Abstract
Aims
Tamsulosin is an α1-adrenoceptor antagonist for the treatment of symptomatic benign prostatic hyperplasia with a tolerability similar to that of placebo in short-term, placebo-controlled studies with limited patient numbers. The present study was designed to test the safety of tamsulosin treatment in a large cohort of men during a prolonged period of time, particularly with regard to comedications.
Methods
A multicentre, open-label phase IIIb study with 1784 patients receiving 0.4 mg o.d. tamsulosin for 6 months was performed according to good clinical practice guidelines. The analysis was performed on an intention-to-treat basis and powered to detect adverse events (AE) occurring in 0.15% of patients with 95% confidence.
Results
During a total drug exposure time of 811 patient years, 386 AE were recorded in 253 patients (14.2%; 95% confidence intervals [CI] 12.0–15.2%). Twenty-nine patients suffered 44 serious AE including five fatal events (CI 0.12–0.73%) due to myocardial infarction (n = 3) and to pneumonia and a car accident (one each), but all deaths were judged to be unlikely to be related to study medication. The frequency of AE in patients without any comedication (n = 1095) was 13.0% (CI 11.3–14.9%). In a logistic regression analysis β-adrenoceptor blockers, converting enzyme inhibitors, antidiabetics and diuretics did not significantly affect the odds ratio for having AE. However, concomitant α-adrenoceptor antagonists (a protocol violation) and treatment with verapamil (which also has α-adrenoceptor antagonist activity) significantly enhanced the odds ratio for having AE to 3.87 (CI 1.52–9.85) and 3.17 (CI 1.52–6.58), respectively. Minor increases in the odds ratio, which did not reach statistical significance, were also observed for Ca2+ antagonists other than verapamil and for nitrates.
Conclusions
We conclude that tamsulosin has a good safety profile relative to AE rates in the placebo arms of previous studies on tamsulosin even in the presence of most potentially complicating comedications. No major unexpected severe AE were recorded during our 6 months study.
Keywords: benign prostatic hyperplasia, drug interaction, tamsulosin, tolerability
Introduction
Benign prostatic hyperplasia is a frequent disease state in the elderly male, which is clinically characterized by lower urinary tract symptoms (LUTS) suggestive of benign prostatic obstruction (BPO). In recent years it has been demonstrated in numerous placebo-controlled studies that α1-adrenoceptor antagonists provide rapid and effective symptom relief in patients with LUTS suggestive of BPO [1, 2], and that α1-adrenoceptor antagonists are more effective in this regard than other forms of medical treatment [3, 4]. Classical α1-adrenoceptor antagonists such as doxazosin and terazosin were originally developed for the treatment of arterial hypertension, and accordingly also lower blood pressure in patients with LUTS suggestive of BPO [5, 6]. While such blood pressure reductions may be small in most patients with LUTS, particularly in those who are normotensive, they may contribute to a significantly increased withdrawal rate due to adverse events relative to placebo [2].
Tamsulosin is an α1-adrenoceptor antagonist for the treatment of LUTS suggestive of BPO [7]. Its efficacy in improving LUTS is similar to that of other α1-adrenoceptor antagonists in direct comparative studies [8, 9] and also in indirect comparison of placebo-controlled studies for the individual drugs [2]. However, tamsulosin does not cause clinically relevant blood pressure lowering in patients with LUTS suggestive of BPO and hence cannot be used to treat hypertension [7]. In direct comparisons of equi-effective doses with regard to LUTS treatment, tamsulosin causes less blood pressure lowering and fewer side-effects [9], less night-time orthostatic hypotension [10] and less vascular α1-adrenoceptor antagonism [11] than terazosin. Accordingly, several double-blind studies over 12–13 weeks have reported that the incidence of adverse events (AE) during treatment of patients with LUTS suggestive of BPO with 0.4 mg o.d. tamsulosin is similar to that of placebo [12–14]. However, these studies which had only 183–254 patients per treatment arm and lasted only 12–13 weeks were not designed or powered to detect rare (less than 1% of patients) but potentially severe AE. Therefore, the present study was designed to detect hitherto unrecognized rare but severe side-effects of tamsulosin which might manifest during extended tamsulosin treatment of large patient numbers; in this regard the study was powered to detect AE occurring in 0.15% of patients with 95% confidence. A secondary aim was to investigate effects of concomitant treatment with other drug classes on the tolerability of tamsulosin.
Methods
A multicentre (287 office-based urologists), open-label phase IIIb study with 1784 patients receiving 0.4 mg o.d. tamsulosin for 6 months was performed according to good clinical practice guidelines between January 1996 and March 1997. The study protocol had been approved by the respective regional ethical review boards, and all patients had given informed consent. Inclusion criteria were the diagnosis of LUTS suggestive of BPO by a board-certified urologist, an International Prostate Symptom Score (I-PSS) > 7, and the ability and willingness of the patient to follow the protocol and complete the I-PSS questionaire (German version) [15] sufficiently. Exclusion criteria were a history of bladder neck or prostate surgery, severe liver insufficiency, acute urinary tract infection or other conditions which may affect micturition, orthostatic dysregulation, clinically relevant pathological lab values, and allergic reactions to previously prescribed α1-adrenoceptor antagonists. Concomitant use of drugs which could potentially influence the pharmacodynamic properties of tamsulosin with regard to the prostate (other α1-adrenoceptor antagonists, 5α-reductase inhibitors, plant extracts intended for the treatment of LUTS suggestive of BPO) was not allowed; if such drugs had been used in the past, they had to be discontinued for at least 3 months prior to study entry. Patients were to be withdrawn from the study in cases of serious AE, if treatment for another disease including urinary tract infection became necessary, which might possibly influence the outcome of the present study, and upon the occurrence of any condition belonging to the list of exclusion criteria; in the case of withdrawal, a complete set of assessments as scheduled for visit 4 had to be performed, if possible.
The study consisted of four office visits. At visit 1, patients who fulfilled the above criteria were included if they wished to participate. They received 0.4 mg tamsulosin (modified release capsules) to be taken once daily in the morning after breakfast for a period of 24 weeks. Visits 2, 3 and 4 were scheduled 8, 16 and 24 weeks later, respectively. Patient compliance was estimated from capsule counts at each visit, and a patient was considered compliant if he had taken at least 80% of the study medication required for the respective period. At each visit, the patient was asked to fill the I-PSS questionnaire and was interviewed regarding concomitant medication and occurrence of AE. Information on AE was collected based on a standardized questionnaire according to good cnical practice guidelines which included type of AE, beginning and duration, intensity, frequency, necessity for AE treatment, assumed relationship with study medication, severity and outcome.
A total of 1784 patients participated in the study; unless otherwise noted, all further data, particularly all analyses related to the tolerability of tamsulosin, refer to this intention-to-treat population. Inspection of the case record forms showed that 543 patients had violated key protocol points and were excluded from the efficacy analysis; the remaining 1241 patients were considered as the key-protocol-point population. Among these, 533 patients had violated the study protocol in minor ways; the remaining 708 patients, who had strictly followed the study protocol, were used as primary population to analyse the efficacy of tamsulosin.
Data are presented as mean ± s.e. mean of n patients. AE incidences are given in percentage with 95% confidence intervals (CI). The association between age and a) comedications and b) AE incidence was analysed by multiple logistic regression; the resulting odds ratios are also presented with 95% confidence intervals. Additionally, P values for potential individual interactions were calculated from the overall analysis. Data handling and biometrical analysis were performed by Medidata GmbH (Konstanz, Germany).
Results
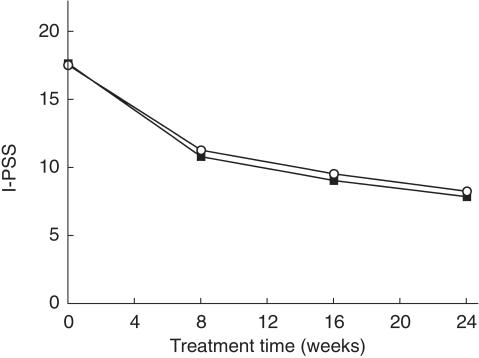
At baseline, mean patient age was 64.1 ± 0.2 (range 21–91) years. Prior to treatment, patients had an I-PSS of 17.6 ± 0.1 and 17.7 ± 0.2 points in the intention-to-treat population and in patients who had strictly adhered to the study protocol, respectively. Tamsulosin treatment similarly lowered the I-PSS in both populations with the majority of the symptom reduction occurring in the first 8 weeks of treatment (Figure 1). The occurrence of acute urinary retention has been used as an indicator of disease progression in patients with BPO [16]. In the present study, acute urinary retention was detected in four cases, i.e. 0.49 per 100 patient years.
Figure 1.
Effect of tamsulosin treatment on I-PSS in the intention-to-treat population and patients who had strictly adhered to the study protocol. Data are mean ± s.e. mean of 1426–1784 patients (○, intention-to-treat population) and 708 patients (▪, strictly per protocol population).
The total drug exposure time was 811 patient years. During this period a total of 386 AE were recorded in 253 patients (14.2%; CI 12.0–15.2%). The most frequent AE, i.e. those occurring in at least five patients (0.3% of all patients) are shown in Table 1. A probable, possible or unlikely relationship to study medication was assessed to have occurred in 36%, 39% and 24% of patients, respectively. A total of 149 patients terminated the study prematurely due to AE, and 29 patients suffered from 44 serious AE including five fatal events (CI 0.12–0.73%). The fatal events were due to myocardial infarction in three cases and to pneumonia and a car accident in one case each; all deaths were judged to be unlikely to be related to study medication. Including these five cases, the following serious AE were noted on the case record forms: death (n = 5), myocardial infarct and dizziness (n = 4 each), cardiovascular disorder and urinary retention (n = 3 each), accidental injury, hypotension, pneumonia and postural hypotension (n = 2 each) and abnormal ejaculation, angina pectoris, cerebrovascular accident, cholelithiasis, coronary artery disorder, epididymitis, gastrointestinal disorder, headache, healing abnormal, heart arrest, hernia, infection, leg cramps, paraesthesia, prostatic carcinoma, shock, and sweating (n = 1 each). With the exception of some episodes of hypotension (including postural hypotension) and abnormal ejaculation, all of the above events were rated by the investigators as unlikely to be related to study medication; even some of the cases of hypotension were judged unlikely to be related since the patient had a history of hypotension or the episode occurred following 4 months of treatment.
Table 1.
Most frequent adverse events during a 6 months treatment with 0.4 mg tamsulosin o.d.
| Adverse event | n | % (95% CI) |
|---|---|---|
| Dizziness | 45 | 2.5 (1.85, 3.36) |
| Abnormal ejaculation | 29 | 1.6 (1.09, 2.33) |
| Headache | 29 | 1.6 (1.09, 2.33) |
| Hypotension | 26 | 1.5 (0.95, 2.13) |
| Gastrointestinal disorder | 18 | 1.0 (0.60, 1.59) |
| Nausea | 17 | 1.0 (0.56, 1.52) |
| Cardiovascular disorder | 11 | 0.6 (0.31, 1.10) |
| Impotence | 10 | 0.6 (0.27, 1.03) |
| Dry mouth | 9 | 0.5 (0.23, 0.96) |
| Sweating | 9 | 0.5 (0.23, 0.96) |
| Arrhythmia | 8 | 0.4 (0.19, 0.88) |
| Postural hypotension | 7 | 0.4 (0.16, 0.81) |
| Pruritus | 7 | 0.4 (0.16, 0.81) |
Five cases (0.3% [95% CI 0.09, 0.65%]) each were seen for death, allergic reaction, asthenia, infection, paraesthesia and tachycardia.
The frequency of AE in patients without any comedication (n = 1095) was 13.0% (CI 11.3, −14.9%). The most frequent comedications were Ca2+ antagonists, β-adrenoceptor blockers, converting enzyme inhibitors, nitrates, antidiabetics and diuretics (Table 2). In a multiple logistic regression analysis none of these comedications significantly affected the odds ratio for having AE (Table 2). However, concomitant α-adrenoceptor antagonists (a protocol violation) and treatment with verapamil (which also has α-adrenoceptor antagonist activity [17]) significantly enhanced the odds ratio for AE (Table 2). Minor increases in the odds ratio, which failed to reach statistical significance, were observed for Ca2+ antagonists other than verapamil and for nitrates (Table 2).
Table 2.
Multiple logistic regression analysis regarding the effect of comedication on adverse events emerging during tamsulosin treatment.
| Iindependent variable | n | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Age (per year) | 1784 | 1.003 (0.991, 1.016) | 678 |
| α-adrenoceptor blockers | 14 | 3.872 (1.523, 9.847) | 17 |
| Verapamil | 27 | 3.166 (1.513, 6.580) | 10 |
| Other Ca2+ antagonists | 99 | 1.632 (1.010, 2.639) | 93 |
| Nitrates | 76 | 1.840 (1.024, 3.309) | 87 |
| Converting enzyme inhibitors | 87 | 0.554 (0.290, 1.055) | 132 |
| β-adrenoceptor blockers | 88 | 1.056 (0.620, 1.797) | 867 |
| Diuretics | 55 | 0.773 (0.368, 1.623) | 568 |
| Oral antidiabetics | 66 | 1.072 (0.555, 2.070) | 863 |
| Insulin | 13 | 1.192 (0.286, 4.969) | 840 |
| Antilipaemics | 43 | 0.841 (0.372, 1.903) | 727 |
Note that odds ratios with 95% CI were calculated for each comedication separately while the P-values were calculated from the overall analysis including all comedications; hence, confidence not including unity for nitrates and ‘other Ca2+ antagonists’ do not correspond to statistical significance at the P < 0.05 level.
Discussion
Four previous double-blind, placebo-controlled studies of 12–13 weeks duration with 183–254 patients each receiving active treatment have demonstrated that the overall incidence of AE during treatment with 0.4 mg tamsulosin o.d. is not significantly different from that during placebo treatment [12–14]. The present study was designed to test the safety of tamsulosin treatment in a large cohort of men during a prolonged period of time, particularly with regard to comedications.
Although the present open-label study was not primarily designed to investigate the efficacy of tamsulosin in the treatment of LUTS suggestive of BPO, the tamsulosin treatment-associated reductions in the I-PSS were similar to the symptom score reductions obtained in the previous double-blind, placebo-controlled studies [12–14], particularly if alterations during the run-in phase of the earlier studies are taken into consideration; the treatment efficacy was similar when the present data were analysed on an intention-to-treat basis or only in those patients who had completely fulfilled all protocol criteria. Thus, we have studied the tolerability of tamsulosin under conditions where its efficacy is similar to that of placebo-controlled studies with tamsulosin or other α1-adrenoceptor antagonists [2].
While LUTS suggestive of BPO markedly affects quality of life, it usually causes little morbidity or mortality. In some cases, however, untreated LUTS may lead to acute urinary retention [16]. It was recently reported from the PLESS study that the 5α-reductase inhibitor, finasteride, probably via its prostate-shrinking effects, can reduce the incidence of acute urinary retention from 2.40 to 0.93 cases per 100 patient years in the first year of treatment [16]. In contrast, α1-adrenoceptor antagonists are believed to primarily inhibit prostate smooth muscle contractility rather than reduce prostate size [1]. The present study, however, has detected only a very low incidence of acute urinary retention, i.e. 0.49 cases per 100 patient years. While this is difficult to interpret in the absence of a placebo group in our study, it should be noted that baseline symptom severity and age in our study were at least as high as in PLESS. Moreover, data from direct comparative studies between finasteride and the α1-adrenoceptor antagonists, alfuzosin [4] and doxazosin [18], as well as from a placebo-controlled study with alfuzosin [19] also indicate that the incidence of acute urinary incidence is similar during α1-adrenoceptor antagonist and finasteride treatment. Since none of these studies was designed and powered to look into effects of α1-adrenoceptor antagonists on the progression of LUTS suggestive of BPO, controlled studies to this effect appear warranted.
The primary aim of the present study was to exclude rare but severe AE. Although our study was powered to detect AE occurring in 0.15% of patients with 95% confidence, no major unexpected severe AE were recorded during our 6 months study. Thus, all serious AE were considered unlikely to be related to study medication except for a few cases of hypotension or abnormal ejaculation, which are well known AE of all α1-adrenoceptor antagonists. Although AE had been documented with a specific questionnaire at each visit, the overall incidence of AE was lower than in the placebo-controlled studies [12–14]. While this may at least partly be explained by the conditions of an open-label study, it should be noted that the AE reported most frequently in the present study, i.e. dizziness, abnormal ejaculation, headache and hypotension, are the same which were seen most frequently in the placebo-controlled trials. While all of these AE are typical for an α1-adrenoceptor antagonist [20], except for abnormal ejaculation none of them occurred more frequently during active compared with placebo treatment in the controlled studies with tamsulosin [12–14]. Since bladder neck, urethra and prostate form a functional unit in control of bladder outlet resistence in males, abnormal (i.e. retrograde) ejaculation may be considered as an indicator of efficacy rather than an AE. Taken together, the present data, in agreement with the smaller placebo-controlled studies of shorter duration [12–14] and postmarketing surveillance data collected in Germany [21] and from the UK green card scheme [22] after the completion of the present study, indicate a very good safety of tamsulosin.
The typical patient receiving medical treatment for LUTS suggestive of BPO is in his mid-sixties, i.e. an age where he is likely to suffer comorbidities and hence receive concomitant other medications. Indeed almost 40% of our patients had some form of comedication, most of them related to cardiovascular and metabolic function. This opens the possibility of pharmacokinetic and/or pharmacodynamic drug interactions. Therefore, it was the secondary aim of our study to investigate effects of concomitant medication on AE incidence. Our data show that only concomitant treatment with other α1-adrenoceptor antagonists, a protocol violation, and with verapamil, which has α1-adrenoceptor antagonist effects at therapeutic doses [17], significantly increased the likeliness for AE. A reduced tolerability upon concomitant treatment with verapamil has also been reported for another α1-adrenoceptor antagonist, terazosin [23]. The concomitant use of other drugs with α1-adrenoceptor antagonist activity is an explicit contraindication for the use of tamsulosin, which is clearly noted in the package insert, and our data emphasize the importance of this contraindication.
A trend for a slight increase in AE incidence, which failed to reach statistical significance in a multiple logistic regression, was seen for other Ca2+ entry blockers and nitrates, whereas converting enzyme inhibitors, β-adrenoceptor antagonists, diuretics, lipid lowering and antidiabetic drugs did not affect the AE incidence during tamsulosin treatment. In a recent postmarketing surveillance analysis of almost 20 000 patients receiving tamsulosin for 4–12 weeks comedication with Ca2+ entry blockers, converting enzyme inhibitors, β-adrenoceptor antagonists or diuretics also failed to significantly affect the tolerability of tamsulosin in a multiple logistic regression and was not associated with enhanced blood pressure responses to tamsulosin treatment [21]. Similarly, in a clinical pharmacology study comedication of tamsulosin with atenolol, nifedipine or enalapril also failed to cause additional blood pressure lowering [24]. While the reasons for the excellent tolerability of tamsulosin and its lack of blood pressure reduction are not fully understood [11], these features clearly discriminate it from other clinically used α1-adrenoceptor antagonists. For example, concomitant medication with haemodynamically active drugs was reported to significantly decrease the tolerability of alfuzosin [25] and exaggerate the blood pressure responses to doxazosin [26–28] or terazosin [23].
In conclusion, our data as well as recent postmarketing surveillance data from Germany [21] and the UK [22] do not provide evidence for rare but severe side-effects of tamsulosin. The good tolerability profile of tamsulosin appears to be maintained even in the presence of blood pressure-lowering comedication, except for drugs with α1-adrenoceptor antagonist activity, which are an explicit contraindication for tamsulosin use. A potential effect of comedication with Ca2+ entry blockers or nitrates on tamsulosin tolerability as well as possible reduction of incidence of acute urinary retention by tamsulosin treatment merit further studies.
Acknowledgments
This manuscript is based on a study funded by Yamanouchi Pharma GmbH (Heidelberg, Germany).
References
- 1.Rane A. Endocrine and adrenergic pharmacological intervention in diseases of the prostate. Br J Clin Pharmacol. 1998;45:329–337. doi: 10.1046/j.1365-2125.1998.t01-1-00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- 3.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 4.Debruyne FM, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. European ALFIN Study Group. Eur Urol. 1998;34:169–175. doi: 10.1159/000019706. [DOI] [PubMed] [Google Scholar]
- 5.Lowe FC. Safety assessment of terazosin in the treatment of patients with symptomatic benign prostatic hyperplasia: a combined analysis. Urology. 1994;44:46–51. doi: 10.1016/s0090-4295(94)80008-1. [DOI] [PubMed] [Google Scholar]
- 6.Kirby RS. Doxazosin in benign prostatic hyperplasia: effects on blood pressure and urinary flow in normotensive and hypertensive men. Urology. 1995;46:182–186. doi: 10.1016/s0090-4295(99)80191-5. 10.1016/s0090-4295(99)80191-5. [DOI] [PubMed] [Google Scholar]
- 7.Wilde MI, McTavish D. Tamsulosin – a review of its pharmacological properties and therapeutic potential in the management of benign prostatic hyperplasia. Drugs. 1996;52:883–896. doi: 10.2165/00003495-199652060-00012. [DOI] [PubMed] [Google Scholar]
- 8.Buzelin JM, Fonteyne E, Kontturi MJ, Witjes WPJ, Khan A. Comparison of tamsulosin with alfuzosin in the treatment of patients with lower urinary tract symptoms sugestive of bladder outlet obstruction (symptomatic benign prostatic hyperplasia) Br J Urol. 1997;80:597–605. doi: 10.1046/j.1464-410x.1997.00205.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, Lee C. Clinical comparison of selective and non-selective α1-adrenoreceptor antagonists in benign prostatic hyperplasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol. 1997;80:606–611. doi: 10.1046/j.1464-410x.1997.00411.x. [DOI] [PubMed] [Google Scholar]
- 10.de Mey C, Michel MC, McEwen J, Moreland T. A double-blind comparison of terazosin and tamsulosin on their differential effects on ambulatory blood pressure and nocturnal orthostatic stress testing. Eur Urol. 1998;33:481–488. doi: 10.1159/000019639. [DOI] [PubMed] [Google Scholar]
- 11.Schäfers RF, Fokuhl B, Wasmuth A, et al. Differential vascular α1-adrenoceptor antagonism by tamsulosin and terazosin. Br J Clin Pharmacol. 1999;47:67–74. doi: 10.1046/j.1365-2125.1999.00856.x. 10.1046/j.1365-2125.1999.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapple CR, Wyndaele JJ, Nordling J, Boeminghaus F, Ypma Afgvm, Abrams P. Tamsulosin the first prostate-selective α1A-adrenoceptor antagonist. A meta-analysis of two randomized, placebo-controlled multicentre studies in patients with benign prostatic obstruction (symptomatic BPH) Eur Urol. 1996;29:155–167. [PubMed] [Google Scholar]
- 13.Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 14.Narayan P, Tewari A Members of United States; –; Study Group. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. J Urol. 1998;160:1701–1706. [PubMed] [Google Scholar]
- 15.Cockett ATK, Aso Y, Denis L, et al. The 2nd International Consultation on Benign Prostatic Hyperplasia (BPH) Channel Island, Scientific Communication International Ltd; 1993. pp. 649–660. [Google Scholar]
- 16.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 17.Motulsky HJ, Snavely MD, Hughes RJ, Insel PA. Interaction of verapamil and other calcium channel blockers with α1- and α2-adrenergic receptors. Circ Res. 1983;52:226–231. doi: 10.1161/01.res.52.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Kirby R, Altwein JE, Bartsch G, Boyle P, Jardin A, Roehrborn CG. Results of the PREDICT (Prospective European Doxazosin and Combination Therapy) trial. J Urol. 1999;161(Suppl):266. [Google Scholar]
- 19.Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P, The BPH-ALF Group. Alfuzosin for treatment of benign prostatic hypertrophy. Lancet. 1991;337:1457–1461. doi: 10.1016/0140-6736(91)93140-5. [DOI] [PubMed] [Google Scholar]
- 20.Carruthers SG. Adverse effects of α1-adrenergic blocking drugs. Drug Safety. 1994;11:12–20. doi: 10.2165/00002018-199411010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Michel MC, Mehlburger L, Bressel H-U, Schumacher H, Schäfers RF, Goepel M. Tamsulosin treatment of 19, 365 patients with lower urinary tract symptoms: does comorbidity alter tolerability? J Urol. 1998;160:784–791. doi: 10.1016/S0022-5347(01)62787-3. [DOI] [PubMed] [Google Scholar]
- 22.Mann RD, Biswas P, Freemantle S, Pearce G, Wilton L. The pharmacovigilance of tamsulosin: event data on 12484 patients. BJU Int. 2000;85:446–450. doi: 10.1046/j.1464-410x.2000.00546.x. 10.1046/j.1464-410x.2000.00546.x. [DOI] [PubMed] [Google Scholar]
- 23.Pool JL. Combination antihypertensive therapy with terazosin and other antihypertensive agents: results of clinical trials. Am Heart J. 1991;122:926–931. doi: 10.1016/0002-8703(91)90813-w. [DOI] [PubMed] [Google Scholar]
- 24.Lowe FC. Coadministration of tamsulosin and three antihypertensive agents in patients with benign prostatic hyperplasia: pharmacodynamic effect. Clin Ther. 1997;19:730–742. doi: 10.1016/s0149-2918(97)80097-5. [DOI] [PubMed] [Google Scholar]
- 25.Lukacs B, Leplege A, Thibault P, Jardin A. Prospective study of men with clinical benign prostatic hyperplasia treated with alfuzosin by general practitioners: 1-year results. Urology. 1996;48:731–740. doi: 10.1016/S0090-4295(96)00302-0. 10.1016/s0090-4295(96)00302-0. [DOI] [PubMed] [Google Scholar]
- 26.Lindner UK, von Manteuffel G-E, Stafunsky M. The addition of doxazosin to the treatment regimen of hypertensive patients not responsive to nifedipine. Am Heart J. 1988;116:1814–1820. doi: 10.1016/0002-8703(88)90235-9. [DOI] [PubMed] [Google Scholar]
- 27.de Planque BA. A double-blind comparative study of doxazosin and prazosin when administered with β-blockers or diuretics. Am Heart J. 1991;121:304–311. doi: 10.1016/0002-8703(91)90863-d. [DOI] [PubMed] [Google Scholar]
- 28.Englert RG, Barlage U. The addition of doxazosin to the treatment regimen of patients with hypertension not adequately controlled by β-blockers. Am Heart J. 1991;121:311–316. doi: 10.1016/0002-8703(91)90864-e. [DOI] [PubMed] [Google Scholar]