Abstract
Aims
We attempted to explore the possible differential involvement of β-adrenoceptor subtypes in the dilator response of the human dorsal hand vein to isoprenaline by examining the ability of bisoprolol, a selective β1-adrenoceptor antagonist, and nadolol, a nonselective β1/β2-adrenoceptor antagonist, to antagonize the response.
Methods
Twelve healthy male volunteers participated in four weekly sessions. In the preliminary session a dose-response curve to the vasoconstrictor effect of phenylephrine was constructed and the dose producing 50–75% maximal response was determined for each individual. In each of the remaining three (treatment) sessions, nadolol (40 mg), bisoprolol (5 mg) or placebo was ingested, and isoprenaline hydrochloride (3.33–1000 ng min−1) was infused locally into the dorsal hand vein along with a constant dose of phenylephrine hydrochloride (to preconstrict the vein) 2 h after the ingestion of the drugs. Changes in vein diameter were monitored with the dorsal hand vein compliance technique. Subjects were allocated to treatment session according to a double-blind balanced cross-over design. Systolic and diastolic blood pressure, and heart rate were also measured.
Results
Isoprenaline produced dose-dependent venodilatation which was antagonized by nadolol but remained unaffected by bisoprolol (anova with repeated measures: P < 0.025; Dunnett's test: placebo vs nadolol, P < 0.01; placebo vs bisoprolol, P = NS). Mean log ED50 (ng min−1) was significantly increased in the presence of nadolol and remained unchanged in the presence of bisoprolol (anova, P < 0.025; Dunnett's test: placebo vs nadolol, P < 0.005; placebo vs bisoprolol, P = NS; differences between mean log ED50 [95% CI]: placebo vs bisoprolol −0.11 [-0.38, 0.16], placebo vs nadolol 0.32[0.09, 0.72], bisoprolol vs nadolol −0.43 [-0.71, −0.15]). Mean Emax did not differ in the three treatment conditions.
Conclusions
The failure of bisoprolol to attenuate isoprenaline-evoked venodilatation in the human dorsal hand vein argues against the involvement of a β1-adrenoceptor-mediated component in the isoprenaline-evoked venodilatory responses. The possibility cannot be excluded that the consequences of β1-adrenoceptor blockade by bisoprolol might have been obscured by a possible venodilator effect of bisoprolol.
Keywords: β-adrenoceptors, bisoprolol, dorsal hand vein, nadolol, noradrenaline
Introduction
The human dorsal hand vein contains both β- and α-adrenoceptors [1–3] and there is evidence that both α1- and α2-adrenoceptors mediate constrictor responses [3, 4] and β2-adrenoceptors mediate dilator responses [3, 5]. It is more controversial, however, whether there are also venodilator β1-adrenoceptors in the dorsal hand vein. Attempts to answer this question have been impeded by the lack of availability of selective β1-adrenoceptor agonists suitable for administration to human subjects, and researchers have been forced to use a more indirect approach involving selective β1-adrenoceptor antagonists and nonselective adrenoceptor agonists. Two approaches have been adopted: (a) investigation of the effect of selective β1-adrenoceptor antagonists on venodilator responses to the nonselective β1-/β2-adrenoceptor agonist isoprenaline, and (b) investigation of the effect of selective β1-adrenoceptor antagonists on venoconstrictor responses to the nonselective α/β-adrenoceptor agonist noradrenaline.
In early studies, practolol, a β1-adrenoceptor antagonist with some intrinsic sympathomimetic (partial agonist) activity, was used. It was reported that practolol could both antagonize isoprenaline-evoked venodilatation and potentiate noradrenaline-evoked venoconstriction [1], suggesting the involvement of venodilator β1-adrenoceptors in the responses to both isoprenaline and noradrenaline. More recently, we have reinvestigated the question of the probable involvement of masked β1-adrenoceptors in the venoconstrictor response to noradrenaline by using bisoprolol, a highly selective β1-adrenoceptor antagonist lacking in intrinsic sympathomimetic activity [6–8]. We found that bisoprolol failed to potentiate the constrictor response to noradrenaline, and that it antagonized the constrictor responses both to noradrenaline and the selective α1-adrenoceptor agonist phenylephrine [9, 10]. These results suggest that bisoprolol, similarly to another selective β1-adrenoceptor antagonist, nebivolol, may dilate the vein. The venodilatation evoked by nebivolol is mediated via the release of nitric oxide [11, 12].
In the present experiment, we extended our previous work with bisoprolol by examining its effect on isoprenaline-evoked venodilatation. Nadolol, a nonselective β1/β2-adrenoceptor antagonist, was used as a control. Some of these results have been communicated to the British Pharmacological Society [13].
Methods
Ethical considerations
The study protocol was approved by the University of Nottingham Medical School Ethics Committee. All volunteers gave their written informed consent following a verbal explanation of the study and after reading a detailed information sheet.
Subjects
Twelve healthy male volunteers aged 18–30 years (mean ± s.e mean, 21.3 ± 2.7) and weighing 58–86 kg (mean ± s.e mean, 74.8 ± 3.2) participated. Each subject completed a brief medical history and underwent a complete physical examination. Subjects had not participated in drug studies within 3 months of the start of the study, and had not used any drug within the 14 days preceding the study. They were requested to stop smoking and to avoid drinking alcohol, coffee and other caffeine-containing beverages for at least 12 h before each experimental session. All subjects were advised to have a light breakfast 2 h before the experimental sessions.
Drugs
Isoprenaline hydrochloride (Saventrine IVR) was obtained from Pharmax Ltd, Kent, UK, phenylephrine hydrochloride (Phenylephrine InjectionR) from Boots Pharmaceuticals, Nottingham, UK, bisoprolol fumarate (Monocor*5R) from Lic. E. Merck, Darmstadt, Germany, nadolol (CorgardR) from Squibb. The sterile solutions of isoprenaline hydrochloride and phenylephrine hydrochloride were infused locally into the vein at a constant rate of 0.3 ml min−1 and over the following dose range: isoprenaline hydrochloride 3.33–1000 ng min−1, phenylephrine hydrochloride 0.033–10 µg min−1. Bisoprolol 5 mg, nadolol 40 mg, and lactose placebo were prepared in identical capsules for double-blind administration.
Tests
The dorsal hand vein compliance technique
The dorsal hand vein compliance technique, as modified by Aellig [14], was used as described previously [15]. Each period of drug-infusion consisted of an initial 3 min with the cuff deflated, followed by a further 2–4 min with the cuff inflated (i.e. a sufficient period of time to ensure that the signal from the Linear Variable Differential Transformer (LVDT) had reached plateau). The baseline venodilatation during saline infusion with the cuff inflated was defined as 100% relaxation, the recording with the cuff not inflated was defined as 100% constriction. As the vein has no resting tone, it was necessary to preconstrict it with phenylephrine prior to the infusion of isoprenaline in order to be able to record dilator responses [16]. Venodilator responses to isoprenaline following the preconstriction with phenylephrine were defined as a percentage of the baseline obtained during initial saline infusion by the formula [(Z−Y)/(X−Y)] × 100, where Z is vein diameter during the coinfusion of each dose of isoprenaline and a fixed (‘preconstricting”) dose of phenylephrine, X is vein diameter (‘baseline’) during infusion of saline and Y is vein diameter (preconstricted) during infusion of phenylephrine. Drug solutions were infused at a constant rate (0.3 ml min−1).
Cardiovascular measures
Systolic and diastolic blood pressure were measured using a mercury sphygmomanometer, and heart rate by feeling the pulse of the radial artery at the wrist for 1 min. All measurements were taken on the arm opposite to the one used for the pharmacological testing.
Experimental design
Subjects were allocated to systemic treatments and sessions according to a double-blind balanced design. Each volunteer participated in four experimental sessions at weekly intervals. In the first (preliminary) session a dose–response curve to phenylephrine was constructed and the dose producing 50–75% maximal response was determined for each individual subject; this dose was used in subsequent experimental sessions in order to ‘set the baseline’ for measuring the responses to isoprenaline. Each of the remaining three sessions was associated with one of the following treatments: bisoprolol (5 mg), nadolol (40 mg), or placebo. After a 30 min acclimatization period, the first cardiovascular testing (blood pressure and heart rate; measurement I) was carried out. Then the subject ingested the drug capsule. 2 h later the second cardiovascular testing (II) was carried out, followed by a 30 min period of saline infusion during which baseline venous diameter was recorded. This was followed by the third cardiovascular testing (III). Then the ‘baseline-setting’ dose of phenylephrine was infused for 5 min, followed by the coinfusion of phenylephrine+isoprenaline (6 doses) for 30 min. Finally, the fourth cardiovascular testing (IV) was carried out. The timings of start of local infusion and post-treatment tests were based on the single-dose pharmacokinetics of bisoprolol and nadolol; it has been reported that peak plasma concentration is attained 2–4 h after oral administration of a single dose of bisoprolol [17, 18], and nadolol [19, 20, 21].
Data analysis
Dorsal hand vein responses
The raw data were analysed with two-way analysis of variance (anova, dose of agonist; systemic drug treatment) with repeated measures on both factors. When a significant overall main effect of drug treatment was identified, individual comparisons were made between placebo vs bisoprolol and placebo vs nadolol with Dunnett's test. The individual dose–response curves obtained in each subject were also analysed by fitting a rectangular hyperbolic function to the data using a computer program based on Wilkinson's method [22]. This analysis yields estimates of the maximal response (Emax) and the dose producing the half-maximal response (ED50). The analysis also provides the index of determination (p2) for each curve; p2 expresses the proportion of the data variance accounted for by the fitted function. The distribution of the ED50 values was normalized by logarithmic transformation, and the geometric mean was calculated for each of the three dose–response curves. Dunnett's test was used to compare the effects of bisoprolol vs placebo, and nadolol vs placebo on Emax and log ED50. The degree of antagonism of the responses by bisoprolol and nadolol was expressed in two ways: by calculating the percentage change in geometric mean ED50 in the presence of bisoprolol and nadolol, and by calculating the dose-ratio [15]. 95% confidence intervals were calculated for the differences between the dose–response curve parameters seen under the three treatment conditions.
Cardiovascular measures
The effects of the three systemic treatments on the cardiovascular measures were calculated both in the absence (difference between measurements II and I) and in the presence (difference between measurements IV and III) of the infusion of isoprenaline (Table 4). One-way anova (repeated measures) was used to detect any significant effect of systemic drug treatment; in the case of significant treatment effects, Dunnett's test was used to compare the effects of the active treatments with those of placebo. 95% confidence intervals were calculated for the differences between the cardiovascular measures seen under the three treatment conditions, both in the presence and in the absence of isoprenaline.
Table 4.
Cardiovascular parameters prior to (A) and following (B) the infusion of isoprenaline.
| A Change from pretreatment baseline (mean ± s.e. mean) | B Change from preinfusion value (mean ± s.e. mean) | |
|---|---|---|
| Heart rate (beats min−1)1 | ||
| Placebo | −5.00 ± 1.72 | 14.33 ± 2.70 |
| Bisoprolol 5 mg | −15.33 ± 2.91** | 4.83 ± 1.85* |
| Nadolol 40 mg | −12.50 ± 1.80* | 1.08 ± 1.24* |
| Systolic BP (mmHg)2 | ||
| Placebo | 0.42 ± 2.34 | 14.42 ± 3.69 |
| Bisoprolol 5 mg | −14.17 ± 2.94** | 1.25 ± 1.52* |
| Nadolol 40 mg | −9.17 ± 1.35* | 0.42 ± 2.08* |
| Diastolic BP (mmHg)3 | ||
| Placebo | 2.33 ± 1.92 | −8.92 ± 2.41 |
| Bisoprolol 5 mg | −2.92 ± 1.41 | −3.17 ± 0.88* |
| Nadolol 40 mg | −1.67 ± 1.68 | −1.00 ± 1.04* |
significance of treatment effect from the anova: 1: P < 0.0001; 2: P < 0.0001; 3: P < 0.0025.
Asterisks: significance of Dunnett's test (difference from placebo condition):
P < 0.01
P < 0.005.
A probability level of P < 0.05 was considered as being of significance for all statistical tests.
Results
Dorsal hand vein responses
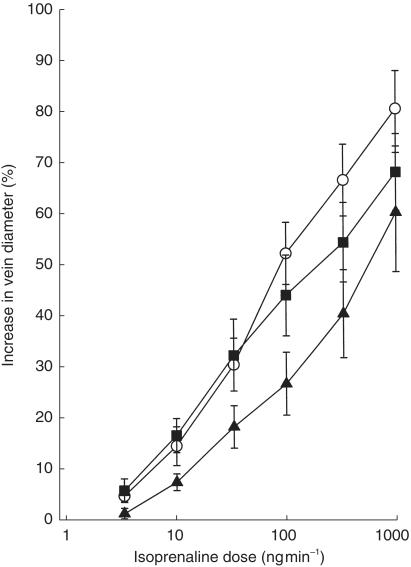
Venous diameter, recorded following the administration of systemic treatments, both prior to and following the local infusion of saline/drug solutions, is shown in Table 1. The effects of the systemic drug treatments on the dose–response curves to isoprenaline are shown in Figure 1. Isoprenaline evoked dose-dependent venodilator responses, which were antagonized by nadolol and remained unaffected by bisoprolol. anova showed significant main effects of both dose of isoprenaline (P < 0.0001) and of the systemic drug treatments (P < 0.025), and a significant interaction (P < 0.05). Dunnett's test revealed a significant effect of nadolol, but not of bisoprolol: placebo vs bisoprolol (P = NS); placebo vs nadolol (P < 0.01). In the case of the individually fitted curves (n = 12) the value of p2 ranged from 0.92 to 0.99 (median 0.97) in the presence of placebo, from 0.72 to 0.99 (median 0.91) in the presence of bisoprolol, and from 0.88 to 0.99 (median 0.95) in the presence of nadolol. The estimated parameters of the dose–response curves (n = 12) are shown in Table 2. Mean log ED50 was significantly increased in the presence of nadolol and remained unchanged in the presence of bisoprolol (anova, P < 0.025; Dunnett's test: placebo vs nadolol: P < 0.005; placebo vs bisoprolol, P = NS).The effects of the two active treatments on the log ED50, compared using Bonferroni-corrected t-test, differed significantly from one another (P < 0.05). Mean Emax did not differ in the three treatment conditions (anova, P = NS). The geometric mean ED50 was increased by approximately 207% in the presence of nadolol, and decreased by approximately 22% in the presence of bisoprolol, and the dose ratios were: placebo vs nadolol 2.08, and placebo vs bisoprolol 0.78. Table 3 shows the differences (mean, 95% CI, n = 12) between the effect of placebo and active drug treatments, and between the two active drug treatments, on the parameters of the dose–response curves.
Table 1.
Venous diameter (mm, mean ± s.e. mean) recorded following the administration of systemic treatments and during the infusion of saline (‘baseline’), phenylephrine hydrochloride solution (‘preconstriction’), and phenylephrine hydrochloride + six different doses of isoprenaline hydrochloride (1–6). See text for details.
| Phenylephrine+isoprenaline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Phenylephrine | 1 | 2 | 3 | 4 | 5 | 6 | |
| Placebo | 0.73 ± 0.11 | 0.19 ± 0.05 | 0.22 ± 0.05 | 0.26 ± 0.05 | 0.34 ± 0.06 | 0.44 ± 0.07 | 0.52 ± 0.08 | 0.59 ± 0.09 |
| Bisoprolol 5 mg | 0.76 ± 0.07 | 0.24 ± 0.05 | 0.28 ± 0.06 | 0.32 ± 0.05 | 0.36 ± 0.05 | 0.43 ± 0.07 | 0.52 ± 0.07 | 0.62 ± 0.07 |
| Nadolol 40 mg | 0.78 ± 0.11 | 0.14 ± 0.03 | 0.14 ± 0.03 | 0.17 ± 0.04 | 0.23 ± 0.04 | 0.31 ± 0.07 | 0.38 ± 0.07 | 0.47 ± 0.09 |
Figure 1.
Dose–response curves for the venodilator effect of isoprenaline during local infusion into the superficial dorsal hand vein (cuff pressure 45 mmHg) 2 h after ingestion of placebo (○), bisoprolol 5 mg (▪), and nadolol 40 mg (▴); mean ± s.e. mean, n = 12. 100% response was defined as the recording during saline infusion with the cuff inflated.
Table 2.
Parameters of the dose-response curves.
| Log ED50 (mean ± s.e. mean) | ED50 (geometric mean) | Emax (%) (mean±s.e. mean) | |
|---|---|---|---|
| Placebo | 1.79 ± 0.10 | 60.98 | 84.13 ± 8.09 |
| Bisoprolol 5 mg | 1.68 ± 0.11 | 47.45 | 68.07 ± 7.91 |
| Nadolol 40 mg | 2.1 ± 0.12* | 126.62 | 68.09 ± 12.81 |
ED50: ng min−1
P < 0.005 (difference from placebo condition).
Table 3.
Parameters of dose–response curves: differences (mean, 95% CI) between placebo (Pl) and bisoprolol 5 mg (BIS5), placebo and nadolol 40 mg (NAD40), and bisoprolol 5 mg and nadolol 40 mg.
| Log ED50 (ng min−1) | Emax (%) | |
|---|---|---|
| Pl/BIS5 | −0.11 (−0.38, 0.16) | −16.06 (−39.11, 6.99) |
| Pl/NAD40 | 0.32 (0.09, 0.72) | −16.05 (−37.70, 5.62) |
| BIS5/NAD40 | −0.43 (−0.71, −0.15) | −0.02 (−27.36, 27.32) |
Cardiovascular measures
The effects of bisoprolol, nadolol, and placebo on cardiovascular measures are summarized in Table 4. Both bisoprolol and nadolol decreased heart rate (anova, P < 0.0025; Dunnett's test: placebo vs bisoprolol P < 0.005; placebo vs nadolol, P < 0.01), and systolic blood pressure (anova, P < 0.0025; Dunnett's test: placebo vs bisoprolol, P < 0.005; placebo vs nadolol, P < 0.01). There was no significant effect of bisoprolol or nadolol on diastolic blood pressure (anova, P = NS). There was no significant difference between the effects of nadolol and bisoprolol on any of the cardiovascular measures. The mean differences (95% CI) between the effect of placebo and each active drug treatment and between the two active treatments on the cardiovascular measures are shown in Table 5.
Table 5.
Effect of systemic drug treatments in the absence and presence of isoprenaline on the cardiovascular measures: heart rate (HR: beats min−1), systolic blood pressure (SBP: mmHg), and diastolic blood pressure (DBP: mmHg), difference (mean, 95% CI, n = 12) between placebo (Pl) and bisoprolol 5 mg (BIS5), placebo and nadolol 40 mg (NAD40), and bisoprolol 5 mg and nadolol 40 mg.
| HR (beats min−1) | SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|
| In the absence of isoprenaline | |||
| Pl vs BIS5 | −10.3 (−16.4, −4.2) | −14.6 (−23.4, −5.8) | −5.3 (−8.8, −1.7) |
| Pl vs NAD40 | −7.5 (−11.5, −3.5) | −9.58 (−16.42, −2.75) | −4.0 (−9.0, 1.0) |
| BIS5 vs NAD40 | 2.8 (−4.0, 9.6) | 5.0 (−1.1, 11.1) | 1.3 (−2.7, 5.2) |
| In the presence of isoprenaline | |||
| Pl vs BIS5 | −9.5 (−13.73, −5.27) | −13.2 (−21.2, −5.1) | 5.8 (1.1, 10.4) |
| Pl vs NAD40 | −13.3 (−18.5, −8.0) | −14.0 (−21.8, −6.2) | 7.9 (2.3, 13.6) |
| BIS5 vs NAD40 | −3.8 (−8.1, 0.6) | −0.8 (−6.9, 5.2) | 2.2 (−0.1, 4.4) |
The effects of isoprenaline on cardiovascular measures in the presence of placebo, bisoprolol, and nadolol are summarized in Table 4. In the presence of placebo, isoprenaline increased heart rate and systolic blood pressure, and reduced diastolic blood pressure. These effects were significantly attenuated by both bisoprolol and nadolol. Table 5 shows the differences (mean 95% CI, n = 12) between placebo and active drug treatments on the cardiovascular measures.
Discussion
The doses of the two β-adrenoceptor antagonists used in the study were selected on the basis of published reports on the effectiveness of these drugs in man. Bisoprolol 5 mg and nadolol 40 mg have been shown to produce a similar degree of antagonism (16.8% and 22.9%, respectively) of exercise-induced tachycardia, a β1-adrenoceptor-mediated response [23], and bisoprolol 5 mg does not block β2-adrenoceptors in man [24].
An oral dose (40 mg) of nadolol antagonized the venodilator responses to isoprenaline leading to a rightward shift in the dose–response curves of isoprenaline, whereas a single oral dose (5 mg) of bisoprolol was without a significant effect. Both nadolol and bisoprolol reduced heart rate and blood pressure, in agreement with previous reports [25–27] and consistent with the well-documented effects of these drugs on the cardiovascular system, i.e slowing of the heart and decrease in myocardial contractility [28].
It was predicted that bisoprolol, a highly selective β1-adrenoceptor antagonist [6, 7], would either antagonize or not affect the dilator responses to isoprenaline, depending on the contribution of venodilator β1-adrenoceptors to the response. Bisoprolol (5 mg) had no effect on isoprenaline-evoked venodilatation, consistent with the hypothesis that β1-adrenoceptors do not contribute significantly to isoprenaline-evoked venodilatation in the dorsal hand vein. It should be noted, however, that bisoprolol may have additional pharmacological properties, which may have masked the antagonism of the responses to isoprenaline. Indeed, in a separate study, we observed that bisoprolol could antagonize both noradrenaline-and phenylephrine-evoked venoconstriction in a dose dependent manner [9]. As there is evidence that the venoconstrictor responses to noradrenaline and phenylephrine are mediated by α1- and α2-adrenoceptors [3], and bisoprolol has no affinity for these receptors [6, 7], the antagonism of the constrictor responses by bisoprolol is likely to reflect functional rather than competitive antagonism due to a possible venodilator effect of the drug [9]. Indeed, there is some indirect evidence that bisoprolol may have exerted such an effect in the present study. There was a tendency for bisoprolol to reduce isoprenaline-induced venodilatation at the higher concentrations of isoprenaline. This observation may reflect an artefactual reduction in the sizes of the responses, due to a ‘floor effect’ [29], manifesting when the sizes of the responses were enhanced by the added effect of bisoprolol-induced venodilatation. A possible venodilator effect of bisoprolol is not excluded by the lack of effect of the drug on the diameter of the untreated vein, since the dorsal hand vein has no tone at rest [16, 30], and hence it has to be preconstricted to reveal the dilator effect of any drug applied locally or systemically [11, 16, 30].
In conclusion, a single oral dose (40 mg) of nadolol attenuated isoprenaline-evoked venodilatation in the dorsal hand vein consistent with its β-adrenoceptor antagonistic properties, while the highly selective β1-adrenoceptor antagonist bisoprolol, a drug recommended for the characterization of β1-adrenoceptors [6], failed to do so. Although the lack of effect of bisoprolol on the responses to isoprenaline would be consistent with the absence of a significant β1-adrenoceptor-mediated component in the isoprenaline-evoked venodilatation, the possibility cannot be excluded that the consequences of β1-adrenoceptor blockade by bisoprolol might have been obscured by some other effect of bisoprolol (e.g. venodilatation).
References
- 1.White CB, Udwadia BP. β-Adrenoceptors in the human dorsal hand vein, and the effects of propranolol and practolol on venous sensitivity to noradrenaline. Br J Clin Pharmacol. 1975;2:99–105. doi: 10.1111/j.1365-2125.1975.tb01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan HYM, Hoffman BB, Pershe RA, Blaschke TF. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986;239:802–807. [PubMed] [Google Scholar]
- 3.Blochl-Daum B, Korn A, Wolzt M, Schmidt E, Eichler H-G. In vivo studies on alpha-adrenergic subtypes in human veins. Naunyn-Schmiedeberg's Arch Pharmacol. 1991;344:302–307. doi: 10.1007/BF00183004. [DOI] [PubMed] [Google Scholar]
- 4.Schulte KL, Laber E, Braun J, Meyer-Sabellek W, Distler A, Gotzen R. Nifedipine vasodilates human forearm arteries and dorsal hand veins constricted by specific α-adrenoceptor stimulation. Gen Pharmac. 1987;18:525–529. doi: 10.1016/0306-3623(87)90074-7. [DOI] [PubMed] [Google Scholar]
- 5.Brodde O-E, Zerkowski H-R, Doetch N, Khamssi M. Subtype selective up-regulation of human saphenous vein β-adrenoceptors by chronic β-adrenoceptor antagonist treatment. Naunyn-Schmiedeberg’s Arch Pharmacol. 1989;339:479–482. doi: 10.1007/BF00736065. [DOI] [PubMed] [Google Scholar]
- 6.Brodde O-E. Bisoprolol (EMD 33512), a highly selective β1-adrenoceptor antagonist: in vitro and in vivo studies. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S29–S35. doi: 10.1097/00005344-198511001-00005. [DOI] [PubMed] [Google Scholar]
- 7.Manalan AS, Besch Hr, Jr, Watanabe AM. Characterization of (±) [3H]-carazolol binding to β-adrenoceptors. Application to study of β-adrenergic receptor subtypes in canine ventricular myocardium and lung. Circ Res. 1981;49:326–336. doi: 10.1161/01.res.49.2.326. [DOI] [PubMed] [Google Scholar]
- 8.Wellstein A, Belz GG, Palm D. Beta adrenoceptor subtype binding activity in plasma and beta blockade by propranolol and beta-1 selective bisoprolol in humans. Evaluation with Schild-plots. J Pharmacol Exp Ther. 1988;246:328–337. [PubMed] [Google Scholar]
- 9.Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Bisoprolol attenuates noradrenaline- and phenylephrine-evoked venoconstriction in man in vivo. Br J Clin Pharmacol. 1997;44:61–68. doi: 10.1046/j.1365-2125.1997.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of nadolol and bisoprolol on noradrenaline-evoked venoconstriction in man in vivo. Br J Clin Pharmacol. 1998;45:271–276. doi: 10.1046/j.1365-2125.1998.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman AJ, Chen CPL-H, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol. 1994;39:199–204. doi: 10.1111/j.1365-2125.1994.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an l-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995;274:1067–1071. [PubMed] [Google Scholar]
- 13.Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Are β1-adrenoceptors involved in the isoprenaline-evoked dilatation of the human dorsal hand vein? Br J Pharmacol. 1999;128:257P. [Google Scholar]
- 14.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of desipramine on noradrenaline- and methoxamine-evoked venoconstriction in man in vivo. Br J Clin Pharmacol. 1995;40:445–451. [PMC free article] [PubMed] [Google Scholar]
- 16.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins-1. Br J Clin Pharmacol. 1994;38:181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leopold G. Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S16–S20. doi: 10.1097/00005344-198511001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Le Coz F, Sauleman P, Poirier JM, et al. Oral pharmacokinetics of bisoprolol in resting and exercising healthy volunteers. J Cardiovasc Pharmacol. 1991;18:28–34. doi: 10.1097/00005344-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Heel RC, Brogden RN, Pakes GE, Speight JM, Avery GS. Nadolol: a review of its pharmacological properties and therapeutic efficacy in hypertension and angina pectoris. Drugs. 1980;20:1–23. doi: 10.2165/00003495-198020010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Dreyfuss J, Griffith DL, Singhui SM, et al. Pharmacokinetics of nadolol, a beta-receptor antagonist: administration of therapeutic single- and multiple- dosage regimens to hypertensive patients. J Clin Pharmacol. 1979;19:712–720. doi: 10.1002/j.1552-4604.1979.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 21.Frishman WH. β-Adrenoceptor antagonists: New drugs and new indications. N Engl J Med. 1981;305:500–506. doi: 10.1056/NEJM198108273050907. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson GN. Statistical estimations in enzyme kinetics. Biochem J. 1961;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipworth BJ, Irvine NA, McDevitt DG. A dose ranging study to evaluate the β1-adrenoceptor selectivity of bisoprolol. Eur J Clin Pharmacol. 1990;40:135–139. doi: 10.1007/BF00280067. [DOI] [PubMed] [Google Scholar]
- 24.Dorrow P. β1/β2-Splitting of bisoprolol. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S65–S68. [PubMed] [Google Scholar]
- 25.Lammers JWJ. Folgering, HThM, van Herwaarden, CLA. Respiratory tolerance of bisoprolol and metoprolol in asthmatic patients. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S69–S73. doi: 10.1097/00005344-198511001-00012. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee SS. The cardioselective and hypotensive effects of bisoprolol in hypertensive asthmatics. J Cardiovasc Pharmacol. 1986;8(Suppl. 1):S74–S77. doi: 10.1097/00005344-198511001-00013. [DOI] [PubMed] [Google Scholar]
- 27.Burkart F, Pfisterer M, Steinmann E. Effects of bisoprolol in relation to metoprolol and bufuralol on left ventricular hemodynamics at rest and during exercise in chronic ischemic heart disease. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S78–S82. doi: 10.1097/00005344-198511001-00014. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman BB, Lefkowitz RJ. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: Hardman Joel G, Limbird Lee E., editors. The Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. pp. 199–248. Chapter 10. [Google Scholar]
- 29.Szabadi E. The influence of the baseline on the size of pharmacological responses: a theoretical model. Br J Pharmacol. 1977;61:492–493P. [PMC free article] [PubMed] [Google Scholar]
- 30.Webb DJ. The pharmacology of human blood vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]