Abstract
Aims
Patients with migraine may receive the 5-HT1B/1D agonist, rizatriptan (5 or 10 mg), to control acute attacks. Patients with frequent attacks may also receive propranolol or other β-adrenoceptor antagonists for migraine prophylaxis. The present studies investigated the potential for pharmacokinetic or pharmacodynamic interaction between β-adrenoceptor blockers and rizatriptan.
Methods
Four double-blind, placebo-controlled, randomized crossover investigations were performed in a total of 51 healthy subjects. A single 10 mg dose of rizatriptan was administered after 7 days' administration of propranolol (60 and 120 mg twice daily), nadolol (80 mg twice daily), metoprolol (100 mg twice daily) or placebo. Rizatriptan pharmacokinetics were assessed. In vitro incubations of rizatriptan and sumatriptan with various β-adrenoceptor blockers were performed in human S9 fraction. Production of the indole-acetic acid-MAO-A metabolite of each triptan was measured.
Results
Administration of rizatriptan during propranolol treatment (120 mg twice daily for 7.5 days) increased the AUC(0,∞) for rizatriptan by approximately 67% and the Cmax by approximately 75%. A reduction in the dose of propranolol (60 mg twice daily) and/or the incorporation of a delay (1 or 2 h) between propranolol and rizatriptan administration did not produce a statistically significant change in the effect of propranolol on rizatriptan pharmacokinetics. Administration of rizatriptan together with nadolol (80 mg twice daily) or metoprolol (100 mg twice daily) for 7 days did not significantly alter the pharmacokinetics of rizatriptan. No untoward adverse experiences attributable to the pharmacokinetic interaction between propranolol and rizatriptan were observed, and no subjects developed serious clinical, laboratory, or other significant adverse experiences during coadministration of rizatriptan with any of the β-adrenoceptor blockers. In vitro incubations showed that propranolol, but not other β-adrenoceptor blockers significantly inhibited the production of the indole-acetic acid metabolite of rizatriptan and sumatriptan.
Conclusions
These results suggest that propranolol increases plasma concentrations of rizatriptan by inhibiting monoamine oxidase-A. When prescribing rizatriptan to migraine patients receiving propranolol for prophylaxis, the 5 mg dose of rizatriptan is recommended. Administration with other β-adrenoceptor blockers does not require consideration of a dose adjustment.
Keywords: drug interactions, metoprolol, migraine headache, nadolol, pharmacodynamics, pharmacokinetics, propranolol, rizatriptan
Introduction
Rizatriptan is a novel 5-HT1B/1D receptor agonist that has been shown to be clinically effective and well tolerated in the treatment of acute migraine attacks with or without aura [1–5]. Recommended doses are 5 or 10 mg for treatment of acute migraine. The pharmacokinetics of rizatriptan are well described [6]. The drug is rapidly absorbed (tmax∼1 h), is approximately 45% bioavailable, and shows a half-life of approximately 2 h. Rizatriptan, like sumatriptan and zolmitriptan, two other 5-HT1B/1D agonists, undergoes metabolism primarily via monoamine oxidase-A, with the cytochrome P450 system playing a minor role [7–10]. In addition to the inactive indole acetic acid metabolite (MAO-A product), a quantitatively minor, but pharmacologically active N-monodesmethyl metabolite is also formed [11].
In addition to receiving acute migraine therapy with agents such as rizatriptan, patients who experience frequent migraine headaches are often prescribed prophylactic therapy in an attempt to reduce the frequency of migraine attacks. The β-adrenoceptor blocker propranolol is frequently used in this fashion [12], giving rise to a potential drug interaction between rizatriptan and propranolol. Furthermore, propranolol is known to alter the pharmacokinetics of several other drugs [13, 14], although it did not affect that of sumatriptan [15], also metabolised by MAO-A.
This report summarizes the results of double-blind, placebo-controlled, crossover investigations evaluating the influence of propranolol, nadolol, and metoprolol on the pharmacokinetics of rizatriptan in healthy subjects. We first investigated the potential for interaction between rizatriptan and propranolol administered together, at a relatively high (for migraine) propranolol dose of 120 mg twice daily. As summarized below, propranolol increased plasma concentrations of rizatriptan. Therefore, to determine whether the interaction was related to β-adrenoceptor blockade, investigations of similar design were performed with two other β-adrenoceptor blockers commonly used for migraine prophylaxis, nadolol and metoprolol. To further characterize the interaction with propranolol, the effect of reducing the dose of propranolol and/or inserting a delay between propranolol and rizatriptan administration on the pharmacokinetics of rizatriptan was also investigated. The mechanism of the interaction was explored in human microsomes.
Methods
Subjects
A total of 51 healthy male or female subjects (18–45 years) were enrolled in four double-blind, placebo-controlled investigations. Inclusion/exclusion criteria were identical for the studies. All subjects were required to be within 20% of ideal body weight based on the Metropolitan Life Height and Weight Tables. Female subjects of childbearing potential were required to have a negative pregnancy test and use barrier or intrauterine device for contraception. Oral contraceptives were not permitted because, at the time of the study, the interaction between rizatriptan and oral contraceptives had not been investigated. Subjects were excluded from the study if they were breast-feeding, or if they had a history of hypertension, or pulmonary, gastrointestinal, cardiovascular, hepatic, neurologic, endocrine, or renal disease. The use of prescription or nonprescription drugs was prohibited within 2 weeks prior to study entry and during the study, and oral contraceptives were prohibited within 1 month of study entry. The studies were approved by the institutional review board of each (of three) participating centre, and each subject gave written informed consent before participating in the study.
Study design
Initial propranolol investigation
This double-blind, randomized, three-period, placebo-controlled, crossover study was conducted at a single centre in 11 healthy subjects (6 males aged 20–31 years) to assess the effects of propranolol on the pharmacokinetics of rizatriptan and the effects of rizatriptan on the pharmacodynamics of propranolol. Eligible subjects each received in random sequence three treatment regimens for 8 days: (A) propranolol 120 mg every 12 h; (B) propranolol-placebo every 12 h; and (C) propranolol 120 mg every 12 h. On days 7 and 8, a single 10 mg dose of rizatriptan (Treatments A and C) or matching placebo (Treatment B) was administered with the morning propranolol/placebo dose. Propranolol was administered as 40 mg hard gelatin red opaque capsules (size 0), each containing four 10 mg propranolol tablets (Inderal®, Zeneca, UK). Matching placebo capsules were used for Treatment B. Each subject received all three 8 day treatment regimens according to a randomized allocation schedule, and each treatment regimen was separated by a washout period of at least 13 days.
For this study, subjects remained on the research unit overnight on day 7 and subsequently underwent a submaximal exercise test on day 8 to determine the effects of rizatriptan on the pharmacodynamics of propranolol. Approximately 2 h after administration of rizatriptan or its placebo on day 8, changes in heart rate and systolic and diastolic blood pressures were measured in response to a work load resulting in a target heart rate on a stationary bicycle (heart rate 120 beats min−1) determined at the screening visit. Subjects drank 200 ml of a glucose drink (Lucozade) approximately 5 min before exercise testing. The product of systolic blood pressure and heart rate was selected as the primary pharmacodynamic index.
Second propranolol investigation
This study was initiated at a different centre to clarify the results of the initial study. It was a double-blind, randomized, five-treatment, three-period, balanced incomplete block, placebo-controlled, crossover study involving 20 healthy subjects (10 males). The study was designed to assess the effects of reducing the propranolol dose and/or staggering the timing of propranolol and rizatriptan dosing on the pharmacokinetics of rizatriptan. In addition, the influence of propranolol on plasma concentrations of the minor, but active N-monodesmethyl (NMDM) metabolite was examined. An incomplete block design was used to limit the number of periods a subject might receive propranolol because, in the initial study, several subjects discontinued due to intolerance of propranolol before receiving rizatriptan. Eligible subjects were randomized to receive the following treatment regimens:
Propranolol-placebo twice daily (days 1–7) + rizatriptan 10 mg administered concomitantly on day 7
Propranolol 60 mg twice daily (days 1–7) + rizatriptan 10 mg administered concomitantly on day 7
Propranolol 60 mg twice daily (days 1–7) + rizatriptan 10 mg 1 hr after propranolol dosing on day 7
Propranolol 120 mg twice daily (days 1–7) + rizatriptan 10 mg 1 hr after propranolol dosing on day 7
Propranolol 120 mg teice daily (days 1–7) + rizatriptan 10 mg 2 hr after propranolol dosing on day 7
Subjects received a total of three of five treatment regimens according to a randomized allocation schedule, and each treatment regimen was separated by a washout period of at least 7 days.
Nadolol/metoprolol investigations
Two double-blind, randomized, two-period, placebo-controlled, crossover investigations were conducted at a third centre in 25 healthy subjects (13 males, aged 22–44 years) to assess the effects of two other β-adrenoceptor blockers used for migraine prophylaxis on the pharmacokinetics of rizatriptan. Eligible subjects were randomized to receive one of three treatment regimens for 7 days: (A) nadolol, 80 mg every 12 h; (B) metoprolol, 100 mg every 12 h; and (C) placebo every 12 h. On day 7, a 10 mg dose of rizatriptan and the other study treatments were administered together. Nadolol was administered as two hard red opaque gelatin capsules (size A1), each containing one 40 mg nadolol tablet; capsules containing one 50 mg metoprolol tablet were prepared in similar fashion, as were matching placebo capsules. Thus each subject received two capsules every 12 h for 7 days in each treatment period, with order of treatments (β-adrenoceptor blocker vs placebo) and β-adrenoceptor blocker (nadolol or metoprolol) assigned according to a randomized allocation schedule.
Pharmacokinetic sampling
Identical procedures were followed in each study for the assessment of rizatriptan pharmacokinetics. On day 7 of treatment with β-adrenoceptor blocker or placebo, subjects reported to the clinic after an overnight fast. They were allowed free access to water, but alcohol or caffeine-containing drinks were not permitted. Subjects were given 200 ml of a glucose drink (e.g. Lucozade) 3 h after the rizatriptan dose. A light lunch was provided at 5 h post dosing and an evening meal at 10 h post dosing. On all other study days, subjects were allowed to consume their normal diets without regard to dosing times. Blood samples (10 ml) were collected from an indwelling intravenous catheter at 0 (predose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 16 h after administration of rizatriptan on day 7 and analysed for rizatriptan and, in the second propranolol study only, for its NMDM metabolite.
Drug analysis
Plasma samples were stored frozen at −20 °C until assayed. Concentrations of rizatriptan and N-monodesmethyl rizatriptan in plasma and urine were determined by a liquid chromatography-mass spectrometric/mass spectrometric (LC-MS/MS) method in the positive ion mode, using the N-diethyl analogue of rizatriptan as internal standard [16]. The assay was run using a Sciex API III mass spectrometer equipped with an upgraded collision cell interfaced via Sciex's heated nebulizer to a Hewlett Packard 1050 liquid chromatography system. For plasma, limits of detection for the assay were 0.5 and 0.2 ng ml−1 for rizatriptan and its metabolite, respectively. Intra- and interassay precision (coefficient of variation, n = ∼5 replicates for intra-assay and 9–10 replicates for interassay assessments) averaged 10% or less for parent and metabolite concentrations of 0.5–100 ng ml−1. For urine, limits of detection for the assay were 10 and 4 ng ml−1 for rizatriptan and its metabolite, respectively. Intra- and interassay precision (coefficient of variation, n = 4–5) averaged 10% or less for parent and metabolite concentrations from 10 to 2500 ng ml−1.
Pharmacokinetic parameters calculated from rizatriptan and metabolite plasma concentration data included area under the concentration-time curve 0 to infinity (AUC(0,∞)), maximal plasma concentration (Cmax), time to achieve maximal plasma concentration (tmax), plasma apparent half-life (t½), and renal clearance (CLr).
Safety assessments
Adverse experiences were monitored throughout the study and were defined as any treatment emergent unfavourable and unintended change in the structure, function, or chemistry of the body, or a worsening of a pre-existing condition. Investigators evaluated all clinical adverse experiences in terms of intensity (mild, moderate, or severe), duration, seriousness, outcome, and relation to test drugs.
Statistical analysis
The statistical significance of differences in pharmacokinetic parameters between treatment regimens was assessed using analysis of variance(anova) models appropriate for each crossover design. The pharmacokinetic parameters, AUC(0,∞) and Cmax of rizatriptan or its NMDM metabolite were natural log transformed prior to analysis. Confidence intervals were calculated from the differences in the least squares means of the natural log transformed data between relevant treatments, and were back-transformed to confidence intervals for the geometric mean ratios. A confidence interval which exceeded (0.7, 1.43) indicated a difference of potential clinical significance. These confidence intervals for the differences in the least squares means of the natural log responses were calculated using the MSE from the appropriate anova referencing a t-distribution. Pharmacodynamic data were compared among treatment groups using analysis of variance(anova) models appropriate for each crossover design.
Specifically, for the balanced incomplete block study, the final anova model including gender, subject (gender), period, and treatment was used to estimate the geometric means, their ratios, and the appropriate confidence intervals for the AUC(0,∞) and Cmax for rizatriptan, its metabolite, and the ratio (rizatriptan/metabolite). Since AUC(0,∞) and Cmax were log transformed, the least square means, their differences and confidence interval limits were exponentiated to determine the geometric means, their ratios, and corresponding confidence limits. The same model was also used to estimate the least square means, the estimated differences between the least square means, and the 95% one-sided confidence intervals for the mean difference (90% two-sided confidence intervals) of the untransformed tmax, elimination rate constant, urinary excretion, and renal clearance of both rizatriptan and its metabolite, NMDM. All of the above comparisons were made for each of the four combination treatment groups (rizatriptan and propranolol) relative to rizatriptan given alone.
All of the pairwise comparisons between the five treatment regimens were calculated for AUC(0,∞) and Cmax of rizatriptan using this final anova model. The 95% two-sided confidence intervals were generated using the least square means from this anova model for the log transformed AUC(0,∞) and Cmax of rizatriptan. The confidence limits were exponentiated to determine the confidence intervals for the appropriate geometric mean ratio.
In vitro studies
Pooled S9 (i.e. microsomal) fractions were prepared from the stored liver sections of two subjects (IIAM, Exton, PA) as follows: Sections of these livers were combined (91 g wet wt. total), homogenized in two volumes of 0.05 m Tris-1.15% potassium chloride, pH 7.5 buffer and centrifuged at 1000 g to remove cell debris. The supernatant was subjected to repeat centrifugation at 9000 g and the resultant supernatant, the S9 fraction, was divided into 11 ml aliquots and stored at −70 °C. The protein concentration was 57.2 mg ml−1. [14C]-rizatriptan and [14C]-sumatriptan (50 µm, ∼4–5% apparent Km) were incubated (1 ml volume) in a medium containing 100 µmol phosphate buffer, 3 µmol MgCl2, pH 7.4, and 0.5 µmol NADPH. After incubation for 1 h at 37 °C, the indole-3-acetic acid metabolites of each compound were quantified by radio-h.p.l.c. Five β-adrenoceptor blockers, propranolol, metoprolol, nadolol, timolol and atenolol, were evaluated at concentrations of 10–250 µm for their effects on formation of the respective indole acetic acid metabolites of rizatriptan and sumatriptan.
Results
Effects of propranolol on rizatriptan pharmacokinetics
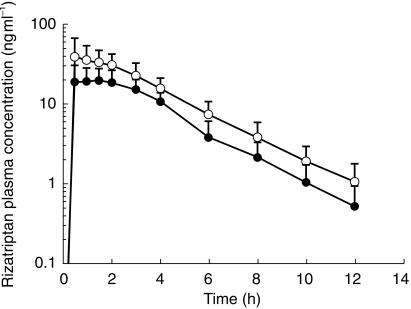
The plasma rizatriptan concentration profile and the principal rizatriptan pharmacokinetic parameters with and without propranolol are shown in Figure 1 and Table 1. Administration of 10 mg rizatriptan resulted in an AUC(0,∞) and Cmax of 94 ng ml−1 h and 26 ng ml−1, respectively. When rizatriptan was given during propranolol treatment (120 mg twice daily for 7 days), the AUC(0,∞) and Cmax for rizatriptan were increased. The geometric mean ratios (rizatriptan with propranolol/rizatriptan alone) for AUC(0,∞) and Cmax were 1.67 (90% CI 1.44, 1.93) and 1.75 (90% CI 1.41, 2.17), respectively. Both geometric mean ratios were significantly greater than 1 (P < 0.001). Based on protocol specified criteria, these results indicate an effect of potential clinical significance (i.e. the upper limit of the confidence interval was greater than 1.43). One subject (subject 4, male) showed a 3.7-fold increase in the AUC(0,∞) for rizatriptan when rizatriptan was administered in combination with propranolol. This subject was otherwise clinically indistinguishable from the other subjects.
Figure 1.
Rizatriptan concentration-time profiles in healthy subjects receiving rizatriptan 10 mg on day 7 with (○) or without (•) co-administration of propranolol (120 mg twice daily for 7.5 days). Each point represents the mean (± s.d.) of 11 subjects (Study 1).
Table 1.
Mean (± s.d.) pharmacokinetic parameters for rizatriptan in healthy subjects receiving rizatriptan 10 mg with or without coadministration of propranolol (120 mg twice daily for 7.5 days), n = 11.
| Parameter | Without propranolol | With propranolol | Geometric mean ratio (90% CI) |
|---|---|---|---|
| AUC(0,∞) (ng ml−1 h) | 94 ± 32a | 159 ± 60 | 1.67 (1.44, 1.93)b |
| Cmax (ng ml−1) | 26 ± 9a | 47 ± 24 | 1.75 (1.41, 2.17). c |
| tmax (h) | 1.4 ± 0.9 | 1.0 ± 0.6 | – |
| Keld (h−1) | 0.33 | 0.25 | – |
| t1/2e (h) | 2.2 | 2.8 | – |
AUC and Cmax means are geometric means.
AUC of rizatriptan with propranolol/AUC of rizatriptan without propranolol
Cmax of rizatriptan with propranolol/Cmax of rizatriptan without propranolol
arithmetic mean
harmonic mean
AUC(0,∞)=area under the concentration-time curve from 0 to infinity; Cmax=maximal plasma concentration; Kel=elimination rate constant; tmax=time to achieve maximal plasma concentration; t½=plasma apparent half-life.
A nonsignificant decrease in tmax for rizatriptan occurred when rizatriptan was administered in combination with propranolol (Table 1). The elimination rate constant for rizatriptan was significantly lower when rizatriptan was administered in combination with propranolol compared with administration with placebo (0.25 vs 0.33 h−1, P = 0.025), corresponding to a minor prolongation of t½ by about 40 min, from 2.2 to 2.8 h.
Effect of rizatriptan on the pharmacodynamic response to propranolol during exercise
During propranolol treatment, resting (pre-exercise) blood pressure and heart rate were reduced. As expected, changes in heart rate and the product of heart rate and systolic blood pressure in response to submaximal exercise (RPP=rate Pressure Product) were attenuated by propranolol administration and were not influenced by rizatriptan. Respective increases in RPP (Mean [s.d.]) were 8210 [2690] and 6046 [2469] beats min−1 mmHg following rizatriptan alone and rizatriptan with propranolol (P < 0.05 for difference in exercise induced increases in RPP on the two treatments). During propranolol alone, RPP increased 5968 [2265] beats min−1 mmHg.
Effect of reducing or delaying propranolol dosing on rizatriptan pharmacokinetics
On review of the results summarized above, it was noted that the dose of propranolol used in the initial study was relatively high, although within the recommended dose range for migraine prophylaxis [12]. Furthermore, rizatriptan was administered simultaneously with propranolol, a situation considered unlikely to occur in clinical practice when migraine occurs at random during the day and prophylactic medications are taken at about the same time each day. Simultaneous administration might be expected to enhance the effect (if any) of propranolol on first pass metabolism of rizatriptan. Accordingly, a follow-up study was designed to investigate the influence of a reduction in propranolol dose and/or the insertion of a delay between propranolol and rizatriptan administrations on the effect of propranolol on rizatriptan plasma concentrations. Table 2 summarizes the results of this study.
Table 2.
Effect of reducing the dose of propranolol and/or inserting a delay between propranolol and rizatriptan administration on pharmacokinetic parameters for rizatriptan in 12 healthy subjects
| Rizatriptan pharmacokinetic parameters (arithmetic means±s.d.) (n = 12) | |||||
|---|---|---|---|---|---|
| Parameter | 10 mg rizatriptan+ placebo propranolol | 10 mg Rizatriptan+ 60 mg propranolol | 10 mg rizatriptan 1 h after 60 mg propranolol | 10 mg rizatriptan 1 h after 120 mg propranolol | 10 mg rizatriptan 2 h after 120 mg propranolol |
| AUC(0,∞) (ng ml−1 h) | 61.8 ± 12.1 | 80.9 ± 16.6 | 84.7 ± 27.8 | 89.3 ± 21.9 | 86.3 ± 22.9 |
| Geometric mean AUC ratio | 1.38* | 1.42* | 1.53* | 1.45* | |
| vs rizatriptan alone | (1.24, 1.54) | (1.27, 1.58) | (1.37, 1.71) | (1.30, 1.61) | |
| (97.5% confidence interval) | |||||
| Cmax (ng ml−1) | 19.5 ± 4.8 | 25.0 ± 4.8 | 27.1 ± 9.9 | 24.8 ± 7.9 | 27.2 ± 10.1 |
| Geometric mean Cmax ratio vs | 1.39* | 1.48* | 1.29* | 1.45* | |
| rizatriptan alone | (1.21, 1.60) | (1.29, 1.70) | (1.12, 1.49) | (1.26, 1.67) | |
| (90% confidence interval) | |||||
| tmax (h) | 0.9 ± 0.5 | 0.8 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.7 | 1.3 ± 0.7 |
| Kela | 0.379 | 0.361 | 0.379 | 0.363 | 0.337 |
| (0.335, 0.423) | (0.317, 0.405) | (0.335, 0.423) | (0.319, 0.407) | (0.293, 0.381) | |
| t½ (h)b | 1.9 | 2.0 | 1.7 | 2.0 | 1.9 |
| Ue (%) | 9.7 ± 3.0 | 11.5 ± 3.2 | 11.0 ± 4.0 | 14.8 ± 4.8 | 10.6 ± 3.3 |
| CLR (ml min−1) | 261.5 ± 64.6 | 242.8 ± 72.8 | 227.4 ± 85.9 | 294.3 ± 119.4 | 229.9 ± 134.8 |
P < 0.01 vs 10 mg rizatriptan+propranolol placebo.
Arithmetic mean (95% confidence interval).
Harmonic mean.
AUC(0,∞)=area under the concentration-time curve from 0 to infinity; Cmax=maximal plasma concentration; tmax=time to achieve maximal plasma concentration; Kel=elimination rate constant; CLR=renal clearance.
All four combination treatments in the second study resulted in a significant increase in AUC(0,∞) for rizatriptan, compared to control. Although the upper bounds of the 90% confidence intervals exceeded 50% in all groups, only the geometric mean ratio for treatment D (120 mg propranolol twice daily, 1 h delay) exceeded the limit defined prospectively as being of potential clinical significance. Subtle, nonsignificant differences in AUC(0,∞) for rizatriptan were observed among the four combination treatments. Overall, reducing the dose of propranolol and/or inserting a 1 or 2 h delay between propranolol and rizatriptan administration failed to markedly reduce the effect of propranolol on rizatriptan AUC(0,∞) and Cmax, although, on the average, geometric mean increases in AUC(0,∞) and Cmax were less in the second study (≤ 53% vs 67% for AUC(0,∞), and ≤48% vs 75% for Cmax). Furthermore, in contrast to the initial study, changes in rizatriptan elimination rate/half-life were not observed (the harmonic mean half-life was ≤ 2 h for all treatments) (Table 2).
The renal clearance of rizatriptan was not measured in the initial study. In the second study, renal elimination of intact rizatriptan was not significantly affected by propranolol treatment (Table 2).
Assuming the effect of propranolol on rizatriptan pharmacokinetics was based on changes in rizatriptan metabolism, it was of interest to determine whether the pharmacokinetics of the usually minor, but active, NMDM metabolite of rizatriptan were altered. Inhibition of rizatriptan metabolism via this pathway might decrease plasma concentrations of this metabolite. Compared with placebo (rizatriptan plus propranolol placebo), none of the four combination treatments resulted in a clinically or statistically significant change in the AUC(0,∞) the active rizatriptan NMDM metabolite. Small but significant decreases in metabolite Cmax were observed in the two treatments that included 120 mg propranolol. The least squares mean Cmax values (95% CI) were 2.01 (1.83, 2.22) ng ml−1 with propranolol placebo and 1.42 (1.29, 1.56) and 1.71 (1.56, 1.89) ng ml−1 for 120 mg propranolol with 1 and 2 h delays, respectively. tmax, t½, and renal clearance of the metabolite were unchanged.
Effects of nadolol and metoprolol on rizatriptan pharmacokinetics
Nadolol, at a maximum recommended dose of 80 mg twice daily and metoprolol, 100 mg twice daily were investigated for their effects on the pharmacokinetics of rizatriptan. A 1 week treatment with either of these β-adrenoceptor blockers did not alter the pharmacokinetics of rizatriptan to a clinically significant degree. Geometric mean AUC ratios and 90% confidence limits (combination/rizatriptan alone) were 1.08 (0.96, 1.21) for nadolol (P = 0.27) and 1.07 (1.00, 1.15) for metoprolol (P = 0.087). Corresponding ratios for Cmax were 1.16 (0.95, 1.43) for nadolol (P = 0.20) and 0.92 (0.81, 1.03) for metoprolol (P = 0.22).
Safety
No serious clinical, laboratory, or other adverse experiences occurred during study I. Three patients reported somnolence, headache, and/or paresthesia on the day that they received rizatriptan. A total of three patients discontinued the initial study (two because of clinical adverse experiences related to propranolol, and one due to an ECG abnormality which was not identified at screening). All of these events occurred prior to administration of rizatriptan. One subject discontinued the nadolol/metoprolol study for hypotension during nadolol treatment.
In vitro incubations
In vitro experiments in human S9 fractions were performed to assess directly the potential for propranolol to alter the metabolism of rizatriptan. For rizatriptan and sumatriptan, propranolol markedly inhibited the formation of this metabolite. Inhibition was concentration-dependent. Thus the addition of 10, 50, 100 and 250 µm propranolol resulted in 21, 38, 71 and 79% inhibition of the formation of the rizatriptan metabolite and 20, 50, 67 and 85% inhibition of the formation of the sumatriptan metabolite, respectively. Metoprolol (100 µm) had a modest effect, causing 26% inhibition of formation of the metabolite of sumatriptan, compared with 67% for propranolol. 11% or less inhibition was observed for atenolol, nadolol and timolol.
Discussion
The first of these double-blind, placebo-controlled, crossover studies in healthy subjects showed that administration of rizatriptan during propranolol treatment resulted in a statistically and potentially clinically significant increase in the AUC(0,∞) and Cmax for rizatriptan and a modest, but statistically significant, prolongation of rizatriptan t½. One subject had a four-fold increase in rizatriptan AUC(0,∞). In the second study, a reduction in the dose of propranolol and/or incorporation of a delay between propranolol and rizatriptan administration did not eliminate the effect of propranolol on the pharmacokinetics of rizatriptan. However, as far as comparisons between studies are reasonable, the effects of propranolol on rizatriptan pharmacokinetics were somewhat less in the second study with the largest increase in rizatriptan AUC being about two-fold. Propranolol did not significantly alter the pharmacokinetics of the minor, but active, N-monodesmethyl metabolite of rizatriptan. Nadolol and metoprolol had no detectable effect the pharmacokinetics of rizatriptan.
In the first study, administration of rizatriptan during propranolol treatment did not alter the effects of the latter on exercise tolerance. With the exception of several subjects who discontinued the study because of known adverse effects of propranolol and nadolol, all treatments were generally well tolerated.
The mechanism(s) whereby propranolol increased the plasma concentrations of rizatriptan cannot be ascertained from this clinical study. However, based on the in vitro data summarized above, one possible mechanism is that propranolol, or a metabolite of propranolol, e.g. N-desmethyl propranolol [17, 18], competitively inhibits monoamine oxidase A, a key enzyme involved in the metabolism of rizatriptan (and sumatriptan) to its indole acetic acid metabolite. The principal route of rizatriptan clearance is oxidative deamination, resulting in approximately 50% to 60% of an oral rizatriptan dose being eliminated in the urine as the indole-3-acetic acid metabolite. Only limited metabolism of rizatriptan occurs by non-MAO pathways such as cytochrome P450. These in vivo and in vitro results are consistent with the hypothesis that propranolol or its metabolite competitively inhibits rizatriptan metabolism via MAO, thereby leading to a decrease in the metabolic clearance and an increase in the oral bioavailability of rizatriptan. The in vitro results were partially confirmed by the absence of detectable effects of nadolol and metoprolol on the pharmacokinetics of rizatriptan.
This effect of propranolol on rizatriptan is not unique to this class of 5-HT1B/1D agonists. Peck and colleagues have shown that propranolol increases the plasma concentrations of zolmitriptan [9]. In that study, pretreatment of healthy volunteers with propranolol (160 mg for 7 days) increased the mean AUC and Cmax of a single 10 mg dose of zolmitriptan by 56% and 37%, respectively, effects which are comparable with those in our second propranolol study. The authors speculated that the increase in plasma concentrations of zolmitriptan resulted from a decrease in the conversion of zolmitriptan to its metabolites.
An effect of propranolol on the pharmacokinetics of sumatriptan might also be predicted from our in vitro data. However, a study by Scott and colleagues reported no effect of propranolol (80 mg twice daily for 7 days) on the pharmacokinetics of sumatriptan (single oral 300 mg dose) [15]. The reason for this lack of interaction is unclear, but it is possible that a combination of a somewhat lower dose of propranolol with the much higher dose of sumatriptan (relative to the doses of rizatriptan and zolmitriptan) may have limited the effect of propranolol on the metabolism of sumatriptan. As the authors point out, the possibility exists that administration of higher doses of propranolol (e.g. 320 mg daily) might have produced an alteration in the pharmacokinetics of sumatriptan. In this regard, it would be of interest to investigate the effects of a higher dose of propranolol, such as that used in the present study, on plasma sumatriptan concentrations following a lower oral sumatriptan dose.
Since rizatriptan undergoes extensive first-pass metabolism following oral administration, a window of up to 1 h might exist during which the competitive interaction between propranolol and rizatriptan would be apparent. In addition, since the 120 mg twice daily dose of propranolol used in the initial study represents a relatively high dose for migraine prophylaxis, we investigated whether administration of a lower, more typical dose of propranolol (60 mg twice daily) and/or inserting a delay between propranolol and rizatriptan dosing might reduce the interaction between propranolol and rizatriptan. The results of this study showed that neither reducing the dose of propranolol nor inserting a delay between propranolol and rizatriptan administration eliminated the effect of propranolol on the AUC(0,∞) or Cmax of rizatriptan, although the extent of the interaction was somewhat less.
As explored in the second study, the effects of propranolol did not extend to the active metabolite of rizatriptan. Although this metabolite is not a product of MAO-A, it is a substrate of the enzyme. The selective MAO-A inhibitor, moclobemide, has been shown to cause a twofold increase in the AUC of rizatriptan and more than a fivefold increase in that of the monodesmethyl metabolite [8]. As a result, rizatriptan, sumatriptan and zolmitriptan are contraindicated during treatment with monoamine oxidase inhibitors. The effects of propranolol on rizatriptan pharmacokinetics are much smaller, although one subject in our first study did show a fourfold increase in rizatriptan AUC(0,∞). Thus these findings suggest that some care should be exercised when administering rizatriptan to patients receiving propranolol for migraine prophylaxis, and use of the 5 mg dose is recommended. This dose provides effective relief of headache and migraine-associated symptoms [1, 3]. We speculate that administration with propranolol might enhance the efficacy of rizatriptan given at this dose. Furthermore, even though in the USA labelling for zolmitriptan does not recommend caution during concommitant treatment with propranolol, the magnitude of the interaction for this compound was comparable with that observed with rizatriptan [9].
In conclusion, propranolol therapy was associated with an increase in the AUC(0,∞) and Cmax for rizatriptan, an effect that persisted in spite of a reduction in the dose of propranolol and/or incorporation of a delay between propranolol and rizatriptan administration. Nevertheless, administration of 10 mg rizatriptan in combination with propranolol was well tolerated and was not associated with an alteration in the pharmacodynamic effects of propranolol during submaximal exercise. In patients receiving propranolol for migraine prophylaxis, it is recommended that the 5 mg dose of rizatriptan should be prescribed. Our data also suggest that the full range of rizatriptan dose can be used during co-treatment with nadolol, metoprolol, atenolol or timolol.
Acknowledgments
Susan Ermlich and Michele Hyman are acknowledged for their assistance in study management. Drs William Prince, William Carey, and Guido Somers (deceased) are acknowledged for conducting the clinical aspects of these studies. J. D. Rogers, Mary Jo Brucker, Debra McLoughlin are acknowledged for their assistance in assay development, and sample and pharmacokinetic analyses. Elaine Landes is acknowledged for her assistance in manuscript preparation.
References
- 1.Teall J, Tuchman M, Cutler N, et al. Rizatriptan (MAXALT) for the acute treatment of migraine and migraine recurrence. A placebo-controlled, outpatient study. Rizatriptan 022 Study Group. Headache. 1998;38:281–287. doi: 10.1046/j.1526-4610.1998.3804281.x. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Matzura-Wolfe D, Polis A, et al. A placebo-controlled crossover study of rizatriptan in the treatment of multiple migraine attacks. Rizatriptan Multiple Attack Study Group. Neurology. 1998;51:773–781. doi: 10.1212/wnl.51.3.773. [DOI] [PubMed] [Google Scholar]
- 3.Gijsman H, Kramer MS, Sargent J, et al. Double-blind, placebo-controlled, dose-finding study of rizatriptan (MK-462) in the acute treatment of migraine. Cephalalgia. 1997 October;17(6):647–651. doi: 10.1046/j.1468-2982.1997.1706647.x. [DOI] [PubMed] [Google Scholar]
- 4.Visser WH, Terwindt GM, Reines SA, et al. Rizatriptan vs sumatriptan in the acute treatment of migraine. A placebo-controlled, dose-ranging study. Dutch/US Rizatriptan Study Group. Arch Neurol. 1996;53:1132–1137. doi: 10.1001/archneur.1996.00550110072014. [DOI] [PubMed] [Google Scholar]
- 5.Cutler NR, Claghorn J, Sramek JJ, et al. Pilot study of MK-462 in migraine. Cephalagia. 1996;16:113–116. doi: 10.1046/j.1468-2982.1996.1602113.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MR, Lowry RC, Musson DG, et al. Lack of Pharmacokinetic and pharmacodynamic interaction between rizatriptan and paroxetine. J Clin Pharmacol. 1999;39:192–199. doi: 10.1177/00912709922007633. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter D, Halpin RA, Davis MR, Maryanchik A, Walsh D, Vyas KP. The in vitro metabolism of rizatriptan by human liver subcellular fractions. ISSX. 1996;10:372. [Google Scholar]
- 8.Van Haarst AD, van Gerven JMA, Cohen AF, et al. The effects of moclobemide on the pharmacokinetics of the 5-HT1B/1D agonist rizatriptan in healthy volunteers. Br J Clin Pharmacol. 1999;48:190–196. doi: 10.1046/j.1365-2125.1999.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peck RW, Seaber EJ, Dixon R, et al. The interaction between propranolol and the novel antimigraine agent zolmitriptan (311C90) Br J Clin Pharmacol. 1997;44:595–599. doi: 10.1046/j.1365-2125.1997.t01-1-00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon CM, Park GR, Tarbit MH. Characterization of the enzyme responsible for the metabolism of sumitriptan in human liver. Biochem Pharmacol. 1994;47:1253–1257. doi: 10.1016/0006-2952(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 11.Vyas KP, Halpin RA, Geer LA, et al. Disposition and pharmacokinetics of the antimigraine drug, rizatriptan, in humans. Drug Metab Dispos. 2000;28:89–95. [PubMed] [Google Scholar]
- 12.Welch KMA. Drug therapy of migraine. N Engl J Med. 1993;329:1476–1483. doi: 10.1056/NEJM199311113292008. [DOI] [PubMed] [Google Scholar]
- 13.Melmon KL, Nierenberg DW. Drug interactions and the prepared observer. N Engl J Med. 1981;304:723–725. doi: 10.1056/NEJM198103193041208. [DOI] [PubMed] [Google Scholar]
- 14.Bax NDS, Lennard MS, Tucker GT. Inhibition of antipyrine metabolism by beta-adrenoreceptor antagonists. Br J Clin Pharmacol. 1981;12:779–784. doi: 10.1111/j.1365-2125.1981.tb01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott AK, Walley T, Breckenridge AM, et al. Lack of an interaction between propranolol and sumatriptan. Br J Clin Pharmacol. 1991;32:581–584. doi: 10.1111/j.1365-2125.1991.tb03955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin DA, Olah TV, Ellis JD, Gilbert JD, Hapin RA. Quantitation of the 5HT1D agonists MK-462 and sumatriptan in plasma by liquid chromatography-tmospheric pressure chemical ionization mass spectrometry. Chromatography. 1996;726:115–124. doi: 10.1016/0021-9673(96)88660-2. [DOI] [PubMed] [Google Scholar]
- 17.Benedetti MS, Dostert P. Contribution of amine oxidases to the metabolism of xenobiotics. Drug Metab Rev. 1994;26:507–535. doi: 10.3109/03602539408998316. [DOI] [PubMed] [Google Scholar]
- 18.Walle T, Walle UK, Olanoff LS. Quantitative account of propranolol metabolism in urine of normal man. Drug Metab Dispos. 1985;13:204–209. [PubMed] [Google Scholar]