Abstract
Aims
To document the population pharmacokinetics of carbamazepine in patients with epilepsy living in Singapore, the majority of whom are of Chinese origin and others of minority races.
Methods
Steady-state plasma carbamazepine concentration data were gathered during routine care from various hospitals in Singapore for patients with epilepsy. Age, body weight, gender, race, formulation and concurrent medication (for other illnesses) were the fixed effects (covariates) tested simultaneously for their influence on the population mean of carbamazepine clearance, using the nonlinear mixed-effects model, in the NONMEM program.
Results
No age, gender, race, or formulation–related effect was found. Body weight (W), age (A) and concurrent medication with phenobarbitone (PB) emerged as the determinants of carbamazepine clearance (CL). The final regression model for carbamazepine clearance found best to describe the data was CL = 40.7 × A0.494 × W−1.17 × 1.44PB where CL is in l day−1 kg−1, A is in years, W is in kg and PB = 0 for a patient on carbamazepine only and PB = 1 for a patient on concomitant PB. The corresponding interindividual variability (CV%) in CL, described by using an exponential model, was 21.4%, and the residual error, described by using an exponential error model, was 18.2%. Predictive performance of this population covariate model was evaluated by Bayesian forecasting in a similar, but independent cohort of patients. There was no statistically significant bias between predicted and measured plasma carbamazepine concentrations. The population mean value of carbamazepine clearance obtained was similar to that previously reported for patients with a very different ethnic (Caucasians and Blacks) or geographical background (South Africa, Europe and USA).
Conclusions
The derived covariate regression model reasonably predicted concentrations in the separate validation Singapore patient data set. The correlation between carbamazepine clearance and patient-specific characteristics may thus allow dosage adjustment to be made to achieve target steady-state plasma concentrations.
Keywords: carbamazepine, children and adults, NONMEM, pharmacogenetics, population pharmacokinetics, Singapore epileptic patients
Introduction
Carbamazepine (CBZ) is an anticonvulsant widely used for the treatment of partial seizures and generalized tonic-clonic seizures [1]. However, it is difficult to establish suitable dosage regimens for this drug because of the lack of a good relationship between the dose and the desired effect, its narrow therapeutic range, and the variation in its pharmacokinetic characteristics [2–4]. The influence of genetic difference, age, sex, race, variable absorption rates, autoinduction, disease-state, and comedication may cause significant changes in blood levels of CBZ and its metabolites [5–8]. Although a therapeutic range for CBZ is generally accepted to be between 4 and 12 mg l−1, certain patients respond to concentrations either below or above these values. Dose/concentration related toxicity may be manifested by dizziness, diplopia, nausea, headache and light-headedness [9]. Determining patient-specific therapeutic concentrations necessitates individualized dosing regimens.
Studies of the pharmacokinetics, pharmacodynamics, efficacy, and toxicity of drugs have traditionally been conducted in primarily Caucasian populations. There are several pharmacokinetic studies and reviews on CBZ in adults and children [2, 4–12]. Ethnic background is usually not considered in decisions regarding drug dosages. However, a growing body of evidence indicates that ethnic differences may affect pharmacokinetics and hence dosage requirements [13, 14]. Differences between Chinese and Caucasian groups are especially well documented for drugs such as propranolol [15], morphine [16], nifedipine [17], diazepam [18], phenytoin [19] and haloperidol [20]. It is thus more appropriate for individualizing dosage of such a drug in patients based on pharmacokinetics derived from the same ethnic patient population. However, very little published information exists concerning CBZ pharmacokinetics (particularly population clearance values) in non-Caucasian patients, for example, in Asians. Such information is useful for optimizing daily dosages of CBZ in these patients. Their welfare and quality of life may be improved by better seizure control or reduction in toxicity.
The present study was thus undertaken to determine the population clearance values of CBZ in epileptic patients living in Singapore (mostly Chinese), using data gathered during routine care from various hospitals in Singapore. The possible influences of certain physiological factors (age, weight and sex) and concurrent medication on CBZ clearance were also examined.
Methods
Patients
Data from 193 epileptic patients with a total of 302 measured plasma CBZ concentrations were collected retrospectively from different hospitals in Singapore. CBZ was prescribed two to four times a day. The daily dose of CBZ was administered orally in the form of conventional tablets or syrup (Tegretol®). Only those blood samples that were collected at least 2 weeks (CBZ concentrations assumed being at steady state) after each dosage adjustment were used for data analysis. Most of the blood samples were drawn shortly before administration of a dose. In addition to dosing information and sampling time, the following demographic data were collected in each patient: age, body weight, gender, race, formulation, and concurrent medication (e.g. phenytoin, phenobarbitone, salbutamol, acetazolamide, metronidazole, aspirin, amitriptyline, vitamin B complex, etc.). The characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of the 193 patients studied. Mean (s.d.).
| Characteristics | Index |
|---|---|
| Number of patients | 193 |
| Female (%) | 47.4 |
| Male (%) | 52.6 |
| Syrup (%) | 62.3 |
| Tablets (%) | 37.7 |
| Number of observations | 302 |
| CBZ dosage (mg kg−1 day−1) | |
| Mean | 16.71 (8.38) |
| Maximum | 66.67 |
| Minimum | 4.23 |
| CBZ concentration (mg l−1) | |
| Mean | 7.81 (3.34) |
| Maximum | 20.5 |
| Minimum | 1.0 |
| Weight (kg) | |
| Mean | 34.90 (20.52) |
| Maximum | 84.5 |
| Minimum | 4.84 |
| Age (years) | |
| Mean | 12.48 (10.08) |
| Medium | 10 |
| Maximum | 51 |
| Minimum | 0.3 |
| Percentage of patients on (%) | |
| Monotherapy | 52.3 |
| Polytherapy | 47.7 |
| Phenytoin | 12.2 |
| Phenobarbitone | 26.5 |
| Phenytoin and phenobarbitone | 2.3 |
| Race (%) | |
| Chinese | 74.3 |
| Indian | 5.3 |
| Malay | 11.5 |
| Others | 8.9 |
| CBZ L/D ratio | |
| Mean | 0.56 (0.30) |
| Maximum | 1.79 |
| Minimum | 0.06 |
| Correlation coefficientγ | 0.816 |
| Apparent clearance (l day−1 kg−1)* | |
| Mean | 2.50 (1.94) |
| Maximum | 16.76 |
| Minimum | 0.56 |
s.d. = standard deviation; CBZ = carbamazepine; L/D = level/dose.
Correlation between age and weight.
Reciprocal of CBZ L/D ratio.
Pharmacokinetic model
It was not anticipated that these steady state data, with a limited number of samples drawn during the absorption phase, would be very informative regarding the absorption rate constant and volume of distribution. In our data set, most of the concentrations measured at steady state can be regarded as average steady state concentrations. Thus, clearance of CBZ was estimated based on the standard steady state clearance equation, as follows:
| Equation 1 |
where C ss is the steady state concentration of CBZ (mg l−1), R is the dosing rate (mg day −1), CL is the plasma clearance of CBZ (l day −1). Because of the lack of injectable CBZ formulation, no precise data exist on the absolute bioavailability of CBZ. Several studies have assumed the bioavailability of CBZ to be 70% [21], 85% [22] and 100% [8, 9,11] for clearance calculation. The present study did not permit determination of bioavailability. For the purposes of this analysis, bioavailability is not assumed; if it is assumed, the term CL in Equation 1 maybe regarded as the apparent oral clearance, CL/F, where F is the bioavailablity of both CBZ preparations.
Data analysis
Data analysis was performed using the population pharmacokinetic package NONMEM (version V, level 1.1). To estimate the pharmacokinetic parameters of CBZ in the investigated population, the following models were used to describe the intersubject variability in clearance:
| Equation 2.1 |
| Equation 2.2 |
where CLj is the plasma clearance of CBZ (l day−1 kg−1) from the ‘j’th patient; CLpop is the population mean value of CL or a known function that describes the expected value of CLj as a function of individual specific covariates, such as age, body weight, gender, race, etc., and the vector of population average parameters. ηj,CL is the independent random error distributed normally with mean zero and variance equal to σCL2, which specifies the interindividual variation around CLpop, as CLj differs between patients.
Residual error (intraindividual variability) in the concentration was modelled in two ways:
| Equation 3.1 |
| Equation 3.2 |
where Rij is the ‘i’th body weight-adjusted dosing rate of CBZ (mg kg−1 day−1) in the ‘j’th patient; Cssij is the steady state plasma CBZ concentration (mg l−1) measured in the ‘j’th patient when receiving dosage Rij. εij is the independent, normally distributed error, known as the residual error (between the predicted and observed concentrations) with mean zero and variance σε2 which accounts for all uncertainties caused by intraindividual variation in CBZ pharmacokinetic parameters, assay and sampling errors, and model misspecification.
Model building procedure
Structural pharmacokinetic basic model
In the first step of the analysis the structural pharmacokinetic basic model without any covariates was derived and fitted to the data. Using NONMEM, the values of the population parameters θ (i.e. CL), σCl2 and σε2 were estimated.
Covariate model
In the second step a regression model was derived to describe the dependence of individual pharmacokinetic parameter estimates (i.e. the elements of CLpop) on the candidate covariates, i.e. to allow nonlinear covariate-parameter relationships to be discovered. Before fitting the regression model by stepwise addition/deletion, we performed an initial screening with SPSS version 9.0. This initial screening gives a first impression of the relative importance of several covariates (i.e. their ability to reduce the residual sum of squares) and of the shape of the relationships between covariates and pharmacokinetic parameters. To carry out this preliminary step, individual estimates of clearance were first obtained and subsequently the significance of each possible covariate in affecting the parameter was evaluated. After the initial screening step, with the estimates of the individual CL values treated as ‘data', a regression model was derived with stepwise regression. This step corresponds to the now classical regression problem of variable selection. The influence of age, body weight, gender, race, formulation and concurrent medication with other drugs were tested according to the following model:
 |
Equation 4 |
where A,W,G,R,FM,PH,PB and PN are values of eight independent variables: age, body weight, gender, race, formulation, concurrent medication with phenytoin, phenobarbitone or nonanticonvulsants, respectively; and θ1 to θ9 are fixed effects parameters. Gender, race, formulation and concurrent medication with phenytoin, phenobarbitone or nonanticonvulsants are dummy variables; for gender, G = 1 if male and 0 otherwise; for race, R = 1 if Chinese and 0 otherwise; for formulation, FM = 1 if tablet and 0 otherwise; for concurrent medication with phenytoin, PH = 1 if concomitant phenytoin and 0 otherwise; for concurrent medication with phenobarbitone, PB = 1 if concomitant phenobarbitone and 0 otherwise; and for concurrent medication with other drugs, PN = 1 if concomitant with nonanticonvulsants and 0 otherwise. Given the hypothesis that when a factor has no effect on CLpop, θ can be constrained and set equal to a constant (e.g. zero or one for θ2, θ3; one for θ1, θ4, θ5, θ6, θ7, θ8, θ9) and thereby a reduced form of Equation 4 can be obtained [23]. The covariate model was derived using a step-wise addition/deletion method from the full model, i.e. Equation 4 At each step, the model was advanced by deletion, addition, or replacement of the covariates until the Akaike Information Criteria (AIC) and Schwartz Criteria (SC) had reached a minimum value.
NONMEM analysis
In the final step of the model-building, the nonlinear mixed effect population model describing the relationship between covariates and CL was built. The NONMEM analysis was resumed in the classic way, i.e. the influence of the demographic factors of interest were entered into the pharmacokinetic basic model, using the regression model (i.e. covariate model) found in the second step as an initial guess for the final population model. The regression model was first started as the initial NONMEM model and thereafter, each covariate was added or dropped in a strategy corresponding to backward elimination and forward addition, until the minimum objective function had been reached. To test which particular fixed effect parameter values rendered the data most probable, objective functions were compared between successive models, using the χ2 distribution with degree of freedom equal to the difference in the number of (unconstrained) parameters between successive models. A P value of < 0.001 was adopted to indicate statistical significance. Model selection was done on the basis of the NONMEM objective function, −2loglikelihood (−2LL). The difference in −2LL between a full and reduced model was chosen as the test statistic. Covariates were added to the model if they significantly decreased the −2LL, or deleted from the model if −2LL did not increase significantly.
Predictive performance
Predictive performance was conducted on a validation data set of 45 observations from an independent cohort of 30 patients randomly selected and deleted from the data set prior to NONMEM analysis. The regression equation from the final NONMEM analysis was used to estimate the individual CBZ Cl values for these patients. In addition, the nonlinear multiple regression program, MULTI2 (BAYES), based on a Bayesian algorithm for microcomputers [24], was also employed in the feedback prediction. The CL values obtained from each method (regression and Bayesian) and the patients' CBZ dosing history were used to predict a concentration at the time of each observed concentration. Predicted concentrations from both methods were compared with observed concentrations. Bias was assessed through the mean prediction error (MPE), whereas precision was assessed through the root mean squared error (RMSE) [25].
Results
Descriptive statistics
There was a statistically significant correlation between dose (mg kg−1 day−1) and CBZ plasma levels (r = 0.157, P < 0.01), although a wide scatter was evident. CBZ plasma level to dose ratios (L/D) appeared to be related to the patient's age (r = 0.2388, P < 0.0005) and body weight (r = 0.2448, P < 0.0005). To evaluate better the influence of age on CBZ L/D ratio, the patients were categorized into the following age groups:
1–24 months
2–12 years, 11 months
13–19 years, 11 months
20–51 years
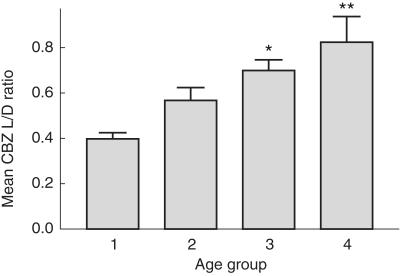
There was a trend that the mean values of CBZ L/D ratios in these age groups increased with age, as shown in Figure 1. Of patients there were 2.9, 20.6, 2.9% among group 1, 17.4, 31.6, 1.9% among group 2, 11.1, 22.2, 3.7% among group 3, and 5.0, 20.0, 1.7% among group 4, receiving phenyton, phenobarbitone, or both, respectively. The CBZ L/D ratio was also found to decrease significantly (r = −0.4206, P < 0.0005) with increasing dose kg−1. CBZ clearance appeared to decline with both advancing age (r = −0.2202, P < 0.0005) and body weight (r = −0.3293, P < 0.0005). Body weight was found to be highly related to age (r = 0.816, P < 0.0005). Patients on polytherapy received significantly higher doses (17.89 mg kg−1 day−1) than that of patients on CBZ monotherapy (15.30 mg kg−1 day−1). Patients on polytherapy were also found to have significantly lower mean plasma CBZ concentrations and mean values of CBZ L/D ratio than those of patients on monotherapy (Table 2).
Figure 1.
Mean plasma carbamazepine concentration to dose (L/D) ratios in various age groups (± s.e. mean). Age group: 1 (1–24 months); 2 (2–12 years,11 months); 3 (13–19 years, 11 months); 4 (20–51 years). *significantly different from age group 1, **significantly different from age group 1 and 2. (P < 0.05, using one-way anova: Post hoc multiple comparison-LSD test.
Table 2.
Comparison of monotherapy and polytherapy. Mean (s.d.).
| Characteristics | Monotherapy | Polytherapy |
|---|---|---|
| Number of patients | 101 | 92 |
| Number of observations | 157 | 145 |
| Female (%) | 49 | 45.5 |
| Male (%) | 51 | 54.5 |
| CBZ dosage (mg kg−1 day−1) | ||
| Mean | 15.30 (6.60) | 17.89 (9.72)* |
| Maximum | 36.95 | 66.67 |
| Minimum | 4.23 | 4.96 |
| CBZ concentration (mg l−1) | ||
| Mean | 8.21 (3.38) | 7.38 (3.26)* |
| Maximum | 20.5 | 20 |
| Minimum | 1 | 1 |
| Weight (kg) | ||
| Mean | 29.77 (18.20) | 40.72 (21.33)* |
| Maximum | 84 | 84.5 |
| Minimum | 4.84 | 8 |
| Age (years) | ||
| Mean | 9.74 (7.78) | 15.45 (11.40)* |
| Medium | 8 | 12 |
| Maximum | 47 | 51 |
| Minimum | 0.3 | 0.5 |
| CBZ L/D ratio | ||
| Mean | 0.63 (0.33) | 0.48 (0.23)* |
| Maximum | 1.79 | 1.18 |
| Minimum | 0.06 | 0.07 |
| Correlation coefficient γ | 0.8411 | 0.7831 |
| Apparent CL (l day−1 kg−1) ** | ||
| Mean | 2.16 (1.65) | 2.86 (2.17)* |
| Maximum | 16.76 | 15.38 |
| Minimum | 0.56 | 0.85 |
s.d. = standard deviation; CBZ = carbamazepine; L/D = level/dose; CL = clearance.
Significantly different from monotherapy.
Correlation between age and weight.
Reciprocal of CBZ L/D ratio.
Population pharmacokinetic analysis
For the basic structural pharmacokinetic model with previously described pharmaco-statistical error models for variability, interpatient variability and intrapatient variability were found to be best described by an exponential and exponential error models, respectively. The basic model ( Equation 1) resulted in an average population CL of 2.10 (l day−1 kg−1), with a variance of 0.134 (CV = 36.6%) and a residual variance of 0.082 (28.6%) for patients who were on CBZ monotherapy.
| Equation 5 |
The results of the preliminary analysis were summarized in Table 3 and showed that gender, race, formulation and concurrent medication with phenytoin or nonanticonvulsants had little or no effect on CL of CBZ. Age, body weight and concurrent medication with phenobarbitone appeared to have a definite influence on CL, as if θ2 and θ3 were constrained to zero and θ8 to one, there was a poor performance in the model fit (i.e. significant increase in the minimum objective function, P < 0.001).
Table 3.
Models tested for factors influencing carbamazepine clearance in the preliminary step.
| Models | AIC | SC |
|---|---|---|
| 1. f(A,W,G,R,FM,PB,PH,PN) | 1897.686 | 1931.079 |
| 2. f(A,W,G,R,FM,PB,PH) | 1895.751 | 1925.435 |
| 3. f(A,W,G,R,PB,PH) | 1893.736 | 1919.709 |
| 4. f(A,W, R,PB,PH) | 1891.780 | 1914.152 |
| 5. f(A,W,PB,PH) | 1889.936 | 1908.488 |
| 6. f(A,W,PB) | 1888.015 | 1902.857 |
| 7. f(W,PB) | 1906.691 | 1917.822 |
| 8. f(A,W) | 1906.778 | 1917.879 |
| 9. f(A,PB) | 1955.311 | 1966.442 |
| 10. f(W) | 1940.617 | 1948.038 |
| 11. f(PB) | 1953.311 | 1960.732 |
| 12. f(A) | 1980.848 | 1988.269 |
A = age; W = weight; G = gender; R = race; FM = formulation
PB = presence of phenobarbitone; PH = presence of phenytoin
PN = presence of nonanticonvulsants; AIC = Akaike Information Criteria
SC = Schwartz Criteria.
The final regression model obtained from the preliminary screening step was used as an initial NONMEM model, i.e. CL = θ1×Aθ2×Wθ3×θ4PB, where 30, 0.40, −1.0, and 1.5 were used as initial estimates for θ1, θ2, θ3, and θ4, respectively. The NONMEM analysis step in the CBZ clearance model building resulted in the following population model for CL:
| Equation 6 |
Table 4 summarized the change in −2LL of the forward addition and backward elimination step performed in NONMEM analysis step. The three important covariates obtained from the preliminary analysis also appeared in final NONMEM model, but age and phenobarbitone comedication had relatively a weak effect when compared with body weight.
Table 4.
Model development in the NONMEM analysis step.
| Models | Change in −2LL | P value |
|---|---|---|
| 1.CL = f(A,W,PB) | ||
| 2. CL = f(A,W,PB,PH) | −0.015 | NS |
| 3. CL = f(A,W,R,PB,PH) | −0.093 | NS |
| 4. CL = f(A,W,G,R,FM,PB,PH) | −0.324 | NS |
| 5. CL = f(A,W,G,R,FM,PB,PH,PN) | −0.434 | NS |
| 6. CL = f(A,W) | 36.03 | 1.94 × 10−9* |
| 7. CL = f(W,PB) | 35.93 | 2.05 × 10−9* |
| 8. CL = f(A,PB) | 130.45 | 2.63 × 10−30* |
| Basic structural CL model | 191.15 | 2.50 × 10−42* |
A = age; W = weight; G = gender; R = race; FM = formulation; PB = presence of phenobarbitone
PH = presence of phenytoin; PN = presence of nonanticonvulsants
−2LL = minus twice the log likelihood
NS = not significant.
Significant.
The summary results of the final fixed-effects and random-effects models were presented in Table 5. The interpatient and intrapatient variability were best described by an exponential and exponential error models, respectively. Interindividual (σCL) and intraindividual (σε) variability values obtained were 21.4% and 18.2%, respectively. The model, a reduced form of equation 4, which best described the overall individual data when incorporated into Equations 2.2 and 3.2 was as follows:
Table 5.
Population pharmacokinetic parameters for carbamazepine.
| Parameters | Meaning | Estimated values | s.e. |
|---|---|---|---|
| θ1 | Coefficient for combination of A, W and PB | 40.7 | 14.4 |
| θ2 | Power for A | 0.494 | 0.133 |
| θ3 | Power for W | −1.17 | 0.168 |
| θ4 | Proportionality constant for PB | 1.44 | 0.159 |
| σCL2 | Inter-patient variance | 0.046 | 0.029 |
| σε2 | Intra-patient variance | 0.033 | 0.012 |
A = age; W = body weight; PB = presence of phenobarbitone; s.e. = standard error.
| Equation 7 |
where CLpop is in l day−1 kg−1, A is in years, and W is in kg.
The results of the predictive performance evaluation were shown in Table 6. As expected, the Bayesian forecasting approach in addition to the derived regression equations provides more precise, less biased estimates than the regression equations without Bayesian feedback. Predictions based on the population covariate model tended to perform better than those based on the basic model.
Table 6.
Predictive performance of carbamazepine population pharmacokinetic models in a group of patients (n = 30). (95% confidence interval).
| Regression | Bayesian | |
|---|---|---|
| Basic model | ||
| MPE (mg l−1) | −0.57 | −0.66 |
| (−1.57–0.43) | (−1.55–0.23) | |
| RMSE (mg l−1) | 3.59 | 3.2 |
| (2.41–4.47) | (2.03–4.04) | |
| Population covariate model | ||
| MPE (mg l−1) | −0.31 | 0.23 |
| (−1.33–0.71) | (−0.28–0.74) | |
| RMSE (mg l−1) | 2.69 | 1.65 |
| (1.51–3.49) | (1.33–1.91) | |
MPE = mean prediction error; RMSE = root mean squared error.
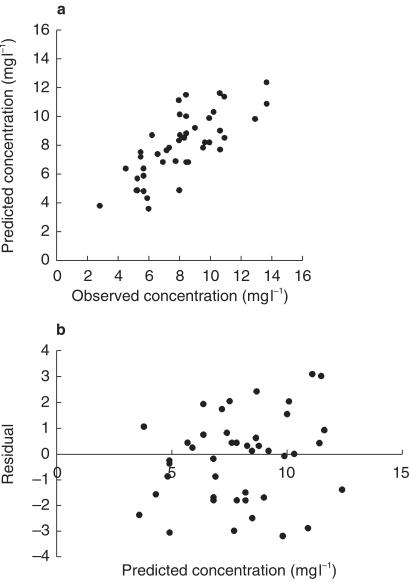
Scatter-plots of observed vs final covariate model-predicted concentration (Figure 2a) and weighted residuals vs final covariate model-predicted concentration (Figure 2b) showed that differences between pairs of observed and predicted values were small in most of the patients selected during validation, whereas a frequency histogram confirmed that these differences followed approximately a normal distribution.
Figure 2.
a) Scatter plot of observed carbamazepine concentration vs final covariate model-predicted concentration. b) Plot of residual vs final covariate model-predicted concentration.
Discussion
The present study, using a NONMEM approach to investigate the influence of demographic fixed-effect parameters on CBZ clearance in Singapore population (mostly are of Chinese origin), yielded a final regression model which relates CL to age, body weight, and phenobarbitone comedication, the only three out of eight covariates tested that emerged as important in our final analysis in determining CBZ clearance.
Although we found a significant correlation between CBZ daily dose and the plasma concentrations, the correlation was poor (r = 0.157) and the scattering of the plasma concentration data for each given dose was such that the relationship had no practical value for predicting the CBZ concentrations in individual patients. Similar results had also been observed by other authors [5].
The results obtained in the present study showed a positive relationship between CL and dose in mg kg−1 (r = 0.6863). Some authors had noted either a negative correlation between dose and CBZ L/D ratios [5, 7, 26] or a positive correlation between dose and CL values derived using such ratios [21, 22]. However, some controversy also exists over the influence of CBZ dose on clearance of the drug. The argument against a relationship between plasma levels and dose was supported by some authors [27–29]. By contrast, several authors had observed a curvilinear relationship between dose and plasma concentrations of CBZ [30, 31]. Possible explanation proposed have included decreased absorption [5] or increased elimination at higher doses [7, 25, 29, 31]. Although the possibility of dose-dependent kinetics cannot be excluded, the nature of the single-point data in the present study precluded the effective exploration of this relationship. In common clinical practice, doses are adjusted to keep concentration within the therapeutic range, only patients with the highest CL receive higher doses. In fact, some authors have suggested an artifact of bias for this influence of the CBZ dose on CL [5, 7, 21, 26]. Martin et al. [10] also recognized the futility of considering dose as a covariate in a population model because the data arise from clinical use of the drug in which doses are titrated to desired serum concentrations.
In the present study, patient's weight and age showed significant positive correlation with CBZ L/D ratios, and significant negative correlation with CBZ CL. This is consistent with the results reported by other authors [5, 7, 21, 26, 32, 33]. These results suggest that drug clearance decreases with maturity, as measured by weight and age. Such a relationship is more evident in children, but is less than in adults [26], as the main pharmacokinetic characteristics do not change after reaching adulthood because the metabolic capacity of the liver remains relatively constant [5]. The present study demonstrated such a relationship because 74% of the patients from the data set were below 16 years of age. It was also found that body weight showed better correlations with CL than did age. The correlation seen between body weight and CL was probably due mainly to the effect of body weight on CL in a nonlinear manner, as well as partly due to the same common cause, age, affecting both variables throughout the period of growth. Hence, age became relatively less important as a fixed effect in the presence of body weight [23]. Therefore, in our final NONMEM analysis step, body weight, but not age, emerged as the most significant covariate, though both were of importance, in determining CBZ CL (Table 4). Again, our results are in concordance with the results of other authors [22, 32, 34].
With regards to the influence of concomitant medication, the final model for CL included the presence of concomitant phenobarbitone only, but not phenytoin, as an important covariate. This is in contrast in part with the results of other studies; both phenytoin and phenobarbitone are well recognized as enzyme inducers, and there is considerable evidence that concomitant therapy with these antiepileptic drugs increases CBZ CL in both adults and children [4, 21, 26, 28, 35, 36]. Nevertheless the present finding is in agreement with some previous reports [7, 10]. It is likely that the relative infrequency of phenytoin comedication in our subjects contributed to the inability to demonstrate an effect (Table 1). A significant difference in mean apparent clearance values between the monotherapy and polytherapy groups is clearly due mainly to comedication with phenobarbitone.
With respect to formulation, it failed to emerge as an important fixed effect on CBZ clearance in the present study, even though most of our young children were on liquid dosage form, rather than solid tablet. CBZ absorption from the conventional tablet is both slow and variable. It has been much reported that a syrup or liquid preparation of CBZ gives a higher and earlier peak concentration compared with that of the tablet form [37–41]. It was believed that CBZ in solution had greater bioavailability [39], but previous studies demonstrated that the two preparations were equally bioavailable [37, 38, 42].
It was found that CL was not significantly influenced by sex. This finding is similar to the data reported in some studies [7, 43, 44]. By contrast, other investigators [4, 8, 21] found that female patients had a lower CL than male patients, probably because in girls, oestrogen secretion increases with maturity, and it is known that oestrogen is able to inhibit microsomal enzymes. Surprisingly, the population mean value of CBZ CL obtained in the present study is similar to the weighted average (i.e. 2.12 l day−1 kg−1) of reported values of that for American boys (n = 25, mean age of 8.84 years, mean body weight 30.85 kg, F = 1, CL = 2.59 l day−1 kg−1) and girls (n = 30, mean age of 8.96 years, mean body weight 30.75 kg, F = 1, CL = 1.728 l day−1 kg−1) on CBZ monotherapy [8].
Carbamazepine is extensively metabolized, primarily through epoxide hydrolase. Racial differences in hepatic drug metabolism are fairly common and account for the majority of the literature on racial difference in pharmacokinetics [45]. There are a number of studies highly suggestive of the difference in hepatic metabolism of specific drugs between Caucasians and Asians [15–17]. Unfortunately, these differences cannot be generalized. For example, in comparisons of Asians with Caucasians, there are examples of higher, lower, and no difference in hepatic clearance between groups. The present finding failed to demonstrate any difference in CBZ CL between Chinese and non-Chinese patients. Perhaps the low incidence of non-Chinese patients in the study might hinder the correct characterization of the influence of this factor. Our non-Chinese group consisted of Malays, Indians, and patients of other races. The population mean value of carbamazepine clearance obtained in the present study was found to differ markedly from that reported for patients in Japan (CL/F = 1.274 l day−1 kg−1 for a patient group with mean age of 14 years, mean body weight 39 kg) [34], but was in close agreement with the other studies of patients who had a very different ethnic or geographical background [8, 9,11, 21, 22] when the differences in F values used and in patients' mean age and body weight between studies were taken into account. It is worthwhile to note that peak instead of trough CBZ levels were used as Css in the Japanese study as blood samples were drawn 2–6 h after the morning dose compared with blood samplings being taken shortly before a dose in the present study as well as in other studies [4, 5,7, 8,10, 22, 26, 43]. In one CBZ population pharmacokinetics study conducted in Spain [22] (for a patient group with mean age of 9.5 years, mean body weight 35 kg, F = 0.85), population mean CL was reported to be 2.37 l h−1 (i.e. 1.91 l day−1 kg−1 for CL/F; which is close to 1.93 l day−1 kg−1 calculated using Equation 7). Summers & Summers [21], conducted a study on the CBZ clearance in Black paediatric epileptic patients (F = 0.7), and found that patients on monotherapy had mean CL value of around 1.53 l day−1 kg−1 (i.e. 2.19 l day−1 kg−1 for CL/F), for a body mass group of 21–30 kg (no age data reported). In another traditional pharmacokinetic analysis on adult patients conducted in France, CL in the monotherapy group (mean age 21 years, mean body weight 59.1 kg, F = 1) was 3.28 l h−1 (i.e. 1.33 l day−1 kg−1, which is slightly lower than 1.55 l day−1 kg−1 simulated using Equation 7) and 5.65 l h−1 (i.e. 2.54 l day−1 kg−1, which is close to 2.51 l day−1 kg−1 calculated using Equation 7) in patients receiving CBZ (mean age 21 years, mean body weight 53.4 kg) in addition to other antiepileptic drugs [9]. The overall findings of the present study suggest that there is little difference in CBZ clearance between the Asians living in Singapore and Caucasians (living in Europe and the United States) as well as Blacks (living in South Africa).
It was considered to be important to assess the predictive performance of the population models in a separate group of patients who had clinical and demographic characteristics similar to those patients used to develop the models. With the use of a confidence limit approach [25], Bayesian forecasts of CBZ concentrations in these patients were tested against observed concentrations. A method using Bayesian feedback provided the best predictions. The values of the mean prediction error (bias) and root mean squared error (precision) between the predicted and observed concentrations were small, with the 95% confidence interval for bias embracing zero. Still, there were a few poor predictions observed in some subjects (see Figure 2A), otherwise the derived covariate regression equation could accurately predict observed CBZ concentrations in a similar, but independent cohort of patients. A number of factors might contribute to such a large deviation between pairs of the predicted and measured CBZ concentrations during validation. Variation in patient compliance could be a factor. Among other factors, variability might have been introduced in the study by treating the bioavailability of CBZ as a constant.
In summary, estimates of CBZ population pharmacokinetics were obtained from a large population of Singapore epileptic patients receiving CBZ. The influence of age, body weight, gender, race, formulation, and concurrent medication on CBZ CL was quantified. Caution is needed with respect to what F value of CBZ is used and patient-specific characteristics when interpreting CBZ CL among different studies.
References
- 1.Holmes GL. Critical issues in the treatment of epilepsy. Am J Hosp Pharm. 1993;50(Suppl 5):S5–S16. [PubMed] [Google Scholar]
- 2.Pynnonen S. Pharmacokinetics of carbamazepine in man: a review. Ther Drug Monit. 1979;1:409–431. doi: 10.1097/00007691-197901030-00014. [DOI] [PubMed] [Google Scholar]
- 3.Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide: an update. Clin Pharmacokinet. 1986;11:177–198. doi: 10.2165/00003088-198611030-00001. [DOI] [PubMed] [Google Scholar]
- 4.Furlanut M, Montanari G, Bonin P, Casara GL. Carbamazepine and carbamazepine-10,11-epoxide serum concentrations in epileptic children. J Pediatr. 1985;106:491–495. doi: 10.1016/s0022-3476(85)80689-2. [DOI] [PubMed] [Google Scholar]
- 5.Battino D, Bossi L, Croci D, Franceschetti S, Gomeni C, Moise A. Carbamazepine plasma levels in children and adults: influence of age, dose, and associated therapy. Ther Drug Monit. 1980;2:315–322. [PubMed] [Google Scholar]
- 6.McKauge L, Tyrer JH, Eadie MJ. Factors influencing simultaneous concentrations of carbamazepine and its epoxide in plasma. Ther Drug Monit. 1981;3:63–70. [PubMed] [Google Scholar]
- 7.Suzuki Y, Cox S, Hayes J, Walson PD. Carbamazepine age-dose ratio relationship in children. Ther Drug Monit. 1991;13:201–208. doi: 10.1097/00007691-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Delgado MR. Influence of sex, age, weight, and carbamazepine dose on serum concentrations, concentration ratios, and level/dose ratios of carbamazepine and its metabolites. Ther Drug Monit. 1994;16:469–476. doi: 10.1097/00007691-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bonneton J, Iliadis A, Genton P, et al. Steady state pharmacokinetics of conventional versus controlled-release carbamazepine in patients with epilepsy. Epilepsy Res. 1993;14:257–263. doi: 10.1016/0920-1211(93)90050-h. [DOI] [PubMed] [Google Scholar]
- 10.Martin ES, Crismon ML, Godley PJ. Postinduction carbamazepine clearance in an adult psychiatric population. Pharmacotherapy. 1991;11:296–302. [PubMed] [Google Scholar]
- 11.Gray L, Botha JH, Miller R. A model for the determination of carbamazepine clearance in children on mono- and polytherapy. J Clin Pharmacol. 1998;54:359–362. doi: 10.1007/s002280050475. [DOI] [PubMed] [Google Scholar]
- 12.Bertillsson L. Clinical pharmacokinetics of carbamazepine. Clin Pharmacokin. 1978;3:128–143. doi: 10.2165/00003088-197803020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kalow W. Ethnic differences in drug metabolism. Clin Pharmacokin. 1982;7:373–400. doi: 10.2165/00003088-198207050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wood AJJ, Zhou HH. Ethnic differences in drug disposition and responsiveness. Clin Pharmacokin. 1990;20:350–373. doi: 10.2165/00003088-199120050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Zhou HH, Koshakji RP, Silberstein DJ, Wilkinson GR, Wood AJJ. Racial differences in drug response: Altered sensitivity to and clearance of propanolol in men of Chinese descent as compared with American whites. N Engl J Med. 1989;320:565–570. doi: 10.1056/NEJM198903023200905. [DOI] [PubMed] [Google Scholar]
- 16.Zhou HH, Sheller JR, Nu H, Wood M, Wood AJJ. Ethnic differences in response to morphine. Clin Pharmacol Ther. 1993;54:507–513. doi: 10.1038/clpt.1993.182. [DOI] [PubMed] [Google Scholar]
- 17.Ahsan CH, Renwick AG, Waller DG, Challenor VF, George CF, Amanullah M. The influences of dose and ethnic origins on the pahrmacokinetics of nifedipine. Clin Pharmacol Ther. 1993;54:329–338. doi: 10.1038/clpt.1993.155. [DOI] [PubMed] [Google Scholar]
- 18.Ghoneim MM, Korttila KK, Chiang CK, et al. Diazepam effects and kinetics in Caucasians and Orientals. Clin Pharmacol Ther. 1981;29:749–756. doi: 10.1038/clpt.1981.106. [DOI] [PubMed] [Google Scholar]
- 19.Chan E, Ti TY, Lee HS. Population pharmacokinetics of phenytoin in Singapore Chinese. Eur J Clin Pharmacol. 1990;39:177–181. doi: 10.1007/BF00280055. [DOI] [PubMed] [Google Scholar]
- 20.Potkin SG, Shen Y, Pardes H, et al. Haloperidol concentrations elevated in Chinese patients. Psychiatry Res. 1984;12:167–172. doi: 10.1016/0165-1781(84)90017-9. [DOI] [PubMed] [Google Scholar]
- 21.Summers B, Summers RS. Carbamazepine clearance in paedetric epilepsy patients. Influence of both mass, dose, sex and co-medication. Clin Pharmacokin. 1989;12:208–216. doi: 10.2165/00003088-198917030-00006. [DOI] [PubMed] [Google Scholar]
- 22.Delgado Iribarnegaray MF, Santos Buelga D, Garcia Sanchez MJ, Otero MJ, Falcao AC, Dominguez-Gil A. Carbamazepine population pharmacokinetics in children. Ther Drug Monit. 1997;19:132–139. doi: 10.1097/00007691-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Chan E, Chan K, Teoh R. Determination of phenobarbitone population clearance values for physically and mentally handicapped Chinese children with epilepsy. J Clin Pharmacol Ther. 1997;22:399–403. doi: 10.1111/j.1365-2710.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamaoka K, Nakagawa T, Tanaka H, Yasuhara M, Okumura K, Hori R. A nonlinear multiple regression program, MULTI2 (Bayes), based on Bayesian algorithm for microcomputers. J Pharmacobiodynam. 1985;8:246–256. doi: 10.1248/bpb1978.8.246. [DOI] [PubMed] [Google Scholar]
- 25.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:296–302. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez A, Duran JA, Serano JS. Steady-state carbamazepine plasma concentration-dose ratios in epileptic patients. Clin Pharmacokin. 1986;11:411–414. doi: 10.2165/00003088-198611050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Huf R, Schain RJ. Long-term experience with carbamazepine (Tegretol) in children with seizures. J Pediatr. 1981;97:310–312. doi: 10.1016/s0022-3476(80)80505-1. [DOI] [PubMed] [Google Scholar]
- 28.Rane A, Hojer B, Silson JT. Kinetics of carbamazepine and its 10,11-epoxide metabolite in children. Clin Pharmacol Ther. 1976;19:276–283. doi: 10.1002/cpt1976193276. [DOI] [PubMed] [Google Scholar]
- 29.Schain RJ, War JW, Guthrie D. Carbamazepine as an anticonvulsant in children. Neurology. 1977;27:476–480. doi: 10.1212/wnl.27.5.476. [DOI] [PubMed] [Google Scholar]
- 30.Eadie MJ, Tyrer JH. Pharmacological Basis and Practice. 2. Edinburgh: Churchill Livingstone; 1980. Anticonvulsant therapy. [Google Scholar]
- 31.Rambeck B, May T, Juergens U. Serum concentrations of carbamazepine and its epoxide and diol metabolites in epileptic patients: the influence of dose and co-medication. Ther Drug Monit. 1987;9:298–303. doi: 10.1097/00007691-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Delgado MR. A comprehensive study of the relation between serum concentrations, concentration ratios, and leve/dose ratios of carbamazepine and its metabolites with age, weight, dose, and clearances in epileptic children. Epilepsia. 1994;35:1221–1229. doi: 10.1111/j.1528-1157.1994.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 33.Harley R, Lucock MD, Ng PC, et al. Factors influencing plasma level/dose ratios of carbamazepine and its major metabolites in epileptic children. Ther Drug Monit. 1990;12:438–444. doi: 10.1097/00007691-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Yukawa E. Population-based investigation of carbamazepine relative clearance using routine clinical pharmacokinetic data in Japan. A multiple peak approach feasibility study for pharmacokinetic screening. Clin Drug Invest. 1995;10:29–39. doi: 10.1111/j.2042-7158.1995.tb03295.x. [DOI] [PubMed] [Google Scholar]
- 35.Eichelbaum M, Kothe KW, Hoffman F, van Unruh GE. Kinetics and metabolism of carbamazepine during combined antiepileptic drug therapy. Clin Pharmacol Ther. 1979;26:366–371. doi: 10.1002/cpt1979263366. [DOI] [PubMed] [Google Scholar]
- 36.Riva A, Contin M, Albani F, et al. Free concentration of carbamazepine and carbamazepine-10,11-epoxide in children and adults: influence of age and phenobarbitone co-medicaiton. Clin Pharmacokin. 1985;10:524–531. doi: 10.2165/00003088-198510060-00005. [DOI] [PubMed] [Google Scholar]
- 37.Rawlins MD, Collste P, Bertilsson L, Palmer L. Distribution and elimination kinetics of carbamazepine in man. Eur J Clin Pharmacol. 1975;8:91–96. doi: 10.1007/BF00561556. [DOI] [PubMed] [Google Scholar]
- 38.Levy RH, Pitlick WH, Troupin AS, Green JR, Neal JM. Pharmacokinetics of carbamazepine in normal man. Clin Pharmacol Ther. 1975;17:657–668. doi: 10.1002/cpt1975176657. [DOI] [PubMed] [Google Scholar]
- 39.Cotter LM, Eadie MJ, Hooper WD, Lander CM, Smith GA, Tyrer JH. The pharmacokinetics of carbamazepine. Eur J Clin Pharmacol. 1977;12:451–456. doi: 10.1007/BF00561065. [DOI] [PubMed] [Google Scholar]
- 40.Meinardi H, van der Kleijin E, Meijer JWA, van Rees H. Absorption and distribution of antiepileptic drugs. Epilepsia. 1975;16:353–365. doi: 10.1111/j.1528-1157.1975.tb06063.x. [DOI] [PubMed] [Google Scholar]
- 41.Morselli PL, Monacco F, Gerna M, Recchia M, Riccio A. Bioavailability of two carbamazepine preparations during chronic administration to epileptic patients. Epilepsia. 1975;16:759–764. doi: 10.1111/j.1528-1157.1975.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 42.Hooper WD, King AR, Patterson M, Dickinson RG, Eadie MJ. Simultaneous plasma carbamazepine and carbamazepine-epoxide concentrations in pharmacokinetics and bioavailability studies. Ther Drug Monit. 1985;7:36–40. doi: 10.1097/00007691-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Lanchote VL, Bonato PS, Campos GM, Rodrigues I. Factors influencing plasma concentrations of carbamazepine and carbamazepine-10,11-epoxide in epileptic children and adults. Ther Drug Monit. 1995;17:47–52. doi: 10.1097/00007691-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Altafullah I, Talwar D, Loewenson R, Olson K, Lockman LA. Factors influencing serum levels of carbamazepine-10,11-epoxide in children. Epilepsy Res. 1989;4:472–480. doi: 10.1016/0920-1211(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JA. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci. 1997;86:1328–1333. doi: 10.1021/js9702168. [DOI] [PubMed] [Google Scholar]