Abstract
Aims
Since relatively little is known about the pharmacokinetics of 6-thioguanine (6TG) in children receiving 6-thioguanine for maintenance therapy of acute lymphoblastic leukaemia (ALL), we studied plasma drug concentrations under standardized conditions and investigated the effect of food on parent drug pharmacokinetics and the accumulation of the active metabolites 6-thioguanine nucleotides (6-TGNs) in red cells.
Methods
Single oral doses of 40 mg of 6-TG were administered both in the fasting and fed state to children with ALL. Pharmacokinetic sampling was performed up to 6 h post dose. Daily oral doses of 40 mg m−2 of 6-TG were administered both fasting and after food over two 4 week periods. Twice weekly samples were taken for metabolite concentrations. The study design was cross-over with each child receiving dosing in either fasted or after food over a 4 week period in each phase.
Results
Eleven patients were studied. A wide interindividual variation in Cmax (median 313 pmol ml−1, range 51–737) and AUC (median 586 pmol ml−1 h, range 156–1306) was observed in the fasted state. Concomitant food administration resulted in a significant reduction in Cmax (median 71 vs 313 pmol ml−1, P = 0.006, CI from 36 to 426), AUC (median 200 vs 586 pmol ml−1 h, P = 0.006, 95% CI from 109 to 692), and time to reach Cmax (median 1.5 vs 3 h, P = 0.013, 95% CI from 0.74 to 2.73). There was no difference in the steady state concentration of red cell 6-TGNs observed after a 4 week period of 6-TG administered fasting or after food.
Conclusions
Children with ALL demonstrate significant interindividual variation in 6-TG pharmacokinetics. Although there would appear to be a reduction in parent drug Cmax and AUC with food there was no difference in 6-TGN concentrations after 4 weeks of 6-TG. Taking the drug on an empty stomach may not be necessary.
Keywords: 6-thioguanine nucleotides, 6-thioguanine, childhood lymphoblastic leukaemia, food, pharmacokinetics
Introduction
Traditionally 6-mercaptopurine (6-MP) has been the main purine antagonist used in the ‘maintenance’ phase of therapy for childhood lymphoblastic leukaemia. Recently there has been increased interest both in Europe and the United States in the use of the closely related compound 6-thioguanine (6-TG, 2-amino 6-mercaptopurine) instead. A major component of the current United Kingdom Medical Research Council trial (MRC ALL 97) for the treatment of childhood ALL is a randomised comparison of 6-MP and 6-TG.
Many studies have investigated 6-MP pharmacokinetics and have shown wide interindividual variation in the plasma concentration of the parent drug [1, 2, 3, 4]. The influence of food on drug pharmacokinetics has been reported to have a variable effect by different investigators. Some have shown reduced absorption with a significant reduction in the plasma concentration and area under the plasma concentration vs time curve (AUC) when the 6-MP dose is taken after food [5, 6]. Others, however, have reported no such effect [7, 8].
6-MP and 6TG have no intrinsic anticancer activity; they are prodrugs which undergo extensive intestinal and hepatic metabolism. Biotransformation occurs via three competing pathways; oxidation, methylation and nucleotide metabolite formation. Oxidation of 6-MP, catalysed by xanthine oxidase, is a catabolic route leading to the formation of thiouric acid. 6-TG functions as a xanthine oxidase substrate only after prior deamination by guanase. Intracellular activation of 6-MP and 6-TG, catalysed by hypoxanthine phosphoribosyltransferase, leads to the formation of active nucleotide metabolites. 6-TG is converted directly into 6-thioguanine nucleotides (6-TGNs) whereas 6-MP nucleotide is oxidized to thioxanthine nucleotide prior to 6-TGN formation. Both 6-MP and 6-TG are incorporated into DNA as the fraudulent base, thioguanine [9].
TPMT catalysed thiopurine S-methylation competes with TGN formation. Both 6-MP and 6-TG serve as substrates for TPMT S-methylation. 6-MP nucleotide (thioinosine monophosphate) is also a good substrate for TPMT, some 18-fold better than TGNs which function as poor substrates [10]. TPMT activity in the red blood cell and other human tissues, including the liver, is under the control of a common genetic polymorphism [11].
The cytotoxic effects of 6-MP and 6-TG are probably mediated through the major metabolites, 6-thioguanine nucleotides (6-TGNs). These can be conveniently measured in red cells and this may reflect the concentration at target tissues [12, 13]. Several factors influence the intracellular concentration of 6-TGNs, including inherited differences in metabolism, absorption and patient compliance [14, 15].
Pharmacokinetic studies on oral 6-TG are sparse [16–18], and there are limited data available on the pharmacokinetics of 6-TG or 6-TG derived 6-TGNs, in children with ALL [18, 19].
The aims of this study were therefore twofold; firstly to investigate the interindividual variation in 6-TG pharmacokinetics in children receiving a standard dose of 6-TG when the drug was taken with food and when the drug was taken on an empty stomach, and secondly to compare the effect of food on parent drug pharmacokinetics and the accumulation of red cell 6-TGNs in the individual child.
Methods
Subjects
All children with ALL aged between 1 and 16 years attending St Bartholomew's Hospital who were prescribed 6-TG for maintenance therapy as part of the ALL 97 pilot study or the MRC ALL 97 trial, were eligible for the study. The local research ethics committee approved the protocol and written consent was obtained from the parents.
Chemotherapy
Each subject received a 5 day intensive module of chemotherapy at weeks 5 and 20 comprising of intravenous (i.v.) daunorubicin, i.v. etoposide, i.v. cytarabine, oral 6-TG, oral prednisolone or dexamethasone and intrathecal (i.t.) methotrexate (MTX). Maintenance therapy comprised of daily 6-TG (commencing at week 8), weekly oral MTX (commencing at week 12) and a monthly pulse of vincristine and a monthly 5 day course of steroids. Maintenance 6-TG was prescribed at a standard protocol dose of 40 mg m−2 and methotrexate 20 mg m−2. During the study period each subject received weekly intrathecal methotrexate as CNS directed therapy from weeks 8–12 and week 23 of therapy. Eight subjects received dexamethasone and three prednisolone as steroid therapy.
Study design
Pharmacokinetic studies were performed following recovery from each 5 day intensive module, this being at weeks 8 or 9 and 23 or 24 of the treatment protocol. Two pharmacokinetic studies were performed on each child. This was at a time when no chemotherapy had been given for 2–3 weeks and when 6-TG was commenced at a standard protocol dose. 6-TG was commenced only if the neutrophil count was > 1.0 × 109 l−1 and the platelet count was > 100 × 109 l−1. Subjects were alternately allocated to receive the 6-TG dose either fasting or after food, i.e. child 1, study 1 was given 6-TG fasting and child 2, study 1 was given 6-TG with food, etc. Subjects 5 and 6 were twins who were studied 1 month apart, hence were both given 6-TG fasting in study 1 for convenience for the family. Each subject was studied fasting and after food.
All subjects were studied in the morning following an overnight fast and either received the first dose of 6-TG on an empty stomach or immediately after a standard breakfast of cereal with milk, toast and a glass of milk. The subjects in the fasting study received the standard breakfast at 1 h with no further restriction of intake. A 3 ml venous blood sample was taken via a central venous catheter, immediately before dosing and at 0.25, 0.5, 0.75, 1.0, 1.5, 2.5, 3.0, 4.0, 5.0 and 6.0 h for the measurement of plasma 6-TG and RBC 6-TGN concentrations. The whole blood was centrifuged immediately (1200 g, 4 °C, 10 min) to prevent cellular metabolism of 6-TG by the RBCs. The RBCs were washed twice in Hanks balanced salts solution (HBSS), resuspended in 1 volume HBSS and counted. The plasma and the washed RBCs were placed in dry ice after processing and stored at −40 °C until the time of assay. Under these conditions, 6TG and the RBC TGNs are stable for at least 6 months [20].
Subjects were then instructed to take their daily dose of 6-TG either 1 h before breakfast or immediately after breakfast for a 4 week period following each pharmacokinetic study. A 5 ml venous blood sample was taken twice weekly when possible, to measure RBC 6-TGN concentrations. All blood samples were obtained at least 4 h post dose. Blood samples were taken when the subject attended hospital for routine visits or in their home.
6-TG and 6-TGN assays
Plasma 6-TG and RBC 6-TGN concentrations were measured in duplicate using an h.p.l.c. assay as previously described [12]. The lower limit of sensitivity was 30 pmol ml−1 for plasma 6-TG and 30 pmol/8 × 108 RBCs for 6-TGNs. The interassay coefficient of variation for quality control samples of 120 pmol 6-TG ml−1 plasma, 300 pmol 6-TG ml−1 plasma, 300 pmol 6-TG/8 × 108 RBCs and 3000 pmol 6-TG/8 × 108 RBCs, taken over 12 assay runs, were 8.4%, 6.5%, 5.1% and 3%, respectively. In addition to these QCs, each assay contained a standard curve ranging from 30 to 1200 pmol 6-TG ml−1 plasma and 30–3000 pmol 6-TGN/8 × 108 RBCs. The CV for 6-TG at 30 pmol ml−1 plasma is 9% and for 6-TGN, at 30 pmol/8 × 108 RBCs, 6%. The lower limit of these assays is based on our routine assays when we inject 50 µl onto the h.p.l.c. from a back-extract of 200 µl 0.1 m HCl. When drug concentrations fell below the 7.5 pmol per 50 µl injection limit the amount of patient sample back extract injected onto the h.p.l.c. was doubled. The standard curve back extracts were diluted 1:1 with 0.1 m HCl and 100 µl injected onto the h.p.l.c. The lower limit of detection is reduced to 15 pmol 6-TG ml−1 plasma (CV 10%) and 15 pmol 6-TGN/8 × 108 RBCs (CV 7%).
Analysis
The AUC was calculated to 6 h for plasma 6-TG using the trapezoidal method. The peak concentration (Cmax), time to peak (tmax), and the plasma concentration at 6 h (C6) were calculated. The AUC for RBC 6-TGN was calculated using the trapezoidal method over matched time periods from time 0 to 25–30 days for both the fasting and after food phases of the study. Fractional time periods were used. The limited pharmacokinetic data on RBC 6-TGN half-life in children with TPMT activity taking 40 mg m−2 6-TG indicate that 6-TGN t1/2 varies from 4.4 to 9 days [18]. The upper value was used to calculate that 6-TGNs would approach, or reach, steady state in approximately 27 days (three half-lives).
Statistical methods
Plasma 6-TG and RBC 6-TGN parameters for the matched pairs were compared by the Wilcoxin signed ranks test. The correlation between plasma 6-TG AUC and steady state RBC 6-TGN concentration were assessed by the Spearman rank correlation coefficient (rs).
Results
Subjects
Eleven children with ALL receiving 6-TG for maintenance were recruited into the study. Ten children completed both pharmacokinetic studies (fasting and after food). One child (subject 6) failed to complete the pharmacokinetic study after food due to problems with venous access. However, some blood samples were collected for red cell metabolites over the subsequent 4 weeks. Subject 3 had inadvertently commenced 6-TG 2 days prior to the pharmacokinetic study after food but had not received a dose on the day of the study. The pharmacokinetic study was performed as usual but the data on 6 h RBC TGN concentrations were not included in the analysis. Subject 4 did not tolerate taking 6-TG on an empty stomach for the 4 week study period but completed the fasting pharmacokinetic study and the study after food without a problem. Subject 7 vomited once 83 min after the dose of 6TG during the pharmacokinetic study in the fasting state and during the 2 days following the pharmacokinetic study after food but settled subsequently with no further episodes.
6-TG pharmacokinetics
The pharmacokinetic parameters for individual subjects are summarized in Table 1. The children were aged between 1.5 and 7 years (median 4) with a surface area of 0.46–0.93 mg m−2 (median 0.75), approximately 9–25 kg (median 18). The peak plasma concentrations varied considerably among individual subjects. There was a significant difference between the level reached in the fasting (median 313 pmol ml−1, range 50–737) and that after breakfast (median 71 pmol ml−1, range 16–213) (median difference 217 pmol ml−1, 95% CI from 36 to 426, P = 0.006). The median time to reach the peak plasma concentration was 1.5 h (range 0.5–3.1) in the fasting state and 3.0 h (range 1.5–6) after food (median difference 1.7 h, 95% CI from 0.74 to 2.73, P = 0.013).
Table 1.
Pharmacokinetic parameters of oral 6-TG (40 mg m−2) administered in the fasting state and after food. *Subject 6 did not complete the pharmacokinetic study after food and hence was excluded from the analysis.
| Fasting | After food | |||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Cmax (pmol ml−1) | tmax (h) | C6 (pmol ml−1) | AUC (pmol l−1h) | Cmax (pmol ml−1) | tmax (h) | C6 (pmol ml−1) | AUC (pmol ml−1 h) |
| 1 | 90 | 1.5 | 36 | 286 | 67 | 5.0 | 50 | 211 |
| 2 | 228 | 0.5 | 0 | 363 | 194 | 3.0 | 0 | 296 |
| 3 | 496 | 1.5 | 28 | 815 | 213 | 3.0 | 40 | 613 |
| 4 | 235 | 1.5 | 0 | 320 | 46 | 2.0 | 26 | 190 |
| 5 | 412 | 1.5 | 28 | 880 | 168 | 1.5 | 0 | 321 |
| 6* | 703 | 1.5 | 30 | 1318 | ||||
| 7 | 50 | 2.5 | 0 | 156 | 16 | 4.4 | 0 | 33 |
| 8 | 737 | 1.5 | 46 | 1306 | 75 | 1.5 | 0 | 96 |
| 9 | 55 | 3.1 | 25 | 168 | 18 | 6.0 | 18 | 25 |
| 10 | 391 | 1.0 | 50 | 809 | 61 | 4.1 | 26 | 117 |
| 11 | 695 | 1.5 | 39 | 1216 | 103 | 3.0 | 36 | 299 |
| Median | 313 | 1.5 | 28 | 586 | 71 | 3 | 22 | 200 |
| Range | 51–737 | 0.5–3.1 | 0–50 | 156–1306 | 16–213 | 1.5–6 | 0–50 | 25–613 |
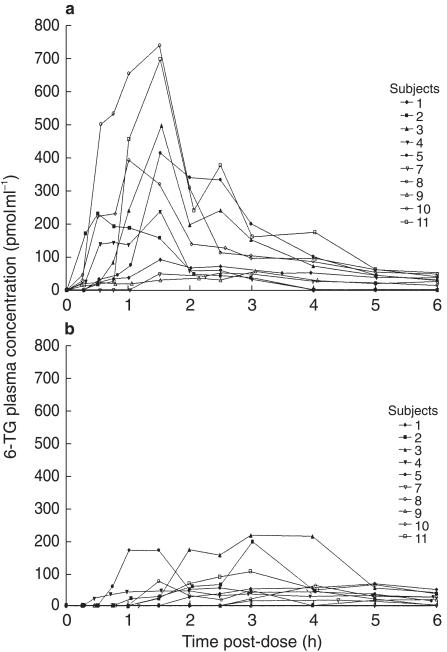
The 6-TG AUC values showed marked interindividual differences both in the fasting state and with food. Figure 1 illustrates the plasma concentration-time profile of 6-TG for the whole group in the fasting state and after food. There was a significant difference between the two studies with lower AUC values in the study with food (median 200 pmol ml−1 h, range 25–613) compared with the fasting study (median 586 pmol ml−1 h, range 156–1306; median difference 380 pmol ml−1 h, 95% CI from 109 to 692, P = 0.006). In all subjects the plasma concentration at 6 h was below 51 pmol ml−1. In three subjects in the fasting study and four in the study after food, the plasma concentrations 6 h after the 6-TG dose was below the limit of sensitivity of the assay. No patient had a detectable plasma concentration of 6-TG at the start of the study.
Figure 1.
Plasma concentration-time profile of 6-TG after a standard oral dose of 40 mg m−2 administered (a) in the fasting state and (b) after food.
RBC 6-TGN concentrations
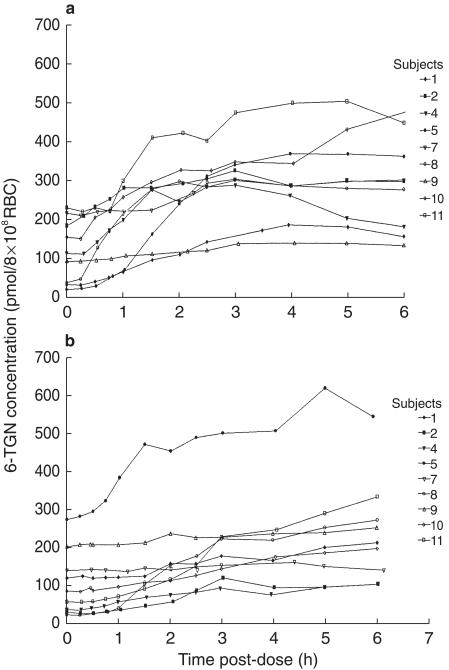
0–6 h In all subjects 6-TGNs gradually accumulated in the RBC over 6 h. The median RBC 6-TGN concentration reached at 6 h was 295 pmol/8 × 108 RBC (range 130–477) in the fasting state and 210 pmol/8 × 108 RBC (103–544) after food, which was not significantly different (P = 0.29).
Figure 2 illustrates the gradual accumulation over 6 h in the fasting state and after food. The RBC concentration at the start of the study ranged from 16 to 225 pmol/8 × 108 RBC (median 108) in the fasting state and 20–269 pmol/8 × 108 RBC (median 83) after food, which reflects 6-TGN remaining in the RBC following the intensive module 2–3 weeks previously. The t1/2 of RBC 6-TGNs following drug withdrawal is approximately 9 days [18]. The difference between the 6 h RBC 6-TGN concentration, when corrected for the time zero value, was not significantly different in the fasted or fed groups (median 123 vs 93 pmol/8 × 108 RBC, P = 0.19).
Figure 2.
Red cell 6-TGN concentration-time profile of 6-TG after a single standard oral dose of 40 mg m−2 administered (a) in the fasting state and (b) after food.
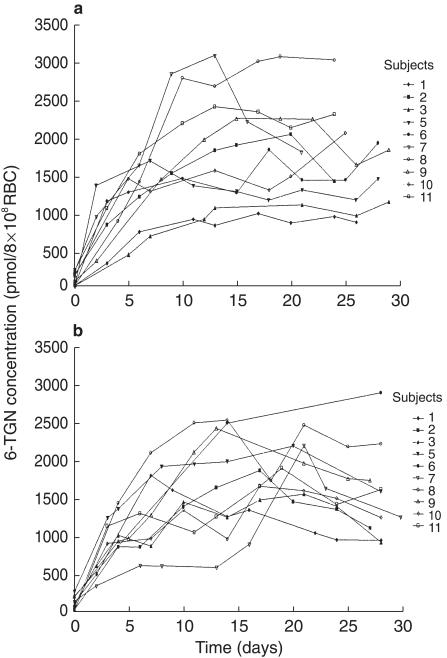
0–30 days There was no clear difference in the pattern of accumulation of RBC 6-TGN with and without food. Figure 3 illustrates the pattern of accumulation for the whole group. Some children demonstrated identical accumulation whereas other children did show differences. In some children higher steady state concentrations were reached when 6-TG was taken on an empty stomach compared with food whereas the converse was true of others. Child 7 was nauseous from day 6–18 in the after food study. Steady state 6-TGN concentrations ranged from 875 to 3026 pmol/8 × 108 RBC (median 1742) when 6-TG was taken before food and 972–2894 pmol/8 × 108 RBC (median 1600) after food, which was not significantly different (P = 0.68).
Figure 3.
Red cell 6-TGN concentration-time profile after a dose of 40 mg m−2 6-TG taken daily over a 4 week period (a) fasting and (b) after food.
The rate of accumulation of 6-TGNs as calculated by the AUC of the 6-TGN concentration vs time (0–30 days), was similar fasting (median 37026 pmol/8 × 108 RBC.days; range 19500–53360) and after food (median 32652 pmol/8 × 108 RBC.days; range 16527–56680). In the group of children studied there were no significant correlations between either plasma 6TG AUC or red cell 6-TGNs at 6 h and steady state RBC 6-TGN concentrations either fasting or after food.
Discussion
In this study of 6-TG pharmacokinetics in children with ALL there was wide interindividual variation in all pharmacokinetic variables. This was more evident when subjects were studied under standard conditions following an overnight fast. The findings confirm earlier studies where considerable differences in interindividual 6-TG pharmacokinetics were found [18, 19]. Similar findings have been reported with 6-MP where plasma 6-MP concentrations show wide interindividual variation after both intravenous and oral administration [1–4].
There was a significant difference between the pharmacokinetic parameters when the 6-TG dose was administered in the fasting state or after food. The peak plasma concentration and the plasma concentration vs time profile were significantly lower and the time taken to reach peak plasma concentration was longer after food. This has been little studied before. An earlier report of adults with AML showed a reduction in plasma 6-TG concentration with concurrent food intake but this was not statistically significant [21]. 6-MP has been studied in this respect. The influence of food has shown a variable effect with reduced absorption reported by some investigators [5, 6] and no effect in other studies [7, 8]. The content of food may be important as has been reported with methotrexate where a milky meal had more effect on pharmacokinetics than a citrus meal [22]. It has been suggested that the high concentration of the enzyme xanthine oxidase found in untreated cows milk may also interfere with the plasma pharmacokinetics of 6-MP [23].
An alternative explanation for the difference between studies is that there may be wide intraindividual variation in the plasma concentration vs time profile when subjects are studied under standard conditions on repeated occasions with the same dose, as has been demonstrated with 6-MP [24, 25]. A study of intraindividual variation in 6-MP kinetics in 18 children revealed a mean coefficient of variation of 58% [26].
The plasma AUC of a thiopurine drug reflects the plasma concentration of the drug absorbed and remaining after first pass metabolism. 6-TG and 6-MP exert their cytotoxic effect through their active metabolites, 6-TGNs, which can be measured in red cells and gradually accumulate to reach steady state over several days or weeks [13]. Factors that influence the accumulation of metabolites are probably of greater clinical importance than those affecting plasma concentration of the parent drug. In this study, overall there was no difference in either the RBC 6-TGN concentration at 6 h or the steady state concentration in the fasting state or after food. There was no clear difference in the pattern of accumulation of RBC 6-TGN with or without food. Some individuals showed identical patterns, others accumulated higher metabolite concentrations in the fasting state compared with food whereas the opposite was true of others. There was no correlation between plasma 6-TG AUC levels and either 6 h or steady-state RBC 6TGN concentrations. The child with the highest RBC 6-TGN concentrations in the after food study had one of the lowest plasma 6-TG AUCs. The RBC can metabolize 6-TG directly to 6-TGNs, but the RBC continues to accumulate 6-TGN metabolites after plasma drug concentrations have fallen below the lower limit of detection [18]. This is also observed with 6-MP [27]. In the TPMT deficient child, RBC 6-TGNs continue to accumulate up to 7 days after a single oral dose of 6-MP [27]. These observations raise questions about the source of 6-TGN metabolites, they could be formed entirely within the RBC and/or be derived from thiopurine metabolites originating elsewhere.
In childhood ALL a prolonged course of maintenance therapy is necessary for a successful outcome and patients who form low intracellular 6-TGN concentrations for whatever reason may be more likely to relapse [13]. A significant number of children on maintenance therapy do not comply with the current recommendations to take their medication on an empty stomach 20 min before food, so we were concerned that this group of patients could be receiving suboptimal therapy. The recommendation could also be a factor contributing to noncompliance with oral maintenance therapy in some children, a problem that might arise in around 10% of UK children [15]. Our present findings would support the argument that it is more important that children take their medication, rather than insist it is taken in a manner that is unacceptable for many children. The time of day however, may be important, as data published by Rivard et al. indicated that children receiving their maintenance therapy in the evening had a better prognosis than individuals receiving a morning schedule [28, 29].
6-TG is a potentially important drug in the maintenance therapy of childhood ALL and may replace the traditional agent 6-MP if current trials demonstrate a therapeutic advantage. To date there are few data reported on the pharmacokinetics of the drug and its major metabolites, 6-TGNs. From this study it would appear that there is wide interindividual variation in the plasma pharmacokinetics of 6-TG and that although food does affect parent drug pharmacokinetics there is no demonstrable effect on the accumulation of cytotoxic 6-TGNs in red cells. It may thus be unnecessary to insist on 6-TG being taken on an empty stomach as has been traditional in the past.
Acknowledgments
This work was supported by the Imperial Cancer Research Fund and the Leukaemia Research Fund of Great Britain.
References
- 1.Zimm S, Collins JM, Riccardi RDON, et al. Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983;308:1005–1009. doi: 10.1056/NEJM198304283081705. [DOI] [PubMed] [Google Scholar]
- 2.Lennard L, Keen D, Lilleyman JS. Oral 6-mercaptopurine in childhood leukemia: parent drug pharmacokinetics and active metabolite concentrations. Clin Pharmacol Ther. 1986;40:287–292. doi: 10.1038/clpt.1986.178. [DOI] [PubMed] [Google Scholar]
- 3.Balis FM, Holcenberg JS, Zimm S, et al. The effect of methotrexate on the bioavailability of oral 6-mercaptopurine. Clin Pharmacol Ther. 1987;41:384–387. doi: 10.1038/clpt.1987.45. [DOI] [PubMed] [Google Scholar]
- 4.Kato Y, Matsushita T, Chiba K, Hijiya N, Yokoyama T, Ishizaki T. Dose-dependent kinetics of orally administered 6-mercaptopurine in children with leukemia. J Pediatr. 1991;119:311–316. doi: 10.1016/s0022-3476(05)80751-6. [DOI] [PubMed] [Google Scholar]
- 5.Burton NK, Barnett MJ, Aherne GW, Evans J, Douglas I, Lister TA. The effect of food on the oral administration of 6-mercaptopurine. Cancer Chemother Pharmacol. 1986;18:90–91. doi: 10.1007/BF00253074. [DOI] [PubMed] [Google Scholar]
- 6.Riccardi R, Balis FM, Ferrara P, Lasorella A, Poplack DG, Mastrangelo R. Influence of food intake on bioavailability of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1986;3:319–324. doi: 10.3109/08880018609031233. [DOI] [PubMed] [Google Scholar]
- 7.Lafolie P, Bjork O, Hayder S, Ahstrom L, Peterson C. Variability of 6-mercaptopurine pharmacokinetics during oral maintenance therapy of children with acute leukemia. Med Oncol Tumor Pharmacother. 1989;6:259–265. doi: 10.1007/BF02985158. [DOI] [PubMed] [Google Scholar]
- 8.Lonnerholm G, Kreuger A, Lindstrom B, Myrdal U. Oral mercaptopurine in childhood leukemia: influence of food intake on bioavailability. Pediatr Hematol Oncol. 1989;6:105–112. doi: 10.3109/08880018909034276. [DOI] [PubMed] [Google Scholar]
- 9.Inamochi H, Higashigawa M, Shimono Y, et al. Delayed cytotoxicity of 6-mercaptopurine is compatible with mitotic death caused by DNA damage due to incorporation of 6-thioguanine into DNA as 6-thioguanine nucleotide. J Exp Clin Cancer Res. 1999;18:417–424. [PubMed] [Google Scholar]
- 10.Deininger M, Szumlanski CL, Otterness DM, Van Loon J, Ferber W, Weinshilboum RM. Purine substrates for human thiopurine methyltransferase. Biochem Pharmacol. 1994;48:2135–2138. doi: 10.1016/0006-2952(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 11.Szumlanski CL, Honchel R, Scott MC, Weinshilboum RM. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isoenzymes. Pharmacogenetics. 1992;2:148–159. [PubMed] [Google Scholar]
- 12.Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583:83–90. doi: 10.1016/0378-4347(92)80347-s. [DOI] [PubMed] [Google Scholar]
- 13.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7:1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [published erratum appears in J Clin Oncol 1990; 8: 567]. [DOI] [PubMed] [Google Scholar]
- 14.Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 15.Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance? Br J Cancer. 1995;72:1004–1006. doi: 10.1038/bjc.1995.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LePage GA, Whitecar Jp., Jr Pharmacology of 6-thioguanine in man. Cancer Res. 1971;31:1627–1631. [PubMed] [Google Scholar]
- 17.Liliemark J, Petterson B, Järnmark M, Peterson C. On the cellular pharmacokinetics of 6-thioguanine in acute myelogenous leukemia. Leuk Lymphoma. 1991;4:271. doi: 10.3109/10428199109068076. [DOI] [PubMed] [Google Scholar]
- 18.Lennard L, Davies HA, Lilleyman JS. Is 6-thioguanine more appropriate than 6-mercaptopurine for children with acute lymphoblastic leukaemia? Br J Cancer. 1993;68:186–190. doi: 10.1038/bjc.1993.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erb E, Harms DO, Janka-Schaub G. Pharmacokinetics and metabolism of thiopurines in children with acute leukaemia receiving 6-thioguanine versus 6-mercaptopurine. Cancer Chemother Pharmacol. 1998;42:266–272. doi: 10.1007/s002800050816. 10.1007/s002800050816. [DOI] [PubMed] [Google Scholar]
- 20.Lennard L, Maddocks JL. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983;35:15–18. doi: 10.1111/j.2042-7158.1983.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 21.Brox LW, Birkett L, Belch A. Clinical pharmacology of oral thioguanine in acute myelogenous leukemia. Cancer Chemother Pharmacol. 1981;6:35–38. doi: 10.1007/BF00253008. [DOI] [PubMed] [Google Scholar]
- 22.Pinkerton CR, Welshman SG, Glasgow JF, Bridges JM. Can food influence the absorption of methotrexate in children with acute lymphoblastic leukaemia? Lancet. 1980;ii:944–946. doi: 10.1016/s0140-6736(80)92105-4. [DOI] [PubMed] [Google Scholar]
- 23.Rivard GE, Lin KT, Leclerc JM, David M. Milk could decrease the bioavailability of 6-mercaptopurine. Am J Pediatr Hematol Oncol. 1989;11:402–406. [PubMed] [Google Scholar]
- 24.Hayder S, Lafolie P, Bjork O, Peterson C. 6-mercaptopurine plasma levels in children with acute lymphoblastic leukemia: relation to relapse risk and myelotoxicity. Ther Drug Monit. 1989;11:617–622. doi: 10.1097/00007691-198911000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Endresen L, Lie SO, Storm-Mathisen I, Rugstad HE, Stokke O. Pharmacokinetics of oral 6-mercaptopurine: relationship between plasma levels and urine excretion of parent drug. Ther Drug Monit. 1990;12:227–234. [PubMed] [Google Scholar]
- 26.Lafolie P, Hayder S, Bjork O, Peterson C. Intraindividual variation in 6-mercaptopurine pharmacokinetics during oral maintenance therapy of children with acute lymphoblastic leukaemia. Eur J Clin Pharmacol. 1991;40:599–601. doi: 10.1007/BF00279977. [DOI] [PubMed] [Google Scholar]
- 27.Lennard L, Lewis IJ, Michelagnoli M, Lilleyman JS. Thiopurine methyltransferase deficiency in childhood lymphoblastic leukaemia: 6-mercaptopurine dosage strategies. Med Pediatr Oncol. 1997;29:252–255. doi: 10.1002/(sici)1096-911x(199710)29:4<252::aid-mpo3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Rivard GE, Infante-Rivard C, Hoyoux C, Champagne J. Maintenance chemotherapy for childhood acute lymphoblastic leukaemia: better in the evening. Lancet. 1985;ii:1264–1266. doi: 10.1016/s0140-6736(85)91551-x. [DOI] [PubMed] [Google Scholar]
- 29.Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM, Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10:201–204. doi: 10.3109/07420529309073888. [DOI] [PubMed] [Google Scholar]