | 1.72 | 1763 | < 60 | < 0.03 |
| 6 | 105 | 0.190 | 239 | 32.8 | 0.14 | 2.59 | 1892 | < 60 | < 0.03 |
| Mean | 76 | 0.28 | 244 | 27.9 | 0.12 | 1.6 | 1985 | < 60 | < 0.03 |
| s.d | 18 | 0.06 | 57 | 6.3 | 0.03 | 0.6 | 478 | ||
| 95%CI | 184,304 | 21.3,34.7 | 0.09,0.14 | 1.0,2.2 | 1484,2488 |
not detected in milk. These are ‘at worst’ estimates.
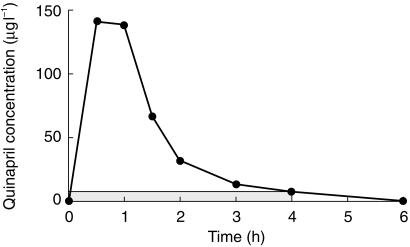
Quinapril concentrations in plasma fell to below quantifiable limits (5.0 µg l−1) by 6 h post dose in all mothers. Quinapril concentrations were only detectable in the milk samples from the first postdose collection period (0–4 h) (Figure 1). In one volunteer (subject 3), there was no quinapril detected in milk at any stage. For the purposes of calculating the mean results and the M/P ratio, this subject was assumed to have concentrations equal to the limit of quantification (i.e. 5.0 µg l−1). This allows for an ‘at worst’ estimation of the M/P ratio.
Figure 1.
Quinapril concentrations in plasma (•) and in milk (shaded area) (subject 2).
Quinaprilat concentrations in plasma were detectable in all subjects up to 12 h, in three of the six at 18 h, and in none at 24 h. Quinaprilat concentrations in milk were below the limit of quantification in all subjects at all time points.
The M/PAUC ratio for quinapril was 0.12 (95% CI 0.09–0.14). For quinaprilat an M/PAUC ratio of < 0.03 can be calculated if concentrations were assumed to be at the limit of detection (5.0 µg l−1). This is an ‘at worst’ situation.
Five of the six subjects had side-effects that may have been related to quinapril. Subject 1 fainted briefly, subject 4 developed hypotension (systolic blood pressure < 85 mmHg), subject 5 developed fatigue, and subjects 3 and 6 had headaches. These side-effects were significant but were not unexpected given that 20 mg is a high ‘starting dose’ for quinapril. Initiation of quinapril would normally be by gradual titration.
Discussion
These results indicate that quinapril administration to a lactating mother is unlikely to cause problems in a suckling infant. The mean dose of quinapril received by the infant over 24 h is 27.9 µg for each litre of milk ingested. Assuming a maternal weight of 70 kg (and therefore a dose of 0.28 mg kg−1) and ingestion of 150 ml kg−1 day−1 of milk, this represents 1.6% (95%CI 1.0,2.2) of the maternal dose, corrected for respective body weights. This is well below the 10% level that is conventionally considered as a ‘safe’ cut-off [3]. Furthermore, since there was no quinapril detected in the milk after 4 h, feeding just prior to, or greater than 4 h after maternal dose ingestion can increase the safety margin.
The active metabolite, quinaprilat was not able to be quantified at all, indicating that the maximum dose of this that an infant might receive over 24 h is less than 5 µg for each litre of milk ingested. Assuming complete conversion of quinapril to quinaprilat, this dose is less than 0.26% of the maternal dose, adjusted for body weight. This is a negligible burden for the infant, and as quinaprilat is the acid salt of quinapril, it is likely that it would be poorly absorbed following oral ingestion.
To our knowledge, there have not been any other studies investigating the safety of quinapril during breastfeeding. Three studies have investigated the transfer of other ACE inhibitors, captopril [4] and enalapril [5, 6] into breast milk. The results of these studies suggest that the infant dose for both drugs is likely to be < 1% of the maternal dose, when adjusted for the difference in body weight. Therefore, the results of the current study are consistent with those of other ACE inhibitors. A potential limitation of this study is that it was a single dose study. However, following multiple doses of quinapril there is little accumulation of quinapril and quinaprilat in plasma. Our results and the principles of diffusion suggest that it is unlikely that accumulation in milk would occur during chronic administration. This does not exclude the possibility of accumulation if excessive doses are used in renal dysfunction.
Taken together, these results suggest that quinapril can be considered compatible with breast-feeding, according to conventional criteria. However, the effect of very small doses of quinapril on the developing infant remain unknown, and it would be expected that quinapril converts to the active quinaprilat in the infant. This should be taken into account along with all other considerations related to risk-benefit assessment in any decision regarding breastfeeding during quinapril administration.
References
- 1.Wadworth AN, Brogden RN. Quinapril. A review of its pharmacological properties, and therapeutic efficacy in cardiovascular disorders. Drugs. 1991;41:378–399. doi: 10.2165/00003495-199141030-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ferry JJ, Horvath AM, Easton-Taylor M, Toothaker RD, Colburn WA. Determination of quinapril and its active metabolite in human plasma and urine by gas chromatography with electron-capture detection. J Chromatogr. 1987;421:187–191. doi: 10.1016/0378-4347(87)80396-1. [DOI] [PubMed] [Google Scholar]
- 3.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and Human Lactation. 2. Elsevier; 1996. pp. 67–74. [Google Scholar]
- 4.Devlin RG, Fleiss PM. Captopril in human blood and breast milk. J Clin Pharmacol. 1981;21:110–113. doi: 10.1002/j.1552-4604.1981.tb01759.x. [DOI] [PubMed] [Google Scholar]
- 5.Huttunen K, Grönhagen-Riska C, Fyhrquist F. Enalapril treatment of a nursing mother with slightly impaired renal function. Clin Nephrol. 1989;31:278. [PubMed] [Google Scholar]
- 6.Redman CWG, Kelly JG, Cooper WD. The excretion of enalapril and enalaprilat in human breast milk. Eur J Clin Pharmacol. 1990;38:99. doi: 10.1007/BF00314815. [DOI] [PubMed] [Google Scholar]