Abstract
Aims
The objective of this study was to estimate the number of fractures attributed to oral corticosteroid use.
Methods
Information was obtained from the General Practice Research Database which contains medical records of general practitioners in the UK. The total number of corticosteroid-related fractures during a course of treatment was estimated using the formula for attributable risk among the exposed.
Results
A total of 244 235 patients was prescribed an oral corticosteroid. The rate of hip fractures increased exponentially with age in both males and females. The excess number of hip fracture cases among females aged 85 years or older using 7.5 mg prednisolone per day or more was 1.4 cases per 100 patients per year. About 47% of all hip and 72% of all vertebral fractures that occurred can be attributed to oral corticosteroid use. Among 10 000 female users of higher doses, 99.7 nonvertebral, 31.6 hip and 45.8 vertebral fractures can be attributed to use of oral corticosteroids.
Conclusions
The targeting of high-risk patients will be important for implementing preventative strategies in a cost-effective manner.
Keywords: fractures, oral corticosteroids, public health
Introduction
Osteoporosis, one of the main complications of oral corticosteroid treatment, can lead to the development of fractures, particularly hip and vertebral fractures [1–6]. These fractures are associated with considerable morbidity and mortality. Various studies have found that between 12 and 40% of all hip fracture cases die within 6 months [7]. Given the widespread use of oral corticosteroids, it becomes essential to consider strategies to prevent fractures. Preventative measures can include changes in life style such as reducing cigarette smoking or alcohol use and may also consist of modification of the oral corticosteroid type, dose or form. Furthermore, bone-active medication such as bisphosphonates can be prescribed in order to minimize corticosteroid-induced bone loss [8]. These preventative strategies could be targeted to the patient groups with the largest burden of corticosteroid-related fractures. The burden of corticosteroid-related fractures is determined not only by the magnitude of excess fracture risk with oral corticosteroids but also by the number and type of patients using this treatment [9]. The objective of this study was to estimate the number of fractures attributed to oral corticosteroid use. The data for our study were obtained from the General Practice Research Database (GPRD) which contains the computerized medical records from a large group of general practices. The population included in GPRD is broadly representative of the general population in England and Wales [10, 11].
Methods
General practitioners (GP) play a key role in the health care system in the United Kingdom as they are responsible for primary health care and specialist referrals. Patients are semipermanently affiliated to a practice which centralizes the medical information not only from the GPs themselves but also from specialist referrals and hospitalizations. The current study included 683 practices from different geographical areas in the UK. The data recorded in the GPRD include demographic information, prescription details, clinical events, preventive care provided, specialist referrals, hospital admissions and their major outcomes [10–15]. Clinical data are stored and retrieved by means of OXMIS codes for diseases that are cross-referenced to the International Classification of Diseases (ICD-9) [10, 14]. Each entry into GPRD is internally validated by cross-checking within the practice and by comparisons with external statistics [10–15]. Only data from practices that pass this quality control are compiled to form the GPRD database. Several independent validation studies have confirmed a high level of completeness and validity of GPRD [16–19]. The GPRD is owned by the Medicines Control Agency in the UK.
Study population
A retrospective cohort study was conducted comparing patients using oral corticosteroids (aged 18 years or older) with control patients. Details of the methods used in the investigation and of the relationship between oral corticosteroid use and risk of fractures are available elsewhere [20]. Briefly, the oral corticosteroid users were patients who received one or more prescriptions for oral corticosteroids. The control patients were patients who received nonsystemic corticosteroid prescriptions (topical, aural, ophthalmic or nasal). The nonsystemic corticosteroids included corticosteroid eye drops and ointments (British National Formulary 11.4.1), ear drops and ointments (12.1.1), nasal sprays (12.2.1), and topical skin creams, ointments and lotions (13.4) [21]. They were matched by age (within 5 years or if no patient found, within 10 years), gender and, if possible, medical practice. Each oral corticosteroid user was followed from start of oral corticosteroid treatment and was censored at the time they sustained a fracture, or until 91 days after the last oral corticosteroid prescription, or until the patient's change of practice, death, or the end of the study (whichever date came first). Documented prescription was a means of ensuring active registration at the practice [20]. The most frequently recorded indication for oral corticosteroid treatment was respiratory disease (40%). Cutaneous or musculoskeletal disorders were recorded in around 6% of the oral corticosteroid users. Patients with arthropathies were most likely to be continuous users [22].
Cases were patients who had a nonvertebral or vertebral fracture recorded in their medical records during follow-up. The classification of fractures was based on International Classification of Diseases (9th revision) categories. Previous studies in GPRD reported a high level of validity of the GPRD with respect to fractures [19, 23]. Incidence rates of fractures were calculated by dividing the number of patients with a fracture by the total number of patient-years of follow-up. Adjusted relative rates were estimated using Cox proportional hazards models that included age, gender, and selected risk factors for fractures [20]. For each oral corticosteroid user, the daily dose over the total treatment period was estimated by dividing the total amount of prescribed prednisolone (or equivalent dose) in milligrams by the treatment time [21]. Three dose categories were assigned: low dose (less than 2.5 mg day−1), medium (2.5 up to 7.5 mg day−1) and high dose (7.5 mg day−1 or more).
Determination of corticosteroid-related fractures
The total number of corticosteroid-related fractures was estimated using the formula for attributable risk among the exposed, i.e. the relative rate minus 1 divided by the relative rate [9, 24, 25]. For each oral corticosteroid user, the duration of the first course of treatment was estimated using the life table method. This was done in order to account for intermittent use. Patients were considered to have discontinued treatment if they did not receive a repeat prescription or died within 3 months of the last oral corticosteroid prescription. Patients were censored if they left the practice or the study ended within this time period. The number of fracture cases was then estimated by multiplying the total duration of treatment with the rate of fractures. The proportion of corticosteroid-related cases was based on the attributable risk among the exposed. As daily dose was a strong determinant of fracture and duration of use, these calculations were stratified by daily dose.
Baseline risk score
As the potential confounding variables concerned a large group of heterogeneous factors with varying degrees of fracture risk, a single index of baseline risk was estimated using Miettinen's multivariate confounder score method [26–28]. In the complete study population of users of any form of corticosteroids, patients with a fracture were identified. A similar number of control patients was randomly selected. These cases and controls were then compared using a logistic regression model that included age, gender, potential confounding variables and interaction terms between these variables and age and gender. The regression model did not include oral corticosteroid use or fracture history. Factors associated with fracture and considered as potential confounding variables included history of rheumatoid arthritis, hyperthyroidism, congestive heart failure, diabetes mellitus, seizures, anaemia, dementia, depression, psychotic disorder, cerebrovascular accident, back pain or falls. Prescriptions in the previous year for anticonvulsants, methotrexate, thiazide diuretics, anxiolytics, antipsychotics, NSAIDs, antidepressants, anti-Parkinson drugs, hormone replacement therapy, bisphosphonates, vitamin D, and calcitonin were also considered as potential confounding variables. Smoking and body mass index were also included [29, 30]. The baseline risk score for each study patient was the predicted odds of fracture, estimated from the final logistic model which was selected after backward selection of the various factors. Separate risk scores at baseline were estimated for nonvertebral and vertebral fractures. The combined cohorts of oral corticosteroid users and controls were divided into five strata on the basis of the baseline risk score and the fracture rate was calculated for each risk stratum.
Results
A total of 244 235 patients was prescribed an oral corticosteroid. The majority of the patients was female (58.6%) and 42.5% of the patients were aged 65 years or older. An average of 6.8 oral corticosteroid prescriptions was prescribed during follow-up. The average duration of a course of treatment was 7.4 months. There were around 50 000 oral corticosteroid users with a low daily dose (less than 2.5 mg of prednisolone per day), 105 000 people with a dose of 2.5–7.5 mg day−1, and 88 000 people with a high dose (7.5 mg or more per day). The course of treatment was on average longest in the high dose oral corticosteroid group (3.9 months in the low dose, 7.9 months in medium dose and 8.8 months in the high dose group).
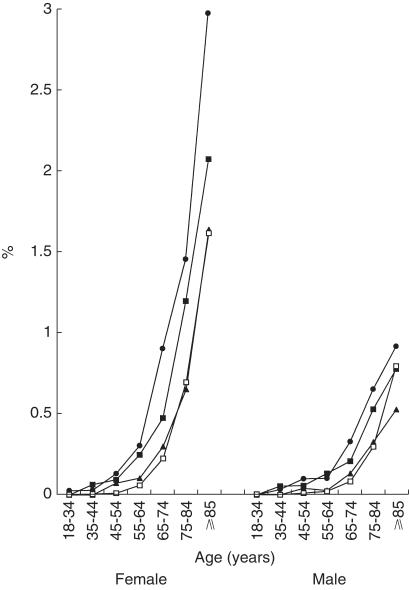
The rate of hip fractures increased exponentially with age in both males and females (Figure 1). Females experienced more hip fractures than males. The rate ranged from 0.003 cases per 100 patient-years to 1.6 for the youngest to the oldest female control patients. For males, the respective figures were 0.004 and 0.8. The use of high or medium doses of oral corticosteroids was associated with increased hip fracture rates, with higher rates of hip fractures in patients using the higher doses. The excess number of hip fracture cases in the high dose group (compared to control) was 1.4 cases per 100 patients per year among females aged 85 years or older compared with 0.1 cases for females aged 45–54 years.
Figure 1.
Annual incidence of hip fractures stratified by daily dose (• high dose, ▪ medium dose, ▴ low dose, □ control), age and gender.
Around 47% of all hip and 72% of all vertebral fractures can be attributed to oral corticosteroid use in patients using this treatment. The highest number of corticosteroid-related fractures occurred among females using daily doses of 7.5 mg or more (Table 1). Among 10 000 female high dose users, approximately 254.2 nonvertebral, 56.5 hip and 56.8 vertebral fractures occurred during treatment. Of these fractures, 99.7 nonvertebral, 31.6 hip and 45.8 vertebral fractures could be attributed to oral corticosteroid use. The male high dose users experienced 131.3 nonvertebral, 17.9 hip and 34.6 vertebral fractures per 10 000. Of those fractures, 51.5 nonvertebral, 10.0 hip and 27.9 vertebral fractures were corticosteroid-related. Vertebral fractures that occurred in the high dose group were primarily related to treatment. About 81% of all vertebral cases and 56% of the hip fractures were corticosteroid-related in this dose group. For the intermediate dose group, these figures were 61% and 43%, respectively.
Table 1.
Number of fractures, overall and corticosteroid-related, per 10 000 males or females during a course of steroid treatment.
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Medium dose (n = 10 000) | High dose (n = 10 000) | Medium dose (n = 10 000) | High dose (n = 10 000) | |||||
| Number of cases overall | Steroid-related cases | Number of cases overall | Steroid-related cases | Number of cases overall | Steroid-related cases | Number of cases overall | Steroid-related cases | |
| Non-vertebral | 186.8 | 49.1 | 254.2 | 99.7 | 87.2 | 22.9 | 131.3 | 51.5 |
| Hip | 40.9 | 17.7 | 56.5 | 31.6 | 13.3 | 5.8 | 17.9 | 10.0 |
| Vertebral | 31.5 | 19.3 | 56.8 | 45.8 | 16.0 | 9.8 | 34.6 | 27.9 |
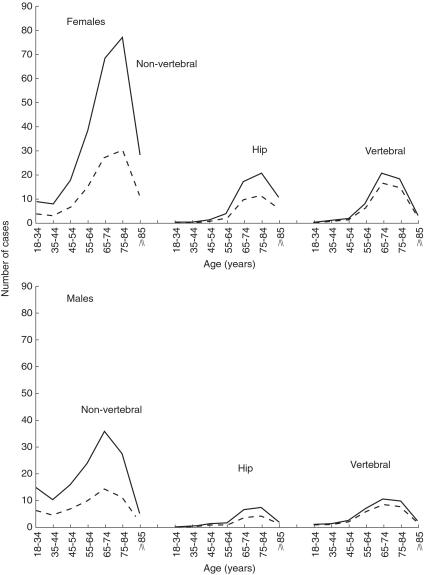
The impact of oral cortic osteroid use on fractures varied substantively across age. Figure 2 provides the number of fracture cases per 10 000 males and females who were using high doses of oral corticosteroids. Among 10 000 females treated with high doses, most corticosteroid-related hip fracture cases were observed in females aged between 75 and 84 years of age (12.1 hip fracture cases in this age group). Corticosteroid-related vertebral fractures occurred most frequently in females aged 65–74 years of age (17.3 cases). Interestingly, the group of patients with the highest absolute risk of fractures (females aged 85 years or older) did not contribute the largest number of corticosteroid-related fractures, with totals of 6.5 corticosteroid-related hip fractures per 10 000 females treated with higher doses.
Figure 2.
Number of fractures, overall (solid line) and corticosteroid-related (dashed line), per 10 000 males and females who start a course of high dose treatment, stratified by age and gender.
Baseline risk wass associated with the rate of fracture. Control patients with the lowest baseline risk had a nonvertebral fracture rate of 0.6 cases per 100 patients per year, compared with a rate of 2.8 in the highest baseline risk group. Similar patterns of increasing fracture rates with higher baseline risks were observed in each of the three corticosteroid dose groups. High dose users had a fracture rate of 1.3 in the lowest and 4.9 in the highest baseline group. The importance of baseline risk is also illustrated by the finding that, of 10 000 high dose oral corticosteroid users, the quintile of patients (2000 patients) with the lowest baseline risk experienced 8.1 nonvertebral, 1.7 hip and 4.4 vertebral corticosteroid-related fractures. Conversely, the quintile of patients with the highest baseline risk had many more corticosteroid-related fractures (29.3 nonvertebral, 11.4 hip and 15.1 vertebral).
As shown in Table 2, only a minority of patients received drug treatment after suffering a fracture (hormone-replacement therapy, bisphosphonates, vitamin D or calcitonin). Patients with vertebral fractures were the most likely to receive drug treatment. About 33.4% of the vertebral fracture cases received bisphosphonate treatment in the 6 months after experiencing the fracture, 5.3% received vitamin D and 3.7% hormone replacement therapy. In contrast, 7.2% patients with hip fractures received bisphosphonates, 3.1% received vitamin D and 2.0% received hormone replacement therapy. Calcitonin was used infrequently after any fracture type.
Table 2.
Use of bone-active treatment in the 6 months following a fracture*
| Non-vertebral (n = 6395) | Forearm (n = 1338) | Hip (n = 1072) | Vertebral (n = 1033) | |
|---|---|---|---|---|
| Bisphosphonates | 253 | 51 | 53 | 276 |
| (4.9%) | (4.4%) | (7.2%) | (33.4%) | |
| Hormone replacement therapy | 251 | 59 | 15 | 29 |
| (4.8%) | (5.1%) | (2.0%) | (3.7%) | |
| Vitamin D | 96 | 14 | 23 | 42 |
| (1.8%) | (1.2%) | (3.1%) | (5.3%) | |
| Calcitonin | 8 | 1 | 1 | 12 |
| (0.2%) | (0.1%) | (0.1%) | (1.5%) |
Patients who died or transferred out within 6 months of the fracture were excluded.
Discussion
The calculation of the impact of an exposure on the disease burden in a population is an important aspect of public health research. Measures of association such as relative rates or rate differences convey information about the strength of the association between exposure and disease but not information on the actual number of cases that are related to the exposure [9]. Public health priorities are influenced by this number of exposure-related cases. In another part of our study, it was found that the use of oral corticosteroids was associated with substantive increases in fracture risk in the study population. In people using doses of 7.5 mg per day or more, the risk of hip fractures was doubled relative to control and the risk of vertebral fractures was increased four-fold [20]. It was also observed that oral corticosteroids were being used frequently and by a heterogeneous group of patients [22]. By integrating the information on fracture risks and utilization, about 47% of all hip and 72% of all vertebral fractures experienced by oral corticosteroid users can be attributed to oral corticosteroid use. The highest number of corticosteroid-related fractures occurred among females using daily doses of 7.5 mg or more. A total of 31.6 hip and 45.8 vertebral fractures attributable to oral corticosteroid use occurred per 10 000 females during a course of high doses.
Recent UK guidelines outline a clinical approach to manage corticosteroid-induced osteoporosis in people taking or anticipated to be taking daily doses of 7.5 mg or more of prednisolone for 6 months or longer. A diagnostic work-up, followed by therapeutic intervention, is advised for patients using oral corticosteroids at doses of 15 mg day−1 or more and those using doses between 7.5 and 15 mg day−1 and with strong risk factors for fracture. All other patients with doses of 7.5 mg or more should, according to these guidelines, be monitored periodically using bone-mass measurements [8]. These guidelines, based on a review of literature, were developed to provide a more systematic approach to the identification of high-risk patients. Most of the studies on the adverse bone effects of oral corticosteroids concern changes in bone density. There are, however, only a few studies that have evaluated the clinically important endpoint of fractures and the majority included small numbers of oral corticosteroid users [31]. Although the UK guidelines were formulated for patients using oral corticosteroids at doses above 7.5 mg day−1, we found that the rate of fracture was increased even at lower doses. Furthermore, the increase in fracture risk following commencement of oral corticosteroid therapy was rapid, with significant increases in risk of non vertebral fracture becoming apparent within the first 3 months of treatment [20].
The findings in our study are consistent with the emphasis in the UK guidelines to identify and target the high-risk patients. When identifying the baseline fracture risks using data on age, gender and medical and prescription histories (no bone density measurements were available in our study population), we found that the quintile of 10 000 oral corticosteroid users with the highest fracture risk at baseline experienced 11.4 hip and 15.1 vertebral corticosteroid-related fractures. This compares with 1.7 hip and 4.4 vertebral corticosteroid-related fractures in the lowest baseline quintile. Several studies have reported that patients with multiple risk factors have an especially high risk of fractures [32, 33].
Therapeutic interventions for corticosteroid-induced osteoporosis include treatments with bisphosphonates, hormone-replacement therapy or calcitriol. The evidence for the efficacy of these therapies has been summarized by Eastell [8]. Bisphosphonates have been most widely studied in patients with corticosteroid-induced osteoporosis. In the three larger randomised studies with bisphosphonates (including more than 100 patients), a significant prevention of bone loss was observed with bisphosphonate therapy. Also, a trend towards decreased fracture rates was observed although this reduction did not reach statistical significance [34–36].
A contributory role of the underlying disease towards the increased fracture risks and detection are the main potential biases of this study. Various analyses did not, however, support the likelihood that the effects of these potential biases were substantive. The relative rate of fracture in oral corticosteroid users compared with controls was similar in patient groups with different underlying diseases (chronic obstructive pulmonary disease, skin conditions, arthropathies, peripheral nervous disorders, and noninfectious enteritis and colitis) [20, 23]. Another potential limitation, specific to this study, was that similar relative rates of fractures were estimated across different groups of patients. To validate this, interaction terms were included in the Cox proportional hazards models. The magnitude of the interactions was generally small and not statistically significant. Prediction of relative rates across different patient characteristics was not improved by including interaction terms. Also, there may have been underrecording of oral corticosteroid use as, for example, casualty departments may provide short-term corticosteroid treatments. The likely effect of this bias would be an underestimate of the fracture risk of oral corticosteroids. Several analyses were conducted to assess the robustness of results to the method of analysis. Restricting the follow-up to the date of the last event recorded for the patient in GPRD did not change results nor did an analysis excluding patients with a hospitalization during follow-up [23].
In conclusion, use of higher doses of oral corticosteroids was associated with a substantive number of fractures, particularly hip and vertebral fractures. Given the mortality, which is associated with these fractures, prevention is important. The targeting of high-risk patients will be important for implementing preventative strategies in a cost-effective manner.
Acknowledgments
Funds for this study were provided by Procter & Gamble Pharmaceuticals. We thank EPIC, the GPRD license holder, for their support.
References
- 1.Adinoff AD, Hollister MD, Roger J. Steroid-induced fractures and bone loss In patients with asthma. N Engl J Med. 1983;309:601–607. doi: 10.1056/NEJM198308043090502. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Coupland C, Mitchell M. Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis. 1995;54:49–52. doi: 10.1136/ard.54.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dykman TR, Gluck OS, Murphy WA, Hahn TJ, Hahn BH. Evaluation of factors associated with glucocorticoid-induced osteopenia in patients with rheumatic diseases. Arthritis Rheum. 1985;28:361–368. doi: 10.1002/art.1780280402. [DOI] [PubMed] [Google Scholar]
- 4.Hooyman JR, Melton Jl, IIII, Nelson AM, O'Fallon MW, Lawrence Riggs B. Fractures after rheumatoid arthritis. Arthritis Rheum. 1984;27:1353–1361. doi: 10.1002/art.1780271205. [DOI] [PubMed] [Google Scholar]
- 5.McDougall R, Sibley J, Haga M, Russell A. Outcome in patients with rheumatoid arthritis receiving prednisone compared to matched controls. J Rheumatol. 1994;21:1207–1213. [PubMed] [Google Scholar]
- 6.Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteriod therapy in rheumatoid arthritis: An analysis of serious adverse events. Am J Med. 1994;96:115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Pitt FA. Epidemiology of osteoporosis. Bone. 1992;13:S7–S15. doi: 10.1016/s8756-3282(09)80004-5. [DOI] [PubMed] [Google Scholar]
- 8.Eastell R, Reid DM, Compston J, et al. A UK consensus group on management of glucocorticoid induced osteoporosis: an update. J Intern Med. 1998;244:271–292. doi: 10.1046/j.1365-2796.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Gefeller O. Definitions of attributable risk. Public Health Rev. 1995;23:343–355. [PubMed] [Google Scholar]
- 10.Lis Y, Mann RD. The Vamp Research Multi-purpose Database in the U. K. J Clin Epidemiol. 1995;48:431–443. doi: 10.1016/0895-4356(94)00137-f. 10.1016/0895-4356(94)00137-f. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell J. General Practice Research Database (GPRD): Scope and Quality of Data. London: Office of Population Censuses and Statistics; 1994. [Google Scholar]
- 12.Hall G. Pharmacoepidemiology using a UK Database of Primary Care Records. Pharmacoepidemiol Drug Saf. 1992;1:33–37. [Google Scholar]
- 13.Mann RD, Hall G, Chukwujindu J. Research implications of computerised primary care. Post Marketing Surveillance. 1992;5:259–268. [Google Scholar]
- 14.Anonymous. Information for Researchers. London: Office for National Statistics; 1996. The general practice research database. [Google Scholar]
- 15.Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350:1097–1099. doi: 10.1016/S0140-6736(97)04248-7. 10.1016/s0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 16.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerised data resource in the United Kingdom. Pharmacoepidemiol Drug Saf. 1992;1:347–349. [Google Scholar]
- 18.Nazareth I, King M, Haines A, Rangel L, Myers S. Accuracy of diagnosis of psychosis on general practice computer system. Br Med J. 1993;307:32–34. doi: 10.1136/bmj.307.6895.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Staa TP, Abenhaim L. The quality of information recorded on a UK database of primary care records: a study of hospitalization due to hypoglycemia and other conditions. Pharmacoepidemiol Drug Saf. 1994;3:15–21. [Google Scholar]
- 20.van Staa TP, Leufkens B, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 21.British National Formulary Number. British Medical Association and the Royal Pharmaceutical Society of Great Britain. Wallingford, England: Pharmaceutical Press; 1996. September, 1996. [Google Scholar]
- 22.van Staa TP, Cooper C, Abenhaim L, Begaud B, Zhang B, Leufkens B. Utilisation of oral corticosteroids in the United Kingdom. Q J Med. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 23.van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HGM. The use of a large pharmaco-epidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf. 2000;9:359–366. doi: 10.1002/1099-1557(200009/10)9:5<359::AID-PDS507>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Nortridge ME. Annotation: public health methods – attributable risk as a link between causality and public health action. Am J Public Health. 1995;85:1202–1204. doi: 10.2105/ajph.85.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinbaum DG, Kupper LL, Morgenstern H. Principles and Quantitative Methods. New York: Van Norstrand Reinhold Company Inc.; 1982. Epidemiologic research. [Google Scholar]
- 26.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104:609–620. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 27.Pike MC, Anderson J. Some insights into Miettinen's multivariate confounder score approach to case-control study analysis. J Epidemiol Community Health. 1979;33:104–106. doi: 10.1136/jech.33.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss D. On Miettinen's multivariate confounder score. J Clin Epidemiol. 1998;51:233–236. doi: 10.1016/s0895-4356(97)00283-7. 10.1016/s0895-4356(97)00283-7. [DOI] [PubMed] [Google Scholar]
- 29.Shane E. Osteoporosis associated with illness and medications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego: Academic Press; 1996. pp. 925–946. [Google Scholar]
- 30.Grisso JA, Capezuti E, Schwartz A. Falls as risk factors for fractures. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego: Academic Press; 1996. pp. 599–611. [Google Scholar]
- 31.Reid IR. Gluco-corticoid-induced osteoporosis and other forms of secondary osteoporosis. In: Meunier PJ, editor. Osteoporosis: Diagnosis and Management. London: Martin Dunitz; 1998. pp. 233–250. [Google Scholar]
- 32.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fractures in white woman. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 33.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 34.Adachi JD, Bensen WG, Brown J, et al. Intermittent etidronate therapy to prevent corticosteroid induced osteoporosis. N Engl J Med. 1997;337:382–387. doi: 10.1056/NEJM199708073370603. [DOI] [PubMed] [Google Scholar]
- 35.Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid induced osteoporosis. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 36.Roux C, Oriente P, Laan R, et al. Randomized trial of effect of cyclical etidronate in the prevention of corticosteroid induced bone loss. J Clin Endocrinol Metab. 1998;83:1128–1133. doi: 10.1210/jcem.83.4.4742. [DOI] [PubMed] [Google Scholar]