Abstract
Aims
Previous studies suggest that estimated creatinine clearance, the conventional measure of renal function, does not adequately reflect changes in renal drug handling in some patients, including the immunosuppressed. The aim of this study was to develop and validate a cocktail of markers, to be given in a single administration, capable of detecting alterations in the renal elimination pathways of glomerular filtration, tubular secretion and tubular reabsorption.
Methods
Healthy male subjects (n = 12) received intravenously infused 2500 mg sinistrin (glomerular filtration) and 440 mg p-aminohippuric acid (PAH; anion secretion), and orally administered 100 mg fluconazole (reabsorption) and 15 mg rac-pindolol (cation secretion). The potential interaction between these markers was investigated in a pharmacokinetic study where markers (M) or fluconazole (F) were administered alone or together (M + F). Validated analytical methods were used to measure plasma and urine concentrations in order to quantify the renal handling of each marker. Plasma protein binding of fluconazole was measured by ultrafiltration. All subjects had an estimated creatinine clearance within the normal range. The renal clearance of each marker (mean±s.d.) was calculated as the ratio of the amount excreted in urine and the area-under-the-concentration-time curve. Statistical comparisons were made using a paired t-test and 95% confidence intervals were reported.
Results
The renal clearances of sinistrin (M: 119 ± 31 ml min−1; M + F: 130 ± 40 ml min−1; P = 0.32), PAH (M: 469 ± 145 ml min−1; M + F: 467 ± 146 ml min−1; P = 0.95), R-pindolol (M: 204 ± 41 ml min−1; M + F: 190 ± 41 ml min−1; P = 0.39; n = 11), S-pindolol (M: 225 ± 55 ml min−1; M + F: 209 ± 60 ml min−1; P = 0.27; n = 11) and fluconazole (F: 14.9 ± 3.8 ml min−1; M + F: 13.6 ± 3.4 ml min−1; P = 0.16) were similar when the markers or fluconazole were administered alone (M or F) or as a cocktail (M + F).
Conclusions
This study found no interaction between markers and fluconazole in healthy male subjects, suggesting that a single administration of this cocktail of markers of different renal processes can be used to simultaneously investigate pathways of renal drug elimination.
Keywords: creatinine clearance, fluconazole, pindolol, renal drug elimination, tubular reabsorption, tubular secretion
Introduction
Fluconazole is an important drug for the treatment of fungal diseases, especially in the immunocompromised. We have previously compared the pharmacokinetics of fluconazole in healthy volunteers and two groups of people with human immunodeficiency virus (HIV) infection with different degrees of immunosuppression [1]. The total clearance of fluconazole was found to be lowest in the group of subjects with a low CD4+ cell count compared with a group with higher CD4+ counts and HIV infection or healthy subjects. A population pharmacokinetic analysis of fluconazole plasma concentration-time data from 113 people HIV seropositive confirmed that the total clearance of fluconazole was significantly lower in people with more severe immunosuppression [2]. Other workers have also reported that the total clearance of fluconazole in HIV seropositive subjects is 25% lower than in healthy volunteers [3].
Renal clearance of unchanged drug makes the overwhelming contribution to the elimination of fluconazole (approximately 80%) [4]. The lower clearance of fluconazole in HIV seropositive people indicates that renal elimination is decreased with HIV infection and that as immunosuppression progresses, renal elimination is further compromised. Importantly, in all HIV seropostive subjects in our previous pharmacokinetic study [1], the predicted creatinine clearance, calculated from the serum concentration of creatinine using a standard nomogram including age, weight and sex [5] was within the normal range and did not suggest that the renal handling of fluconazole would be impaired. A number of researchers have found that available nomograms overestimate the measured creatinine clearance in people with HIV [6–8].
Therefore methods which can predict renal drug handling better than the classical estimation of creatinine clearance are needed to predict accurately dosage requirements. Ideally to study renal drug handling all mechanisms contributing to the renal excreton of therapeutic drugs should be characterized, i.e. glomerular filtration, tubular secretion and tubular reabsorption [9].
The present study has coadministered markers for each of these renal elimination processes: sinistrin, a compound closely related to inulin, to measure glomerular filtration [10, 11], p-aminohippurate sodium (PAH) to measure renal plasma flow and net tubular anion secretion [12–14], pindolol (as a racemic mixture) to measure net tubular cation secretion [15, 16] and fluconazole as an indicator of reabsorption [4]. It is a requirement for using such a cocktail of markers that they be safe and that the components should not interact. This study has been performed in healthy subjects with normal renal function to quantify each of these pathways and to ascertain whether it is possible to administer these markers concomitantly without altering the pharmacokinetics, in particular the renal clearance, of any individual compound.
Methods
Subjects
The study was approved by the Medical Research Ethics Committee of Royal North Shore Hospital, Sydney (Protocol no. 960–008 M). All volunteers gave written informed consent prior to commencing the study. Twelve healthy male subjects (age: mean 24 years, range 19–41 years; weight: mean 72 kg, range 63–95 kg; height: mean 178 cm, range 175–186 cm) were selected after a medical examination which included normal results for haematology and biochemistry tests, including CD4+ counts as well as a negative HIV screen. In addition the creatinine clearance, estimated using the Cockcroft-Gault nomogram [5], was greater than 100 ml min−1 for all subjects.
Dose administration and sampling protocol
Each subject was studied on three occasions, separated by intervals of at least 2 weeks. The subjects did not take any medicines for 5 days and abstained from alcohol intake for 36 h prior to each dose and throughout the blood and urine collecting intervals. Caffeine was restricted for 12 h prior to and 24 h following marker administration. The subjects also fasted from 22.00 h on the evening prior to each study. On study mornings intravenous cannulae were inserted in each arm, one for administration of marker compounds and one for withdrawing blood samples. In order to assess accurately GFR using sinistrin and effective renal plasma flow using PAH, the manufacturer's recommendations for water loading were followed to ensure a urine flow rate greater than 2–3 ml min−1. To ensure that urine flow rate was comparable during all three arms of the study, this intake was maintained when fluconazole was studied alone. Thirty minutes prior to marker administration each subject drank 500 ml water and the bladder was emptied just prior to marker administration. Each subject received in randomised order:
Fluconazole (F): 100 mg capsule (DiflucanR, Pfizer, Sydney, Australia) orally.
Markers (M): Sinistrin 2500 mg (InutestR, Laevosan, Linz, Austria) administered as a slow intravenous injection over 30 s followed by PAH 440 mg (Aminohippurate Sodium USP; Merck, Sharp and Dohme, West Point, PA, USA) administered as a slow bolus injection over 1 min and rac-pindolol 15 mg (ViskenR, Sandoz, Australia, i.e. 7.5 mg of each pindolol enantiomer) orally. This combination has previously been shown not to interact [17, 18].
Markers and Fluconazole (F + M): Fluconazole 100 mg orally plus PAH 440 mg intravenously, sinistrin 2500 mg intravenously and rac-pindolol 15 mg orally as described above.
Oral doses were taken with 200 ml water and an additional 100 ml of water was ingested each half hour for the first 4 h after dosing. Subjects remained semirecumbant for this time interval. A standardized lunch was served 4 h after dosing and dinner 10 h after dose. Blood samples (10 ml) were taken predose and at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6 and 8 h postdose on all 3 days. When markers were administered further blood samples were taken at 5 min, 10 min, 12 h and 24 h. After administration of fluconazole, additional samples were taken by venepuncture at 24, 32, 48, 72, 96 and 120 h postdose. All urine voided was collected as 0–3, 3–6 and 6–24 h samples and following fluconazole administration additional 24–48, 48–72, 72–96 and 96–120 h samples were collected. Blood samples were collected into heparinized tubes, centrifuged, the plasma aliquoted and stored at −20 °C pending analysis. The volume of each urine sample and pH was measured and aliquots were stored at −20 °C pending analysis. Creatinine concentrations were measured in plasma samples taken prior to the administration of markers and/or fluconazole and, in the two arms of the study where fluconazole was given, in the plasma samples collected every 24 h up to 120 h. Creatinine concentrations were also measured in all urine samples collected. All markers were administered under medical supervision. Heart rate and blood pressure were monitored at the time of each blood sampling and all subjects were encouraged to report any adverse effects experienced using open ended questions.
Plasma protein binding
Fluconazole plasma protein binding was determined by ultrafiltration using Amicon Centrifree® Microconcentrators (Millipore Corporation, Bedford, MA, USA). Aliquots (1 ml) of pooled plasma samples from each subject after administration of fluconazole alone or with markers were pipetted into the Centrifree® ultrafiltration devices and centrifuged at 37 °C for 40 min at 1500 g. The free fraction (fu) was calculated by dividing the fluconazole concentration in the ultrafiltrate by the plasma concentration prior to ultrafiltration.
Analytical techniques
Details of analytical methods and their validation have been previously published [19]. There was no analytical interference from markers and drugs used in the cocktail and the coefficients of variation for the interday and intraday precision of all analytical techniques was less than 12%.
Data treatment
Creatinine clearance (CLCR) was estimated from plasma creatinine concentrations and the demographic parameters age (years) and body weight (kg) using the equation of Cockroft & Gault (1976) [5]. The creatinine clearance was also calculated by dividing the urinary excretion rate of creatinine (calculated over 24 h and expressed in mg h−1) by the average plasma creatinine concentration (determine over 24 h and expressed in mg ml−1).
The maximum plasma concentration observed (Cmax) and the time at which it occurred (tmax) were determined by inspection of the data. The slope of the terminal portion of the log concentration-time profile (λz) was calculated by linear regression and the half-life estimated as 0.693/λz. The area under the plasma concentration-time curve to the last plasma concentration measured (Ct), AUC(0,t) was calculated using the linear trapezoidal rule and extrapolated to infinity (AUC) by adding Ct/z. The total clearance (CL) after intravenous dosing and the apparent oral clearance (CL/F) after oral dosing were calculated as Dose/AUC.
The total urinary recovery (Ae) of unchanged analytes for each study arm was calculated as the sum of the amount excreted (urine concentration × volume) in each sample collection period. The renal clearance (CLR) was calculated as Ae/AUC, except for fluconazole which was calculated using the 0–120 h urinary recovery and AUC(0,120 h). The nonrenal clearance (CLNR) was calculated as CL – CLR (apparent nonrenal clearance, CLNR/F, was calculated for fluconazole and pindolol). The renal clearances of sinistrin and PAH were taken to be the glomerular filtration rate (GFR) and effective renal plasma flow, respectively. The renal clearance of the unbound (free) marker (CLuR) was calculated as CLR/fu. The glomerular filtration of the unbound marker was calculated as fu × GFR. The net renal secretion of PAH was calculated as CLR – (fu × GFR), assuming an unbound fraction in plasma of 0.83 [20] and was a marker of tubular anion secretion. The net renal secretion of the enantiomers of pindolol was calculated as CLR – (fu × GFR) and represented tubular cation secretion. The unbound fraction of S-and R-pindolol was assumed to be 0.45 [15]. The net reabsorption of fluconazole (using 0,120 h data) was calculated as (fu×GFR) – CLR. Fluconazole pharmacokinetic parameters were also calculated based on 0–24 h data in order to allow comparison with the concentration-time data collected over 120 h.
Statistical analysis
Relationships between the indices of renal drug handling were investigated using linear regression analyses. Pharmacokinetic parameter estimates are presented as mean±s.d. (range) and 95% confidence intervals (95% CI). Differences between the pharmacokinetic parameters for the markers when given alone (M) or coadministered with fluconazole (F + M) or those for fluconazole when given alone (F) or with the markers (F + M) were investigated using a paired t-test (Statistica 4.5, Statsoft Inc. OK, USA) and the 95% CI of the percentage difference. This approach was also used to assess stereoselectivity in pindolol pharmacokinetics, the agreement between fluconazole renal clearance calculated using 0–24 h and 0–120 h data and whether the indices of glomerular filtration rate were similar. One way anova (Statistica 4.5) was used to assess whether creatinine clearance and urine flow rate were similar on all three study days. A probability of less than 0.05 was considered significant.
Results
No serious adverse effects were reported after the administration of the markers alone, fluconazole alone or their combination. One subject (3) reported a headache in the Markers only arm. Subject 3 also took a single dose of methacycline 300 mg during the fluconazole only arm. No substantial clinically significant changes in blood pressure or heart rate were observed after the single dose of pindolol however, most subjects recorded an apparent slight decrease in both heart rate and blood pressure. The urine flow rates for all three arms of the study were comparable (anova; P > 0.05) for the first 3 h (M: 7.3 ± 2.5 ml min−1; F: 5.8 ± 2.6 ml min−1; F+M: 7.4 ± 2.3 ml min−1), 3–6 h (M: 3.0 ± 0.9 ml min−1; F: 2.3 ± 0.8 ml min−1; F+M: 3.0 ± 1.4 ml min−1) and 6–24 h (M: 0.6 ± 0.2 ml min−1; F: 0.9 ± 0.3 ml min−1; F+M: 0.8 ± 0.5 ml min−1) after administration of markers and/or fluconazole. The pH of urine samples was found to be in the normal range expected for urine.
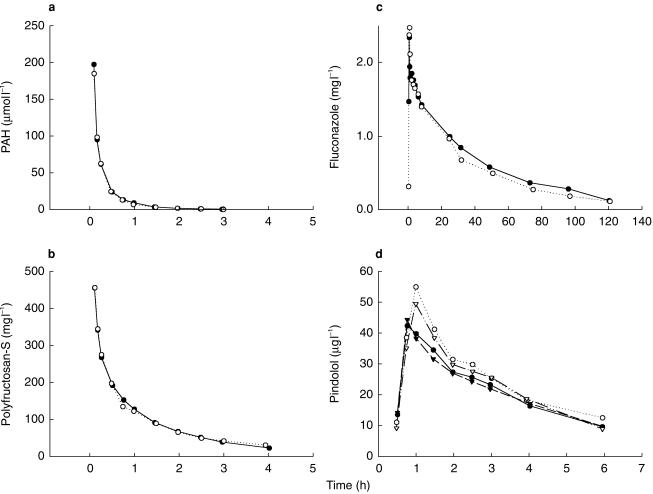
The pharmacokinetic and urine excretion parameters describing the disposition and renal handling of PAH and sinistrin as well as the creatinine clearance, both measured and calculated, are presented in Table 1. The disposition parameters for the enantiomers of pindolol are presented in Table 2 and for fluconazole in Table 3. The pindolol plasma concentration-time data were not included for one subject (6) and the pindolol data treatment is based on the results in 11 subjects. Subject 6 was excluded because the estimate for pindolol clearance (both renal and nonrenal) was markedly different (greater than 2 s.d.s) from the other 11 subjects and published pharmacokinetic data for pindolol [15, 21, 22]. The reason for this difference is unclear but re-analysis of selected samples confirmed it was not related to an analytical error. Plasma concentration-time profiles for each compound in a representative subject are shown in Figure 1.
Table 1.
A comparison of the indices of renal handling of creatinine, PAH and sinistrin between the study arms (F, M, F + M). Creatinine clearance measured from plasma and urine creatinine concentrations and estimated using the Cockcroft and Gault equation is given. The urinary recovery (Ae), clearance (CL) and renal clearance (CLR) of sinistrin and PAH, as well as the net renal secretion (CLsecr) of PAH, are also given (mean ± s.d.; (range)).
| Parameter | Fluconazole (F) study arm | Markers@ (M) study arm | Markers@ + Fluconazole (F + M) study arm | P value* (95% CI of difference) |
|---|---|---|---|---|
| CLcr measured | 145 ± 26 | 136 ± 21 | 138 ± 23 | 0.672† |
| (ml min−1) | (103–178) | (101–163) | (98–174) | |
| CLcr estimated | 142 ± 22 | 146 ± 32 | 144 ± 26 | 0.939† |
| (ml min−1) | (115–180) | (104–216) | (116–186) | |
| CLcrsecr | 17 ± 25 | 8 ± 33 | 0.473 | |
| (ml min−1) | (−33–45) | (−40–50) | (−15,–33) | |
| Sinistrin CL | 139 ± 44 | 143 ± 45 | 0.482 | |
| (ml min−1) | (82–234) | (81–227) | (−16,–7) | |
| Sinistrin Ae | 2286 ± 335 | 2447 ± 213 | 0.116 | |
| (mg) | (1509–2645) | (2122–2760) | (−347,–24) | |
| Sinistrin CLR | 119 ± 31 | 130 ± 40 | 0.317 | |
| (ml min−1) | (80–187) | (76–207) | (−31,–9.6) | |
| PAH CL | 697 ± 183 | 663 ± 166 | 0.398 | |
| (ml min−1) | (449–1021) | (483–1061) | (−42,–111) | |
| PAH Ae | 1379 ± 124 | 1459 ± 141 | 0.069 | |
| (µmol) | (1210–1630) | (1190–1685) | (−158,−2) | |
| PAH CLR | 469 ± 145 | 467 ± 146 | 0.945 | |
| (ml min−1) | (284–713) | (337–855) | (−107,–111) | |
| Net PAH CLsecr | 370 ± 130 | 359 ± 134 | 0.631 | |
| (ml min−1) | (187–583) | (252–729) | (−41,–63) |
Paired t-test: Markers (M) vs Markers + Fluconazole (F + M) a. 95% confidence interval of differences.
anova: Fluconazole (F) vs Markers (M) vs Markers + Fluconazole (F + M) @Markers were sinistrin, PAH and pindolol.
Table 2.
Pharmacokinetic parameters (mean ± s.d.; (range)) for R-and S-pindolol, markers of renal cation secretion in healthy subjects.
| Markers (M) study arm | Markers + Fluconazole (F + M) study arm | P value* | (95% CI of difference) | |||
|---|---|---|---|---|---|---|
| Parameter | R | S | R | S | R | S |
| Cmax | 32 ± 12 | 34 ± 12 | 34 ± 13 | 34 ± 10 | 0.464 | 0.812 |
| (µg l−1) | (14–46) | (16–48) | (16,58) | (19,49) | (−6,2.6) | (−4.8,3.8) |
| tmax (h) | 1.4 ± 0.6 | 1.4 ± 0.6 | 1.6 ± 0.7 | 1.6 ± 0.7 | 0.252 | 0.444 |
| (0.8–2.5) | (0.8–2.5) | (0.8,3.0) | (0.8,3.0) | (−0.62,0.14) | (−0.55,0.23) | |
| Ae | 1979 ± 790 | 2397 ± 808 | 2135 ± 868 | 2579 ± 892 | 0.229 | 0.232 |
| (mg) | (683–3024) | (784–3334) | (875,3444) | (1148,4329) | (−396,84) | (−462,100) |
| CL/F | 1005 ± 615 | 834 ± 402 | 820 ± 401 | 674 ± 154 | 0.101 | 0.131 |
| (ml min−1) n = 11 | (528–2388) | (416–1872) | (448,1768) | (434,862) | (−16,386) | (−31,352) |
| CLR | 204 ± 41 | 225 ± 55 | 190 ± 41 | 209 ± 60 | 0.394 | 0.270 |
| (ml min−1) n = 11 | (140–261) | (153–296) | (141,260) | (153,297) | (−17,45) | (−10,41) |
| CLuR (ml min−1) | 452 ± 92 | 499 ± 121 | 421 ± 92 | 465 ± 132 | 0.394 | 0.270 |
| n = 11 | (342–580) | (341–692) | (314,577) | (264,660) | (−38,100) | (−23,92) |
| CLfilt (ml min−1) | 53 ± 15 | 58 ± 19 | 0.423 | |||
| n = 11 | (35–84) | (34–92) | (−14–6) | |||
| Net CLsecr | 150 ± 49 | 171 ± 60 | 132 ± 45 | 152 ± 70 | 0.296 | 0.197 |
| (ml min−1) n = 11 | (91–203) | (69–253) | (66,225) | (33,253) | (−14.2,50.7) | (−8,47) |
Paired t-test: Markers (M) vs Markers + Fluconazole (F + M) study arms.
Table 3.
Pharmacokinetic parameters (mean ± s.d.; (range)) describing fluconazole disposition and renal handling when administered alone and concomitantly with the markers in 12 healthy subjects.
| Parameter | Fluconazole (F) study arm | Markers + Fluconazole (F + M) study arm | P value* (95% CI of difference) |
|---|---|---|---|
| Cmax (mg l−1) | 1.96 ± 0.44 | 1.92 ± 0.34 | 0.634 |
| (1.15–2.48) | (1.55–2.53) | (−0.11–0.19) | |
| tmax (h) | 2.1 ± 1.6 | 2.3 ± 1.3 | 0.578 |
| (0.5–6.0) | (0.7–4.0) | (−0.80–0.44) | |
| Ae (mg) | 70 ± 14 | 67 ± 14 | 0.524 |
| (41–91) | (50–88) | (−6–12) | |
| fu | 0.842 ± 0.024 | 0.822 ± 0.024 | 0.005 |
| (0.787–0.871) | (0.775–0.860) | (0.01–0.03) | |
| CL/F (ml min−1) | 21.4 ± 4.3 | 20.3 ± 3.7 | 0.117 |
| (14.3–31.0) | (12.5–25.5) | (−0.2–2.4) | |
| CLR (ml min−1) | 14.9 ± 3.8 | 13.6 ± 3.4 | 0.159 |
| (10.7–22.2) | (9.0–17.7) | (−0.4–2.9) | |
| CLNR (ml min−1) | 6.5 ± 4.3 | 6.7 ± 3.5 | 0.874 |
| (1.9–18.2) | (2.3–12.8) | (−2.3–2.0) | |
| CLuR (ml min−1) | 17.7 ± 4.5 | 16.6 ± 4.3 | 0.278 |
| (12.5–26.0) | (10.7–22.8) | (−0.8–3.1) | |
| Clfilt (ml min−1) | 106 ± 31 | ||
| (64–161) | |||
| Clreabs (ml min−1) | 93 ± 29 | ||
| (55–142) |
Paired t-test: Fluconazole (F) vs Markers + Fluconazole (F + M) study arms.
Figure 1.
Plasma concentration-time profiles after administration of markers or fluconazole alone (•) and coadministration of markers and fluconazole (○) in a representative subject (number 11). a) p-aminohippurate, b) sinistrin, c) fluconazole, d) R-pindolol (•, ○) and S-pindolol (▾, ▿).
Creatinine clearance, measured and estimated using the Cockcroft & Gault nomogram, was comparable in all three study arms and in addition the values obtained by measurement and calculated using the Cockcroft-Gault equation were not different (P = 0.176). These values were significantly, but weakly, correlated (slope=0.66, r2 = 0.34, P = 0.0002, n = 36), despite the relatively narrow range of values in healthy volunteers.
All pharmacokinetic parameters describing the disposition and renal handling of sinistrin and PAH were similar when the markers were administered alone or with fluconazole. The plasma and renal clearances of sinistrin were correlated (slope = 0.72; r2 = 0.78 P < 0.001). Sinistrin plasma clearance was 12% higher (P < 0.001; 95% CI of difference: 8,24) than the renal clearance, suggesting a small contribution of nonrenal elimination in the clearance of sinistrin. As a consequence, sinistrin renal clearance was taken as the marker of glomerular filtration rate. Sinistrin renal clearance (GFR) was significantly lower than the measured creatinine clearance (P = 0.044; 95% CI of difference: −24, −1), indicating a small and variable contribution of secretion to the renal elimination of creatinine (average 8%). The correlations of GFR and measured creatinine clearance were significant but not strong (slope = 0.94, r2 = 0.33, P = 0.003, n = 24). Sinistrin plasma clearance was similar to both measured (P = 0.626; 95% CI of difference: −11,18) and predicted (P = 0.612; 95% CI of difference: −19,11) creatinine clearance. As anticipated net secretion of PAH was observed. Nonrenal clearance of PAH accounted for 31% of the total PAH clearance.
The disposition of both R-and S-pindolol was not influenced by coadministration of fluconazole (Table 2). The urinary recovery of S-pindolol was greater (P < 0.001 M; 95% CI of difference −554, 282; P < 0.001 F+M; 95% CI of difference −571, 317) than that of the R-enantiomer indicating stereoselective renal elimination. Net renal secretion of R-and S-pindolol was observed and the two were correlated (slope = 1.04, r2 = 0.58, P < 0.001, n = 22). As fluconazole did not influence pindolol disposition, enantioselectivity was investigated using pooled data for each enantiomer. The apparent clearance of R-pindolol was greater than S-pindolol (P = 0.02; 95% CI of difference 36, 282), principally due to the higher (P = 0.005; 95% CI of difference 69,289) nonrenal clearance of R-than S-pindolol. The renal handling of pindolol was stereoselective. The renal clearance (P = 0.032; 95% CI of difference −38, 3), renal clearance of unbound drug (P = 0.032; 95% CI of difference −84, 7) and net renal secretion (P = 0.032; 95% CI of difference −38, 3) of S-pindolol were higher than the R-enantiomer. Other pindolol pharmacokinetic parameters were not stereoselective (P > 0.05).
The pharmacokinetic parameters describing the disposition of fluconazole were not different when the drug was administered alone (F) or coadministered with the markers (F+M) (Table 3). The unbound fraction was significantly (P = 0.005; 95% CI of difference 0.01,–0.03) lower when fluconazole was given with the markers (Table 3). This small difference in binding resulted in the observation of no significant change in renal clearance of unbound fluconazole when coadministered with markers. As anticipated the data indicate that the net reabsorption of fluconazole was high. Surprisingly, renal clearance accounted for only 70% of the apparent clearance of fluconazole, indicating either incomplete bioavailability or that nonrenal clearance made a significant contribution to fluconazole elimination. When calculated using data collected over the 0–24 h time interval, fluconazole renal clearance (F 15.9 ± 3.9 ml min−1; F+M 15.4 ± 3.9 ml min−1; 95% CI of difference −1.76,2.82) and unbound renal clearance (F: 18.9 ± 4.6 ml min−1 and F+M: 18.8 ± 4.8 ml min−1; 95% CI of difference −2.55,2.87) were similar to those calculated using data collected over 120 h (Table 3). This indicates that fluconazole renal clearance can be reliably estimated by sampling for 24 h after dosing, rather than the lengthy and more invasive 120 h collection procedure required to fully characterize fluconazole disposition.
Discussion
As renal excretion plays an important role in the elimination of many drugs, changes in renal function can have an important influence on drug pharmacokinetics [23]. Dose adjustment is required for drugs with narrow therapeutic indices in patients with altered renal drug handling to avoid adverse drug effects as a consequence of elevated plasma concentrations of parent drugs or metabolites. The classical marker for the integrity of renal drug handling is creatinine clearance, determined routinely by calculation from plasma creatinine concentrations and patient characteristics. It is recognized that significant declines in renal function can occur while plasma creatinine concentrations remain within the normal range [13, 24]. Creatinine is eliminated by glomerular filtration and tubular secretion and its use as a marker of renal integrity assumes that both activities decline in parallel when renal impairment occurs, an assumption supported by the intact nephron hypothesis. However it is known that secretion makes an increasing contribution to creatinine elimination as renal function declines [24, 25]. Creatinine clearance may therefore not accurately reflect the capacity of each of these individual processes and their changes with disease [26]. A number of studies have demonstrated that in people with HIV the standard nomograms provide an overestimation of the measured creatinine clearance [6–8]. This finding is consistent with our pharmacokinetic studies on fluconazole in this population [1, 2] and indicates that a better predictor of renal drug handling is required for some drugs.
The ultimate aim of this investigation has been to measure the activity of each of the processes contributing to the renal excretion of drugs, using appropriate markers administered concomitantly, in order to assess whether any one of these processes is altered in disease. The cocktail of markers must be easy to administer, free of adverse effects and the markers must not interact to affect renal drug handling. Previous studies confirmed that coadministration of three of the markers using in this study (pindolol, inulin and PAH) did not lead to an interaction [17, 18] and this combination represented the markers (M) of this investigation. There were no changes observed in the pharmacokinetic parameters of sinistrin, PAH, R-and S-pindolol or fluconazole following the single dose administration of the cocktail (F+M) relative to administration of the markers (M) or fluconazole (F) alone. The sole significant change observed was a small decrease in fluconazole unbound fraction with coadministration of markers, which did not alter the renal handling of unbound fluconazole (Table 3). Pindolol, PAH and fluconazole pharmacokinetic parameters were similar to those reported previously in healthy volunteers [13, 15, 21, 27, 28].
Sinistrin, or polyfructosan S, a polysaccharide similar to inulin, is a well characterized marker of glomerular filtration rate [29]. It was selected for this study as it is more water soluble than inulin and therefore does not require warming to facilitate dissolution prior to administration. The renal clearance of sinistrin observed (Table 1) is in close agreement with that reported previously in the literature [10, 11, 29]. A higher plasma than renal clearance was noted, suggesting some nonrenal excretion of this marker. For this reason sinistrin renal clearance was selected as the marker of GFR. As frequently reported in the literature [23–25], GFR was lower than creatinine clearance, the difference presumably reflecting the tubular secretion of creatinine. In the healthy volunteers studied the measured creatinine clearance and the value predicted using the Cockcroft-Gault equation were in goodagreement and significantly, but not strongly, correlated.
PAH was selected to measure effective renal plasma flow and tubular anion secretion [13, 14, 18]. In agreement with literature reports [27], the decline in PAH plasma concentrations in most subjects was nonlinear, and total clearance of PAH continued to decline as the plasma concentration fell. Net tubular secretion made the greatest contribution to PAH clearance. PAH nonrenal clearance, principally reflecting acetylation [27] was noted. At the intravenous dose administered in this study the maximum plasma concentration was less than 100 µg ml−1, the concentration known to saturate anion transport in the kidney [14]. Bolus intravenous doses of PAH and sinistrin are convenient and were used as previous studies have demonstrated that they can provide reliable estimates of renal clearance, provided the plasma concentrations are adequately characterized [10, 11, 13, 14, 27, 29].
Pindolol was selected as a marker of tubular secretion by the organic cation transporter. Previous studies have shown that concomitant administration of 15 mg pindolol orally with intravenous PAH and inulin does not alter either the effective renal plasma flow or the glomerular filtration rate [17, 18] and it was therefore considered appropriate for in vivo marker when coadministered with PAH and sinistrin. As the net tubular secretion of pindolol is stereoselective [15, 21, 22], insight into the mechanism responsible for changes in function with disease may be obtained by also measuring pindolol stereoselectivity. In agreement with the literature the net tubular secretion of pindolol was stereoselective, favouring the S-enantiomer, whereas nonrenal clearance, principally metabolism, favoured R-pindolol [15, 21].
Fluconazole is extensively reabsorbed by the renal tubule [4]. It was used as the marker of tubular reabsorption as its clearance is decreased in HIV seropositive people who have creatinine clearances within the normal range [1–3]. The long biological sampling time required to fully characterize fluconazole elimination is a disadvantage of the cocktail developed. However as the renal clearance of fluconazole over a 0–24 h interval is comparable with that calculated based on sampling for 120 h, samples need only be collected for 24 h postdose, simplifying the test procedure considerably.
The study design was sufficiently sensitive (with a power of 80%) to detect a 22.5% change in sinistrin renal clearance, a 25% change in PAH renal clearance, an 18% and 21% change in R-and S-pindolol renal clearances, respectively, and a 20% change in fluconazole renal clearance with an α error of 0.05 and β error of 0.2 [30]. These values also represent the alterations in renal handling processes which this cocktail of markers could detect when given to patients suspected of having diminished renal drug handling. These changes are of a magnitude which may be considered clinically important for drugs eliminated principally by renal excretion. The results obtained in this study using healthy volunteers provide baseline data against which potential changes associated with disease can be assessed.
The individual markers have been widely used in patients and healthy volunteers without significant side-effects and none were noted after coadministration in the present study. The cocktail of markers can therefore be administered to patients and in population studies with relative safety. Of course administration of the β-adrenoceptor antagonist pindolol is contraindicated in people with a history of asthma and careful supervision would be required in some patients, such as the elderly. However, the potential risks of adverse events are limited given the small, single dose administration required.
In recent years much new information has been obtained identifying and characterizing the proteins which transport drugs at the basolateral and brush-border membranes of renal epithelial cells. These active transporters can have a limited capacity for secretion and drug interactions as a consequence of competition for transport can be clinically important [9, 31, 32]. Reabsorption of drugs in the renal tubule has traditionally been thought to be a passive process following the concentration gradient resulting when water is reabsorbed. However cation transporters have recently been identified on the luminal membrane of the distal renal tubule [31] which could play a role in active reabsorption of drugs such as pindolol [33]. Proteins of the ABC (ATP-binding cassette) family including P-glycoprotein (MDR1) have also been identified in the kidney [9] and contribute to the renal secretion of drugs such as digoxin. Fluconazole resistance in some yeast strains is related to the expression of an ABC transport protein, which pumps fluconazole out of cells [34]. Future studies will determine whether fluconazole may also be actively secreted or reabsorbed in the kidney by a protein of this family.
In summary, this study has demonstrated that sinistrin, PAH, pindolol and fluconazole can be coadministered in order to measure the individual processes contributing to renal drug handling. Plasma and urine samples can be collected for 24 h postdose. No adverse effects have been observed following single doses of the markers. Baseline measurements for each of the renal drug handling processes in healthy volunteers, which can be compared with immunocompromised patients or those thought to have impaired renal drug handling, have been reported.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia (CARG Project Grant 960714). PAH and fluconazole were generously provided by Merck, Sharp and Dohme Australia Pty Ltd and Pfizer Australia, respectively. The authors thank Professor G. Shenfield, Department of Clinical Pharmacology, Royal North Shore Hospital, Sydney for support and assistance. The support of the Fondation Pour la Recherche Medicale (ASG) is gratefully acknowledged.
References
- 1.Tett S, Moore S, Ray J. Pharmacokinetics and bioavailability of fluconazole in two groups of males with human immunodeficiency virus (HIV) infection compared with those in a group of males without HIV infection. Antimicrob Agent Chemother. 1995;39:1835–1841. doi: 10.1128/aac.39.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLachlan AJ, Tett SE. Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. Br J Clin Pharmacol. 1996;41:291–298. doi: 10.1046/j.1365-2125.1996.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeates RA, Ruhnke M, Pfaff G, Hartmann A, Trautmann M, Sarnow E. The pharmacokinetics of fluconazole after a single intravenous dose in AIDS patients. Br J Clin Pharmacol. 1994;38:77–79. doi: 10.1111/j.1365-2125.1994.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debruyne D, Ryckelynck J-P. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24:10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;15:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Noormohamed SE, Katseres JK, Stapleton JT. Poor correlation between published methods to predict creatinine clearance and measured creatinine clearance in asymptomatic HIV infected individuals. Ren Fail. 1998;20:627–633. doi: 10.3109/08860229809045156. [DOI] [PubMed] [Google Scholar]
- 7.Huang E, Hewitt RG, Shelton M, Morse GD. Comparison of measured and estimated creatinine clearance in patients with advanced HIV disease. Pharmacotherapy. 1996;16:222–229. [PubMed] [Google Scholar]
- 8.Smith BL, Sarnoski TP, Dennis S, Luke DR. Failure of predicted creatinine clearance equations in HIV-seropositive patients. Int J Clin Pharmacol Ther Toxicol. 1992;30:394–399. [PubMed] [Google Scholar]
- 9.Bonate PL, Reith K, Weir S. Drug interactions at the renal level. Clin Pharmacokinet. 1998;34:375–404. doi: 10.2165/00003088-199834050-00004. [DOI] [PubMed] [Google Scholar]
- 10.Buclin T, Pechere-Bertschi A, Sechaud R, et al. Sinistrin clearance for determination of glomerular filtration rate: a reappraisal of various approaches using a new analytical method. J Clin Pharmacol. 1997;37:679–692. doi: 10.1002/j.1552-4604.1997.tb04355.x. [DOI] [PubMed] [Google Scholar]
- 11.Estelberger W, Petek W, Zitta S, et al. Determination of the glomerular filtration rate by identification of sinistrin kinetics. Eur J Clin Chem Clin Biochem. 1995;33:201–209. doi: 10.1515/cclm.1995.33.4.201. [DOI] [PubMed] [Google Scholar]
- 12.Chan K, Miners JO, Birkett DJ. Direct and simultaneous high-performance liquid chromatographic assay for the determination of p-aminobenzoic acid and its conjugates in urine. J Chromatogr B. 1988;426:103–109. doi: 10.1016/s0378-4347(00)81931-3. [DOI] [PubMed] [Google Scholar]
- 13.Hirata-Dulas CAI, Awni WM, Matzke GR, Halstenson CE, Guay DRP. Evaluation of two intravenous single-bolus methods for measuring effective renal plasma flow. Am J Kid Dis. 1994;23:374–381. doi: 10.1016/s0272-6386(12)80999-1. [DOI] [PubMed] [Google Scholar]
- 14.Kinowski JM, Rodier M, Bressolle F, et al. Bayesian estimation of p-aminohippurate clearance by a limited sampling strategy. J Pharm Sci. 1995;84:307–311. doi: 10.1002/jps.2600840309. [DOI] [PubMed] [Google Scholar]
- 15.Hsyu P-H, Giacomini KM. Stereoselective renal clearance of pindolol in humans. J Clin Invest. 1985;76:1720–1726. doi: 10.1172/JCI112161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross AS, Somogyi AA. Interaction of the stereoisomers of basic drugs with the uptake of tetraethylammonium by rat renal brush-border membrane vesicles. J Pharmacol Exp Ther. 1994;268:1073–1080. [PubMed] [Google Scholar]
- 17.Wainer E, Boner G, Rosenfeld JB. Effects of pindolol on renal function. Clin Pharmacol Ther. 1980;28:575–580. doi: 10.1038/clpt.1980.205. [DOI] [PubMed] [Google Scholar]
- 18.Pasternack A, Pörsti P, Pöyhönen L. Effect of pindolol and propranolol on renal function of patients with hypertension. Br J Clin Pharmacol. 1982;13:241S–244S. doi: 10.1111/j.1365-2125.1982.tb01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLachlan AJ, Gross AS, Beal JL, Minns I, Tett SE. Analytical validation for a series of marker compounds used to assess each renal elimination process. Ther Drug Monit. 2001;23:39–46. doi: 10.1097/00007691-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Aminohippurate Sodium Injection. West Point PA, USA: Product Datasheet Merck Sharp and Dohme; [Google Scholar]
- 21.Somogyi AA, Bochner F, Sallustio BC. Stereoselective inhibition of pindolol renal clearance by cimetidine in humans. Clin Pharmacol Ther. 1992;5:379–387. doi: 10.1038/clpt.1992.37. [DOI] [PubMed] [Google Scholar]
- 22.Ujhelyi MR, Bottorff MB, Schur M, et al. Aging effects on the organic base transporter and stereoselective renal clearance. Clin Pharmacol Ther. 1997;62:117–128. doi: 10.1016/S0009-9236(97)90059-X. [DOI] [PubMed] [Google Scholar]
- 23.Lam YWF, Banerji S, Hatfield C, Talbert RL. Principles of drug administration in renal insufficiency. Clin Pharmacokinet. 1997;32:30–57. doi: 10.2165/00003088-199732010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Smith CL, Hampton EM. Using estimated creatinine clearance for individualizing drug therapy: a reassessment. Ann Pharmacother. 1990;24:1185–1190. doi: 10.1177/106002809002401209. [DOI] [PubMed] [Google Scholar]
- 25.Kim KE, Onesti G, Ramirez O, Brest AN, Swartz C. Creatinine clearance in renal disease. A reappraisal. Br Med J. 1969;4:11–14. doi: 10.1136/bmj.4.5674.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori R, Okumura K, Nihira H, Nakano H, Akagi K, Kamiya A. A new dosing regimen in renal insufficiency: application to cephalexin. Clin Pharmacol Ther. 1985;38:290–295. doi: 10.1038/clpt.1985.173. [DOI] [PubMed] [Google Scholar]
- 27.Prescott LF, Freestone S, McAuslane JAN. The concentration-dependent disposition of intravenous p-aminohippurate in subjects with normal and impaired renal function. Br J Clin Pharmacol. 1993;35:20–29. [PMC free article] [PubMed] [Google Scholar]
- 28.Grant SM, Clissold SP. Fluconazole – a review of the pharmacokinetic properties and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 29.Buclin T, Sechaud R, Bertschi AP, et al. Estimation of glomerular filtration rate by sinistrin clearance using various approaches. Ren Fail. 1998;20:267–276. doi: 10.3109/08860229809045111. [DOI] [PubMed] [Google Scholar]
- 30.Stolley PD, Strom BL. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39:489–490. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- 31.Koepsell H. Organic cation transporters in intestine, kidney, liver and brain. Ann Rev Physiol. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- 32.Roch-Ramel F. Renal transport of organic anions. Curr Opin Nephrol Hypertens. 1998;7:517–524. doi: 10.1097/00041552-199809000-00006. 10.1006/scdb.1996.0065. [DOI] [PubMed] [Google Scholar]
- 33.Balant L, Muir K, Dayer P, et al. Simultaneous tubular excretion and reabsorption of pindolol in man. Eur J Clin Pharmacol. 1981;21:65–72. doi: 10.1007/BF00609590. [DOI] [PubMed] [Google Scholar]
- 34.Moran GP, Sanglard D, Donnelly SM, Shanley DB, Sullivan DJ, Coleman DC. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agent Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]