Abstract
Aims
To evaluate the pharmacokinetic interaction between ritonavir and mefloquine.
Methods
Healthy volunteers participated in two separate, nonfasted, three-treatment, three-period, longitudinal pharmacokinetic studies. Study 1 (12 completed): ritonavir 200 mg twice daily for 7 days, 7 day washout, mefloquine 250 mg once daily for 3 days then once weekly for 4 weeks, ritonavir restarted for 7 days simultaneously with the last mefloquine dose. Study 2 (11 completed): ritonavir 200 mg single dose, mefloquine 250 mg once daily for 3 days then once weekly for 2 weeks, ritonavir single dose repeated 2 days after the last mefloquine dose. Erythromycin breath test (ERMBT) was administered with and without drug treatments in study 2.
Results
Study 1: Ritonavir caused less than 7% changes with high precision (90% CIs: −12% to 11%) in overall plasma exposure (AUC(0,168 h)) and peak concentration (Cmax) of mefloquine, its two enantiomers, and carboxylic acid metabolite, and in the metabolite/mefloquine and enantiomeric AUC ratios. Mefloquine significantly decreased steady-state ritonavir plasma AUC(0,12 h) by 31%, Cmax by 36%, and predose levels by 43%, and did not affect ritonavir binding to plasma proteins. Study 2: Mefloquine did not alter single-dose ritonavir pharmacokinetics. Less than 8% changes in AUC and Cmax were observed with high variability (90%CIs: −26% to 45%). Mefloquine had no effect on the ERMBT whereas ritonavir decreased activity by 98%.
Conclusions
Ritonavir minimally affected mefloquine pharmacokinetics despite strong inhibition of CYP3A4 activity from a single 200 mg dose. Mefloquine had variable effects on ritonavir pharmacokinetics that were not explained by hepatic CYP3A4 activity or ritonavir protein binding.
Keywords: cytochrome P450 3A4, erythromycin breath test, mefloquine, pharmacokinetic interaction, ritonavir
Introduction
Human immunodeficiency virus (HIV) disease is a global pandemic and is present in countries where Plasmodium falciparum malaria, including that caused by chloroquine-resistant strains, is endemic. As more persons with HIV are increasingly well from antiretroviral drug therapy, they are travelling more frequently to destinations that require antimalarial prophylaxis. Mefloquine is the recommended drug for malaria prophylaxis in areas where chloroquine-resistant malaria is widespread [1]. Mefloquine is administered as a racemate of (+)-RS and (–)-SR enantiomers, which are both active against P. falciparum malaria in vitro [2]. Protease inhibitors such as ritonavir contribute to the improved health of HIV-positive individuals, and their inclusion in antiretroviral regimens is commonplace. However, protease inhibitors are often involved in clinically important drug interactions resulting from alteration of cytochrome P450 (CYP) metabolism.
Ritonavir is mainly metabolized by the CYP3A4 isoenzyme and has a high binding affinity to P-glycoprotein [3]. The drug is also an inducer and potent inhibitor of CYP3A4-mediated metabolism and a modest agent for blocking P-glycoprotein binding [3, 4], and is responsible for elevating plasma concentrations of a number of CYP3A4 and P-glycoprotein substrates such as saquinavir and ketoconazole [5, 6]. Mefloquine does not interact with many compounds, although there are in vitro data suggesting it is a substrate and inhibitor of CYP3A4 and P-glycoprotein [7–9] and animal data indicating it reduces bile production in rats [10]. The main metabolite of mefloquine in humans is an inactive carboxylic acid derivative, the formation of which is catalysed by CYP enzymes [9, 11].
This paper presents data on the pharmacokinetics of mefloquine, its carboxylic acid metabolite and two enantiomers, and ritonavir. The objectives of this study were to evaluate the bidirectional pharmacokinetic interaction between ritonavir and mefloquine, including their effect on hepatic CYP3A4 activity, and to assess the safety and tolerance of these agents when used concurrently.
Methods
Participants
Healthy nonsmoking men and women who were between the ages of 18 and 50 years and within 20% of their ideal body weight for height and gender (Metropolitan Life Scale) were eligible to participate in the study. Subjects were excluded from participating if they met one of the following criteria: pregnancy, breast feeding, history of hypersensitivity reactions to protease inhibitors, mefloquine or similar agents (quinine, chloroquine, quinidine, quinolones), use of any medications that could potentially interact with either study drug (including cardiac medications such as β-adrenoceptor blockers and any agents that affect CYP), serum creatinine greater than 1.5 times the upper limit of normal, any liver function test more than three times the upper limit of normal, or evidence by history or physical examination of gastrointestinal, psychiatric, cardiovascular or neurological disorders. Women who were taking oral contraceptives that contained ethinyloestradiol were required to also practice barrier contraceptive methods because of potential reductions in ethinyloestradiol concentrations caused by ritonavir [12]. All participants gave written informed consent, and approval was obtained from the Ottawa Hospital Research Ethics Board and Radiation Safety Committee.
Study design
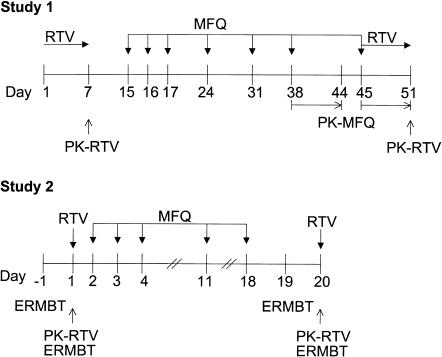
This was an open-label, nonfasting, three-treatment, three-period, longitudinal (fixed sequence) design in two separate, sequential pharmacokinetic studies (Figure 1). The first study, designated as study 1, enrolled seven male and seven female Caucasian volunteers. The second study, designated as study 2, enrolled five male and seven female Caucasian subjects after the first study was completed, eight of whom had participated in the first study.
Figure 1.
Design of studies 1 and 2. Blood samples were collected over 12 h for ritonavir and over 168 h (7 days) for mefloquine. RTV: ritonavir 200 mg every 12 h; MFQ: mefloquine 250 mg; ERMBT: erythromycin breath test; PK: pharmacokinetics.
At the start of each blood collection period in both studies, participants fasted from midnight of the day before. In the morning, 30 min before the next scheduled dose, subjects reported to the Clinical Investigation Unit of the Ottawa Hospital and received a standard full breakfast (566 kcal; 17% protein, 41% fat, 42% carbohydrate) in study 1 and a light breakfast (443 kcal; 11% protein, 17% fat, 72% carbohydrate) in study 2. Each dose was given within 10 min after the subject finished breakfast and was administered with about 200 ml of water. Subjects remained in the Unit for 12 h with supervision for blood sampling and, if necessary, returned on subsequent days for single blood collections as described later.
Study 1 In period 1, participants received 13 doses of 200 mg ritonavir (Norvir, 2.5 ml of an 80 mg ml−1 solution) by oral syringe, twice daily at 08.00 h and 20.00 h for 6 days (days 1–6) as outpatients and at 08.00 h on the morning of day 7 in the Unit. Blood samples were collected after the last dose over the 12 h dosing interval for ritonavir baseline pharmacokinetics. In period 2, following a 7 day washout, a 250 mg tablet of mefloquine (Lariam) was administered by mouth at 08.00 h once daily for 3 days (days 15–17) as a loading dose, then once weekly for 3 weeks (days 24, 31, 38) as maintenance dosing. Subjects took the first five doses as outpatients. On day 38, after the third weekly (6th) dose, blood sampling was performed over the 1 week mefloquine dosing interval (days 38–44) for mefloquine baseline pharmacokinetics.
In period 3, volunteers were restarted on ritonavir 200 mg twice daily for 1 week (days 45–51) and received one further weekly dose of mefloquine (day 45). The first dose of ritonavir was administered simultaneously with the final (7th) mefloquine dose on day 45. Blood sampling was again performed over 1 week (days 45–51) for mefloquine pharmacokinetics in the presence of ritonavir, and over 12 h during the morning of the last (13th) ritonavir dose (day 51) for ritonavir pharmacokinetics in the presence of mefloquine.
Safety follow-up included physical examinations and blood work on 4 separate study days (days 7, 38, 45, 51). Electrocardiograms were performed at baseline, at steady-state mefloquine (day 38), and after 1 week of mefloquine and ritonavir coadministration (day 51). Further follow-up safety evaluations were scheduled at the discretion of the physician.
Study 2 This study followed the same procedures as those in study 1 except ritonavir was given as a single dose and with no washout period after ritonavir alone in period 1, mefloquine was dosed for two instead of four weekly doses and at 20.00 h instead of 08.00 h, and ritonavir was given at 2 instead of 7 days after the last mefloquine dose. Subjects received a single 200 mg dose of ritonavir on day 1 (period 1) followed by three daily (days 2–4) and two weekly (days 11, 18) 250 mg doses of mefloquine (period 2). In period 3, 2 days later, a single 200 mg dose of ritonavir was administered in the presence of mefloquine. Serial blood samples were collected over 24 h for ritonavir pharmacokinetics on day 1 (drug alone) and day 20 (with mefloquine) and for mefloquine measurements on day 20.
Erythromycin breath test
To test hepatic CYP3A4 enzyme activity, the erythromycin breath test (ERMBT) was performed at 09.00 h on the day before the start of study 2 (day −1), at 12.00 h on day 1 (i.e. 4 h after administration of ritonavir alone), at 09.00 h on day 19 (i.e. 13 h after the last dose of mefloquine alone), and at 12.00 h on day 20 (i.e. 4 h after administration of ritonavir in the presence of mefloquine). Subjects received the ERMBT in a fasted state on days −1 and 19 and before lunch was served (4.5 h after a light breakfast) on days 1 and 20. The ERMBT was delayed on days 1 and 20 to allow for maximum ritonavir plasma concentrations to be achieved and to avoid the effects of food (breakfast) on the ERMBT. A light breakfast was served on these days to accommodate administration of ritonavir. Subjects were given 3 µCi (0.04 mg) of [14C-N-methyl]erythromycin (Metabolic Solutions, Inc., Nashua, NH) intravenously while at rest, and breath samples were collected at 20 min after injection. The exhaled radiolabeled carbon dioxide (14CO2) was measured by liquid scintillation counting as previously described [13]. Average background 14C activity in the CO2 trapping solution was determined from three separate samples into which a control subject exhaled without prior injection of the radiolabeled erythromycin. The disintegrations per minute of 14C in each sample were corrected for mean background activity. ERMBT results were expressed two ways: (1) as the percentage of administered 14C exhaled min−1 at 20 min (%M min−1) [13], and (2) as the percentage of administered 14C exhaled during the first hour after injection (%M h−1). The %M h−1 values were estimated from the %M min−1 values by a quadratic equation [14]. Although ERMBT values are usually reported as %M h−1, the error in estimates of %M h−1 increases as the values of %M min−1 approach background levels with strong CYP3A4 inhibitors. Therefore both values are reported.
Blood sampling procedure
Blood samples (5 ml) were collected into Vacutainer tubes (Becton Dickinson, Rutherford, NJ) containing ethylenediamine tetraacetic acid as the anticoagulant. In study 1, blood samples for measurements of mefloquine, the (+)-RS and (–)-SR enantiomers of mefloquine, and MMQ were taken over the weekly dosing interval immediately before and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 96, 144 and 168 h after administration of mefloquine, and blood samples for ritonavir measurements were collected over the 12 h dosing interval immediately before and at 0.5, 1, 2, 3, 3.5, 4, 4.5, 5, 6, 8 and 12 h after the dose. The same ritonavir time points were used in study 2 except hour 4 (time of ERMBT) and with an additional sample collected at 24 h after the single dose. Blood was equilibrated at 20 °C for 10 min and then centrifuged (1500 g at 20 °C for 10 min) to separate plasma. The plasma aliquots were stored at −70 °C or less until analysis.
Drug analysis
Concentrations of total ritonavir, racemic mefloquine, and MMQ in plasma were measured simultaneously with high-performance liquid chromatography (h.p.l.c.) with u.v. detection after an ion-pair liquid-liquid extraction. The extraction procedure was a modification of the procedure of Bergqvist et al. [15]. Clomipramine internal-standard solution (0.2 ml of 0.2 mol l−1 citric acid/0.1 mol l−1 potassium dihydrogen phosphate/methanol (1 : 1 : 2, v/v/vol)) and ion-pairing solution (0.7 ml of 0.3 mol l−1 tetrabutylammonium hydrogen phosphate) were added to plasma samples (1 ml). The mixture was basified with 10% ammonium hydroxide (0.5 ml) and extracted with methyl t-butyl ether (6 ml). The organic extract was isolated and evaporated to dryness, and the residue was dissolved in 0.1 mol l−1 potassium dihydrogen phosphate in 50% methanol (0.3 ml). The solution was washed with hexane (3 ml) and the hexane wash was re-extracted with 0.1 mol l−1 citric acid in 50% methanol. The aqueous extracts were combined and analysed (0.075 ml) by h.p.l.c. The h.p.l.c. conditions were as follows: column, Supelcosil ABZ-plus, 3 µm particle size, 4.6 × 150 mm; column temperature, 45 °C; mobile phase composition, 0.02 mol l−1 potassium dihydrogen phosphate and 0.02 mol l−1 octylsulphonic acid adjusted to pH 2.25/methanol/acetonitrile (42 : 29 : 29, v/v/vol); flow rate, 1 ml min−1; and u.v. detector wavelength, 240 nm for ritonavir and 280 nm for the other analytes. The retention times of clomipramine, mefloquine, MMQ, and ritonavir were 4.3, 4.9, 6.4 and 8.4 min, respectively. The lower limits of quantification for mefloquine, MMQ, and ritonavir were 230, 250 and 30 ng ml−1, respectively.
The mefloquine h.p.l.c. peak was isolated automatically by fraction collection, and the (+)-RS and (–)-SR enantiomers were measured with h.p.l.c. with u.v. detection after derivatization with (–) – (9-fluorenyl)ethylchloroformate according to the method of Bergqvist et al. [16]. The percentage of each enantiomer in the racemic mixture was determined from a plot of area ratio of RS/SR peaks vs milligram percentage of RS in a standard enantiomeric mixture.
Total and unbound plasma concentrations of ritonavir for protein binding determinations were measured with h.p.l.c. with tandem mass-spectrometric detection [17]. For each patient per treatment, plasma samples (1 ml) were pooled over the dosing interval into a single aliquot, and a portion was ultrafiltered (2000 g, 25 °C, 20 min) through an Amicon 1 ml capacity ultrafiltration tube (Centrifree Micropartition Device, no. 4104, Amicon Inc., Beverly, MA) to remove protein. The lower limit of quantification of the assay was 0.2 ng ml−1.
Assay data
The 90% confidence limits around the mean assay biases of duplicate quality-control samples were determined at low, medium, and high concentrations from h.p.l.c. data pooled over 12 batches for mefloquine analytes and 17 batches for ritonavir. Values at the three levels ranged from −14.8% to 10.9%, −21.5% to 16.0%, and −12.5% to 10.2% for mefloquine, MMQ, and ritonavir, respectively. The coefficient of variation (CV) of the slope of standard curves for the batches was less than 5% for mefloquine and its enantiomers, 18.3% for MMQ, 9.3% for multiple-dose ritonavir, and 2.2% for single-dose ritonavir, and was a reflection of between-batch imprecision for peak-response data.
Pharmacokinetic analysis
The plasma concentration (C) vs time (t) data of ritonavir, mefloquine, mefloquine enantiomers, and MMQ were analysed with noncompartmental methods. The highest concentration (Cmax), hour 0 concentration (predose C0), hour 12 concentration of ritonavir (postdose C12), hour 168 concentration of mefloquine (postdose C168), and the time to reach Cmax (tmax) were obtained directly from the observed data. For ritonavir, the apparent terminal disposition half-life (t1/2,z) after single dose or the clinically relevant t1/2,z within one 12 h dosing interval was calculated from the final slope (– λz) of the log-linear concentration-time curve (ln C, t) by least-squares linear regression. The slope was estimated from the data set (n ≥ 3 points) with the smallest 90% confidence interval around the slope. Area under the plasma concentration-time curve (AUC) from time zero to 24 h (AUC(0,24 h)) for single-dose ritonavir or over the dosing interval (τ) (AUC(0,τ)) where τ = 12 h for ritonavir and 168 h for mefloquine) was calculated with the linear trapezoidal method. After single dose, AUC(0,∞) was estimated by adding C24/λz to AUC(0,24 h), where C24 is the predicted plasma concentration at 24 h that was calculated from the linear regression equation. The extrapolated tail segments (24 h to ∞) of total AUC were < 11%. The apparent oral plasma clearance of the analytes (CL/F, where F is the bioavailability) was calculated by dividing the dose by AUC(0,∞) or AUC(0,τ).
Statistical analysis
Differences in mean pharmacokinetic parameters between treatment periods were analysed by analysis of variance (anova) appropriate for a longitudinal study. The anova model included the effects of subject-nested-within-gender, gender, period, and gender-by–period interaction. All parameters except tmax were logarithmically (ln) transformed before analysis, and anova summary statistics were based on least-squares geometric means. The 90% confidence limits around the ratio of geometric means were calculated relative to the reference drug-alone treatment. Median tmax values were compared by the Wilcoxon signed-rank test. The significance level for each comparison was set at 0.05.
Differences between the treatment periods in geometric mean ERMBT parameters were evaluated as above by anova, followed by Dunnett's test for multiple comparisons of the treatments in periods 1, 2 and 3 against baseline measurements. Dunnett's test was used to maintain the experimentwise error rate at 5% (α = 0.05) for the three pairwise comparisons, with a resultant P value of < 0.020 considered statistically significant at the 5% level. The 90% confidence limits around the ratio of geometric means were calculated relative to the baseline mean with use of the critical t-value from the Dunnett's test.
For logarithmically transformed data, intraindividual CV was calculated as 100% × (eMSR−1)1/2 and interindividual CV was estimated as 100% × (e(MS – MSR)/n – 1)1/2, where MS is the subject mean square and MSR is the mean-square residual in the anova model, and n is the number of periods being compared (n = 2 for pharmacokinetic comparisons and n = 4 for ERMBT comparisons).
Correlations between ERMBT and ritonavir pharmacokinetic parameters were evaluated by the Pearson correlation coefficient from simple linear regression analysis and the Spearman correlation coefficient from analysis of ranks. The relationship between change in multiple-dose ritonavir AUC(0,12 h) in the presence of mefloquine and mefloquine AUC(0,168 h) over the 7 day dosing interval from days 45–51, MMQ AUC(0,168 h) over the same period, and change in ritonavir fraction unbound to plasma proteins was explored by stepwise multiple-regression analysis. The significance level for each test of zero correlation was set at 0.05.
Results
Study 1
Six men and six women completed the study. The mean±s.d. age and weight of the subjects were 26.6 ± 7.1 years (range, 19–40 years) and 77.5 ±11.8 kg (range, 56.5–96.7 kg), respectively. All subjects experienced reversible mild adverse effects that were primarily neurological or gastrointestinal: six individuals had headache or abdominal pain, five had insomnia, abnormal dreams, or diarrhea, and three had abdominal pain. Three persons had changes from baseline in their electrocardiogram (one with first degree atrioventricular block and two with sinus dysrhythmia), although none was considered clinically significant. Two subjects were withdrawn from the study, one because of gastrointestinal intolerance to ritonavir during the first period of the study, and the second because of side-effects of dizziness, syncope, and palpitations (no electrocardiographic changes) attributed to mefloquine during the second period of the study. Both subjects recovered without sequelae. All subjects who completed the study had evaluable pharmacokinetics except one female who inadvertently took an extra ritonavir dose before coming to the Unit in period 3 and one male who was nonadherent with his last ritonavir dose as an outpatient in period 3.
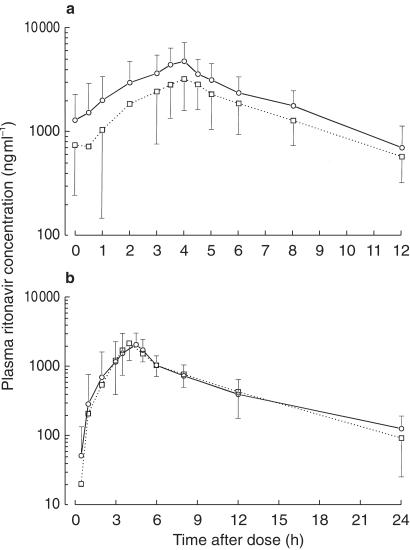
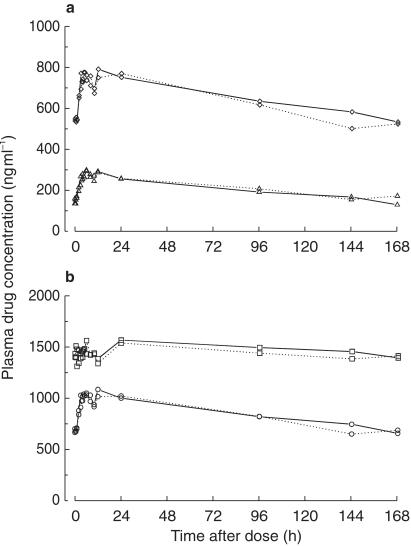
Mean or median plasma pharmacokinetic data for ritonavir, mefloquine, its two enantiomers, and MMQ are summarized in Table 1. Mean plasma concentration-time data of multiple-dose ritonavir are illustrated in Figure 2a and those of the mefloquine analytes are shown in Figure 3. From AUC data, plasma concentrations of MMQ were about 1.8-fold (range, 0.5–4.3) higher than those of mefloquine and levels of the (–)-SR enantiomer were about 3-fold (range, 2.2–4.7) higher than those of the (+)-RS enantiomer. In the presence of ritonavir, with the exception of trough levels of (+)-RS mefloquine, less than 7% changes were observed with high precision (90% CIs: −14% to 15%) in the pharmacokinetic parameters of the mefloquine analytes. Ritonavir significantly increased C168 values of the (+)-RS enantiomer by 34% (range, −3% to 202%).
Table 1.
Study 1: Mean multiple-dose plasma pharmacokinetic parameters of racemic mefloquine, mefloquine enantiomers and carboxylic acid metabolite, and ritonavir for the three treatments: ritonavir alone (period 1), mefloquine alone (period 2), and ritonavir with mefloquine (period 3)
| Parameter | Treatment* Ritonavir and mefloquine (Period 3) | Drug alone (Period 1 or 2) | anova for longitudinal design Point estimate (90% CI)† (%) | P value | Pooled Intra-subject %CV | Pooled Inter-subject %CV |
|---|---|---|---|---|---|---|
| Ritonavir | ||||||
| AUC(0,12 h) (µg ml−1 h) | 19.4 ± 9.3 | 27.5 ± 11.7 | 68.8 (55.7–84.9) | 0.011 | 25.3 | 51.6 |
| Cmax (ng ml−1) | 3463 ± 1842 | 5063 ± 2468 | 63.5 (47.1–85.6) | 0.022 | 36.2 | 57.1 |
| C0 (ng ml−1) | 739 ± 499 | 1288 ± 1001 | 57.0 (41.3–78.6) | 0.012 | 39.3 | 114 |
| C12 (ng ml−1) | 585 ± 259 | 714 ± 446 | 88.1 (68.9–113) | 0.366 | 29.6 | 44.3 |
| t1/2,z (h)‡ | 3.1 ± 0.8 | 3.1 ± 0.7 | 100 (89.1–113) | 0.950 | 13.2 | 18.2 |
| CL/F (ml min−1) | 229 ± 146 | 146 ± 76.1 | 145 (118–180) | 0.011 | 25.3 | 51.6 |
| tmax (h) | 4.0 (2.0, 5.0) | 4.0 (3.0, 5.0) | 0 h | 0.05 | 15.1 | 8.2 |
| fu (%) | 0.43 ± 0.19 | 0.45 ± 0.15 | 93.5 (65.4–134) | 0.735 | 47.8 | ne |
| Mefloquine | ||||||
| AUC(0,168 h) (µg ml−1 h) | 140 ± 26.7 | 144 ± 30.7 | 98.0 (91.6–105) | 0.598 | 9.1 | 17.7 |
| Cmax (ng ml−1) | 1211 ± 210 | 1212 ± 312 | 101 (93.1–110) | 0.795 | 11.4 | 17.6 |
| C0 (ng ml−1) | 664 ± 167 | 699 ± 217 | 95.7 (86.0–107) | 0.471 | 14.6 | 23.3 |
| C168 (ng ml−1) | 689 ± 143 | 664 ± 167 | 105 (95.0–115) | 0.411 | 13.2 | 20.3 |
| CL/F (ml min−1) | 30.6 ± 5.6 | 30.1 ± 5.3 | 102 (95.4–109) | 0.598 | 9.1 | 17.7 |
| (+)-RS Mefloquine | ||||||
| AUC(0,168 h) (µg ml−1 h) | 34.6 ± 7.2 | 33.5 ± 7.0 | 103 (96.7–111) | 0.388 | 9.2 | 17.0 |
| Cmax (ng ml−1) | 326 ± 64.4 | 341 ± 94.3 | 97.3 (88.3–107) | 0.613 | 13.1 | 18.4 |
| C0 (ng ml−1) | 128 ± 40.0 | 152 ± 62.5 | 88.3 (70.1–111) | 0.351 | 32.1 | 26.5 |
| C168 (ng ml−1) | 171 ± 46.8 | 128 ± 40.0 | 134 (116–156) | 0.005 | 20.7 | 20.5 |
| CL/F (ml min−1) | 62.4 ± 11.6 | 64.5 ± 12.3 | 96.7 (90.4–104) | 0.388 | 9.2 | 17.0 |
| (–)-SR Mefloquine | ||||||
| AUC(0,168 h) (µg ml−1 h) | 106 ± 23.3 | 109 ± 26.5 | 96.7 (90.5–103) | 0.391 | 9.1 | 19.9 |
| Cmax (ng ml−1) | 895 ± 195 | 902 ± 246 | 100 (90.9–110) | 0.984 | 13.1 | 19.7 |
| C0 (ng ml−1) | 533 ± 141 | 544 ± 165 | 98.3 (89.3–108) | 0.755 | 13.0 | 24.4 |
| C168 (ng ml−1) | 524 ± 127 | 533 ± 141 | 98.8 (90.0–109) | 0.826 | 12.7 | 23.3 |
| CL/F (ml min−1) | 20.5 ± 4.1 | 19.8 ± 3.7 | 103 (96.7–111) | 0.391 | 9.1 | |
| AUC SR/AUC RS | 3.1 ± 0.6 | 3.3 ± 0.6 | 93.6 (90.2–97.0) | 0.007 | 4.9 | 16.4 |
| MMQ | ||||||
| AUC(0,168 h) (µg ml−1 h) | 244 ± 82.6 | 254 ± 119 | 99.4 (92.5–107) | 0.875 | 9.7 | 40.6 |
| Cmax (ng ml−1) | 1835 ± 700 | 1791 ± 831 | 104 (98.0–111) | 0.247 | 8.3 | 38.0 |
| C0 (ng ml−1) | 1403 ± 543 | 1436 ± 609 | 98.4 (89.3–108) | 0.770 | 13.3 | 41.5 |
| C168 (ng ml−1) | 1422 ± 556 | 1403 ± 543 | 102 (92.2–112) | 0.772 | 13.3 | 40.1 |
| AUC MMQ/AUC MFQ | 1.81 ± 0.76 | 1.85 ± 0.94 | 101 (93.5–110) | 0.764 | 11.0 | 50.7 |
MFQ, mefloquine; MMQ, mefloquine carboxylic acid metabolite; CI, confidence interval; ne, not estimable.
Ritonavir pharmacokinetic measurements were taken after the 13th ritonavir dose (200 mg every 12 h for 7 days, 13 doses) alone and with mefloquine after the 7th dose of mefloquine (250 mg daily for 3 days then weekly for 4 weeks, 7 doses). Mefloquine pharmacokinetic measurements were taken after the 6th mefloquine dose alone and with ritonavir after the 7th mefloquine dose. Values of treatment means are expressed as arithmetic mean ± s.d. (n = 10, ritonavir, except n = 11 for tmax, t1/2,z and fu; n = 12, mefloquine, MMQ and enantiomers); tmax values are expressed as medians (with minimum and maximum given in parentheses)
Percentage ratio of the least-squares geometric treatment mean of the coadministered treatment in period 3 relative to that of the drug-alone treatment in period 1(ritonavir) or 2 (mefloquine). The value for tmax is the point estimate of the absolute difference in medians, relative to the drug-alone treatment. The level of significance was set at 0.05.
Harmonic means are 2.9 h for both arms.
Figure 2.
Mean (± s.d.) plasma concentrations of (a) multiple-dose ritonavir in 10 healthy subjects (study 1), and (b) single-dose ritonavir in 11 healthy individuals (study 2) in the absence (○) and presence (□) of mefloquine. Missing error bars are outside the lower range of the y-axis.
Figure 3.
Mean plasma concentrations of (a) the (+) RS (triangles;) and (–) SR (diamonds) enantiomers of mefloquine, and (b) racemic mefloquine (circles) and the carboxylic acid metabolite of mefloquine (squares) in 12 healthy subjects in the absence (solid lines) and presence (dotted lines) of ritonavir.
Steady-state coadministration of mefloquine resulted in significant increases of 45% in ritonavir CL/F (range, −17% to 141%). Correspondingly, there were significant decreases of −31% in steady-state AUC(0,12 h) (range, −59% to 20%), of −36% in Cmax (range, −73% to 19%), and of −43% in C0 (range, −78% to 38%), with no change in half-life values of ritonavir (range, −28% to 35%). Changes in AUC and Cmax were greater than 20% in seven of the 10 evaluable subjects. There were small and insignificant decreases of −6% in the fraction of ritonavir unbound to plasma proteins (fu: range, −63% to 175%) and of −12% in C12 (range, −48% to 97%) values, but the intraindividual variability was large (> 29%) in these parameters. Mean values of ritonavir fu were less than 0.5% in the absence or presence of mefloquine.
The anova indicated no gender effects in any pharmacokinetic parameter of ritonavir or mefloquine, and no differential effect of mefloquine on change in ritonavir pharmacokinetics in the five males compared with the five females. There was no correlation (P > 0.1) between change in ritonavir AUC (ratio) and either mefloquine or MMQ AUC in period 3 or change in ritonavir protein binding.
Study 2
Five men and six women completed the study. The mean±s.d. age and weight of the subjects were 29.0 ± 7.7 years (range, 19–41 years) and 75.2 ± 10.6 kg (range, 60.5–93.0 kg), respectively. Seven individuals experienced reversible mild to moderate neurological (n = 3) or gastrointestinal (n = 4) adverse events that were similar to those observed in study 1. One subject withdrew from the study during period 2 because she experienced chest pain, palpitations, dyspnoea, fatigue, insomnia, nausea, and diarrhea while taking mefloquine alone. For this person, only baseline and period 1 ERMBT data were evaluable, and comparison of ritonavir pharmacokinetics was not possible.
For single-dose ritonavir, mean or median plasma pharmacokinetic data are summarized in Table 2 and mean plasma concentration-time data are shown in Figure 2b. Mefloquine plasma exposures were similar to those in study 1 over the same 24 h period, but mefloquine did not alter single-dose ritonavir pharmacokinetics. Less than 8% changes in AUC and Cmax were observed with high variability (90%CIs: −26% to 45%). Ritonavir peak concentrations were significantly higher by 60% in women than in men over the two periods (2513 vs 1573 ng ml−1, P = 0.031). Changes in single-dose and multiple-dose AUC or Cmax parameters (point estimates) of ritonavir in the presence of mefloquine in the subjects who participated in both studies were not linearly correlated (P > 0.24, Pearson r2 < 0.33).
Table 2.
Study 2: Mean single-dose plasma pharmacokinetic parameters of ritonavir for the two treatments: ritonavir alone (period 1), and ritonavir with mefloquine (period 3).
| Treatment* | anova for longitudinal design | |||||
|---|---|---|---|---|---|---|
| Parameter | Ritonavir and mefloquine (Period 3) | Drug alone (Period 1) | Point estimate (90% CI)† % | P value | Pooled Intra-subject %CV | Pooled Inter-subject %CV |
| AUC(0,∞) (µg ml−1 h) | 14.0 ± 6.3 | 13.5 ± 7.1 | 108 (80.2–145) | 0.650 | 39.1 | 32.9 |
| Cmax (ng ml−1) | 2225 ± 900 | 2259 ± 1190 | 104 (73.8–145) | 0.851 | 45.3 | ne |
| t1/2,z (h)‡ | 4.4 ± 1.1 | 4.2 ± 1.6 | 107 (95.4–120) | 0.309 | 14.7 | 33.0 |
| CL/F (ml min−1) | 292 ± 143 | 333 ± 230 | 92.7 (68.9–125) | 0.650 | 39.1 | 32.9 |
| tmax (h) | 4.5 (3.0, 5.0) | 4.5 (1.0, 5.0) | 0 h | 0.05 | 19.0 | 11.6 |
CI, confidence interval; ne, not estimable.
Pharmacokinetic measurements were taken after a single 200 mg dose of ritonavir alone and with mefloquine after the 5th mefloquine dose (250 mg daily for 3 days then weekly for 2 weeks, 5 doses). Values of treatment means are expressed as arithmetic mean±s.d. (n = 11); tmax values are expressed as medians (with minimum and maximum given in parentheses)
Percentage ratio of the least-squares geometric treatment mean of the coadministered treatment in period 3 relative to that of the drug-alone treatment in period 1. The value for tmax is the point estimate of the absolute difference in medians, relative to the drug-alone treatment. The level of significance was set at 0.05.
Harmonic means are 4.1 h (with mefloquine) and 3.6 h (without mefloquine).
ERMBT The ERMBT results expressed as percentage dose metabolized per minute at 20 min and over the first hour are tabulated in Table 3. Mefloquine alone had no effect on the ERMBT with less than 20% variability in changes from baseline measurements as determined from 90% confidence intervals around mean ratios. A single 200 mg dose of ritonavir alone or in the presence of mefloquine caused more than a 95% decrease from basal activity, regardless of the parameter units. However, changes from baseline were overestimated by about 70% using values expressed in percentage dose metabolized over the first hour. There was no correlation between CYP3A4 activity (measured as the percentage of basal 14CO2 activity from baseline to period 1) and the concentration at 4.5 h postdose or AUC of single-dose ritonavir in period 1 (P > 0.3, Pearson r2 < 0.12). Basal activity was significantly higher by 55% in women than in men (2.76% vs 1.77% dose metabolized per hour, P = 0.0002).
Table 3.
Erythromycin breath test results in study 2 in the absence (baseline) and presence of three treatments: ritonavir alone (period 1), mefloquine alone (period 2), and ritonavir with mefloquine (period 3).
| Treatment | anova for longitudinal design | |||||
|---|---|---|---|---|---|---|
| Parameter | Baseline | Ritonavir alone (Period 1) | Mefloquine alone (Period 2) | Ritonavir and mefloquine (Period 3) | Pooled Intra-subject %CV | Pooled Inter-subject %CV |
| %M h−1 | 2.38 ± 0.68 | 0.094 ± 0.017 | 2.39 ± 0.70 | 0.10 ± 0.02 | 17.5 | 7.7 |
| Point estimate (90% CI)† | 4.2 (3.6–4.9)‡ | 101 (86.2–118) | 4.6 (3.9–5.4)‡ | |||
| %M min−1 | 0.046 ± 0.014 | 0.001 ± 0.0003 | 0.046 ± 0.014 | 0.001 ± 0.0003 | 23.3 | 10.1 |
| Point estimate (90% CI)† | 2.4 (1.9–2.9)‡ | 101 (81.5–124) | 2.7 (2.2–3.3)‡ | |||
%M h−1 and %M min−1, percentage of administered carbon-14 exhaled as 14 CO2 over the first hour and exhaled per minute as 14 CO2 at 20 min, respectively, after injection of 3 µCi of [14C-N-methyl]erythromycin; CI, confidence interval.
*Erythromycin breath test was administered in the absence of drugs on the day before the start of study 2 (baseline), at 4 h after a single dose of 200 mg ritonavir alone (period 1), at 13 h after the last (5th) 250 mg dose of mefloquine alone (period 2), and at 4 h after a single dose of 200 mg ritonavir in the presence of the last 250 mg dose of mefloquine (period 3). Values of treatment means are expressed as arithmetic mean±s.d. (n = 12, baseline and period 1; n = 11, periods 2 and 3).
Percentage ratio of the least-squares geometric treatment mean in period 3, 2 or 1 relative to that of the baseline treatment. Confidence intervals were calculated with use of Dunnett's critical t-value.
Significant by Dunnett's test at the 5% level (P < 0.020).
Discussion
The first study was designed to evaluate the potential interaction between ritonavir and mefloquine after multiple doses. The regimen of 250 mg mefloquine once weekly is the standard maintenance dose for prophylaxis of P. falciparum malaria. The low ritonavir dose (200 mg) was selected to minimize toxicity yet still provide strong inhibition of CYP metabolic pathways of mefloquine as it does for other drugs [5, 18]. The duration of treatment was expected to provide near steady-state conditions for both drugs. Ritonavir autoinduction is minimal at a low dose of 200 mg every 12 h and steady-state is achieved by 1 week with this regimen [19]. The long mefloquine half-life of about 18 days in Caucasians requires three daily loading doses of 250 mg to achieve 94% of steady-state by the third weekly dose [20, 21]. The minimal changes in mefloquine pharmacokinetics after the third weekly dose indicated that steady-state was achieved by this time. Coadministration of ritonavir and mefloquine over 7 days was estimated to be enough time to detect an effect of ritonavir on mefloquine pharmacokinetics. This was supported by the rapid and substantial inhibitory effect of ritonavir on the ERMBT in the presence of mefloquine. However, months of concurrent therapy would be necessary for the full extent of an interaction to be realized because of the long half-life of mefloquine. Any inhibitory effects of ritonavir are expected to alter systemic clearance and half-life of mefloquine because mefloquine has a high bioavailability with minimal first-pass metabolism [21].
Ritonavir was expected to increase mefloquine plasma levels because ketoconazole, another strong inhibitor of CYP3A4, inhibits the formation of MMQ in vitro [8]. However, ritonavir did not alter mefloquine pharmacokinetics, despite potent inhibition of CYP3A4 activity from a single 200 mg dose as demonstrated by the ERMBT. The lack of correlation between CYP3A4 activity and ritonavir plasma exposures suggests that even the reduced ritonavir exposures in the presence of mefloquine in our subjects would have provided strong inhibition of CYP3A4 activity and would have been adequate to increase mefloquine concentrations if CYP3A4 is a major elimination pathway. The lack of change in mefloquine oral clearance by ritonavir suggests that CYP3A4-mediated metabolism of mefloquine is not a major route of elimination in vivo. Alternatively, a compensatory effect of CYP3A4 inhibition and induction by ritonavir may explain the lack of change in mefloquine pharmacokinetics. Even though we observed small changes of less than 4% with low intraindividual variability in mefloquine and MMQ Cmax values, the inhibitory effects of the initial dose of ritonavir on mefloquine peak levels may be minor for highly bioavailable drugs like mefloquine. Short-term dosing of ritonavir (four doses of 200 mg) caused no change in peak levels but large increases in AUC and half-life values of alprazolam [22], whereas longer-term dosing of ritonavir (12 days titrated upwards to 500 mg twice daily) produced a small decrease in AUC consistent with net induction of metabolism [23]. The impact of the low dose ritonavir in our study on CYP3A4 induction was expected to be less than that at 500 mg [19].
Mefloquine increased multiple-dose clearance of ritonavir by an average of 45% with no change in half-life. However, not all subjects showed decreased ritonavir concentrations and single-dose pharmacokinetics were not altered with mefloquine. Study 2 was designed after the results of study 1 were available to help determine the mechanism of interaction by investigation of the effect of mefloquine and ritonavir on the ERMBT. Study 2 was not designed to formally compare results from single and multiple doses of ritonavir. Therefore, the discordance could arise from the differences in design of the two studies. Ritonavir was not evaluated during the same phase of mefloquine pharmacokinetics or after comparable duration of mefloquine exposures. The disparity could also have resulted from the unusually large decrease in baseline ritonavir clearance values from single to multiple dose, which may be partly related to the different breakfasts served in the two studies. Additionally, the high intraindividual pharmacokinetic variability of ritonavir in the absence and presence of mefloquine in the two studies, and the greater intraindividual variability of ritonavir after single dose complicate the interpretation of the results. The intraindividual CVs for AUC and Cmax were greater than 25% with values higher by 14% and 9%, respectively, after single dose than during multiple dosing. The increased variability in these parameters after single dose compared with multiple doses is often observed for drugs with nonlinear pharmacokinetics [24], and reduces the power of detecting changes in pharmacokinetic variables.
Various mechanisms such as decrease in plasma protein binding, decrease in absorption, or enzyme induction were considered to be potential causes of the lowering in total (unbound plus bound) ritonavir exposure. A reduction in protein binding from displacement interactions for low clearence drugs with a large volume of distribution (> 0.4 l kg−1) and high binding (> 98%), like ritonavir and mefloquine [3, 21], or a decrease in bioavailability will produce decreases in total AUC with no change in half-life [25]. The long elimination half-life of mefloquine also makes it an ideal candidate for protein displacement interactions. However, no changes in protein binding were detected.
The lack of change in ritonavir half-life and the lack of effect by mefloquine on the ERMBT support the non involvement of hepatic, and likely gut, CYP3A4 induction. A decrease in bioavailability appears responsible for the reduced levels. The solubility of ritonavir is greatly improved in the presence of ionic and nonionic surfactants [26], suggesting that the secretion of bile acids will increase the solubility and absorption of ritonavir in the small intestine. Because mefloquine decreases bile production [10], this mechanism may explain the reduced absorption of ritonavir. Reduced bile production has been proposed to explain the 30–40% decrease in plasma concentrations of lumefantrine in the presence of mefloquine [27]. Also, because ritonavir is a substrate of P-glycoprotein [4], induction of gut P-glycoprotein may contribute to the decreased drug absorption. However, the ability of mefloquine and MMQ to upregulate P-glycoprotein expression or change the protein to a more efficient conformation is unknown, although mefloquine has the molecular structural properties to induce P-glycoprotein [28]. Further study is required to determine if induction of P-glycoprotein or other efflux membrane proteins or reduction in bile production is a likely mechanism, or if the changes in ritonavir pharmacokinetics result from variability rather than a true drug interaction.
In summary, mefloquine and ritonavir were safely coadministered at the studied dosages for the duration of the study. Ritonavir did not influence mefloquine pharmacokinetics despite strong inhibition of CYP3A4 activity from a single 200 mg dose. Mefloquine had variable effects on ritonavir pharmacokinetics, reducing steady-state concentrations but not levels after single-dose administration.
Acknowledgments
We thank Isabelle Seguin, Donna Rehel-Giordano and Kathy Fyke for conducting the clinical trials and Dr David Kwok for performing the mass spectrometric measurements. Portions of this work were supported by a grant from the AIDS Program Committee of the Ontario Ministry of Health.
References
- 1.Centers for Disease Control and Prevention. Atlanta, GA: 1999. Health Information for International Travel.−2000, U.S. Department of Health and Human Services. [Google Scholar]
- 2.Basco LK, Gillotin C, Gimenez F, Farinotti R, Le Bras J. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br J Clin Pharmacol. 1992;33:517–520. doi: 10.1111/j.1365-2125.1992.tb04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu A, Granneman GR, Bertz RJ. Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Profit L, Eagling VA, Back DJ. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 5.Landmann R, Peytavin G, Leibowitch J, et al. Conference Record of the 12th World AIDS Conference. Geneva, Switzerland: 1998. Ritonavir (RTV) low dosages increases dramatically the saquinavir (SQV-HGC) bioavailability: A PK study in healthy volunteers (HV) p. 824. abstract no. 42257. [Google Scholar]
- 6.Bertz R, Wong C, Carothers L, et al. Evaluation of the pharmacokinetics of multiple dose ritonavir and ketoconazole in combination. Clin Pharmacol Ther. 1998;63:228. [Google Scholar]
- 7.Ayub M, Scott TA. Inhibition of human placental aromatase by mefloquine. J Steroid Biochem. 1988;29:149–151. doi: 10.1016/0022-4731(88)90389-5. [DOI] [PubMed] [Google Scholar]
- 8.Bangchang KN, Karbwang J, Back DJ. Mefloquine metabolism by human liver microsomes. Effect of other antimalarial drugs. Biochem Pharmacol. 1992;43:1957–1961. doi: 10.1016/0006-2952(92)90638-y. [DOI] [PubMed] [Google Scholar]
- 9.Riffkin CD, Chung R, Wall DM, et al. Modulation of the function of human MDR1 P-glycoprotein by the antimalarial drug mefloquine. Biochem Pharmacol. 1996;52:1545–1552. doi: 10.1016/s0006-2952(96)00556-4. [DOI] [PubMed] [Google Scholar]
- 10.Coleman MD, Fleckenstein L, Heiffer MH. Primaquine disposition in the isolated perfused rat liver: effect of mefloquine induced bile flow reduction. Biopharm Drug Dispos. 1989;10:153–164. doi: 10.1002/bdd.2510100205. [DOI] [PubMed] [Google Scholar]
- 11.Håkansson A, Landberg-Lindberg A, Björkman A. Comparison of the activity in vitro of mefloquine and two metabolites against Plasmodium falciparum. Trans Roy Soc Trop Med Hyg. 1990;84:503–504. doi: 10.1016/0035-9203(90)90014-6. [DOI] [PubMed] [Google Scholar]
- 12.Ouellet D, Hsu A, Qian J, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–116. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. J Clin Invest. 1989;83:688–697. doi: 10.1172/JCI113933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner D. CYP3A4 and the erythromycin breath test (letter) Clin Pharmacol Ther. 1998;64:129. doi: 10.1016/S0009-9236(98)90031-5. [DOI] [PubMed] [Google Scholar]
- 15.Bergqvist Y, Hellgren U, Churchill FC. High-performance liquid chromatographic assay for the simultaneous monitoring of mefloquine and its metabolite in biological samples using protein precipitation and ion-pair extraction. J Chromatogr. 1988;432:253–263. doi: 10.1016/s0378-4347(00)80650-7. [DOI] [PubMed] [Google Scholar]
- 16.Bergqvist Y, Doverskog M, Al Kabbani J. High-performance liquid chromatography determination of (SR) and (RS) enantiomers of mefloquine in plasma and capillary blood samples on paper after derivatization with (-) -1-(9-fluorenyl) ethyl chloroformate. J Chromatogr. 1994;652:73–81. doi: 10.1016/0378-4347(93)e0386-5. [DOI] [PubMed] [Google Scholar]
- 17.Khaliq Y, Gallicano K, Venance S, Kravcik S, Cameron DW. Effect of ketoconazole on ritonavir and saquinavir concentrations in plasma and cerebrospinal fluid from patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2000;68:637–646. doi: 10.1067/mcp.2000.112363. [DOI] [PubMed] [Google Scholar]
- 18.Ouellet D, Hsu A, Granneman GR, et al. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther. 1998;64:355–362. doi: 10.1016/S0009-9236(98)90065-0. [DOI] [PubMed] [Google Scholar]
- 19.Hsu A, Granneman GR, Witt G, et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellgren U, Berggren-Palme I, Bergqvist Y, Jerling M. Enantioselective pharmacokinetics of mefloquine during long-term intake of the prophylactic dose. Br J Clin Pharmacol. 1997;44:119–124. doi: 10.1046/j.1365-2125.1997.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer K, Holliday SM, Brogden RN. Mefloquine: a review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1993;45:430–475. doi: 10.2165/00003495-199345030-00009. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt D, von Moltke LL, Harmatz JS, et al. Alprazolam–ritonavir interaction: implications for product labeling. Clin Pharmcol Ther. 2000;67:335–341. doi: 10.1067/mcp.2000.105757. [DOI] [PubMed] [Google Scholar]
- 23.Frye R, Bertz R, Granneman GR, et al. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Toronto, Canada: 1997. Effect of ritonavir on the pharmacokinetics and pharmacodynamics of alprazolam. abstract no. A-59. [Google Scholar]
- 24.Blume HH, Elze M, Potthast H, Schug BS. Practical strategies and design advantages in highly variable drug studies: multiple dose and replicate administration design. In: Blume HH, Midha KK, editors. Bio-International 2 Bioavailability, Bioequivalence and Pharmacokinetic Studies. Stuttgart, Germany: Medpharm Scientific Publishers; 1995. pp. 117–122. [Google Scholar]
- 25.MacKichan JJ. Protein binding drug displacement interactions fact or fiction? Clin Pharmacokinet. 1989;16:65–73. doi: 10.2165/00003088-198916020-00001. [DOI] [PubMed] [Google Scholar]
- 26.Abbott Laboratories, AbbottPark IL personal communication
- 27.Lefèvre G, Bindschedler M, Ezzet F, Schaeffer N, Meyer I, Thomsen MS. Pharmacokinetic interaction trial between co-artemether and mefloquine. Eur J Pharm Sci. 2000;10:141–151. doi: 10.1016/s0928-0987(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 28.Österberg T, Norinder U. Theoretical calculation and prediction of P-glycoprotein-interacting drugs using MolSurf parametrization and PLS statistics. Eur J Pharm Sci. 2000;10:295–303. doi: 10.1016/s0928-0987(00)00077-4. [DOI] [PubMed] [Google Scholar]