Abstract
Aims
To study the influence of CYP2D6*10 on the formation of p-hydroxymexiletine (PHM) and hydroxymethylmexiletine (HMM) using microsomes from human liver of known genotypes.
Methods
Microsomes from human livers of genotype CYP2D6*1/*1 (n = 5), *1/*10 (n = 6) and *10/*10 (n = 6) were used in this study. The formation of PHM and HMM was determined by high-performance liquid chromatography.
Results
The formation rates of PHM and HMM were decreased by more than 50% and 85% in CYP2D6*1/*10 and *10/*10 microsomes, respectively, compared with *1/*1 microsomes.
Conclusions
The metabolism of mexiletine to form PHM and HMM appears to be impaired to a significant extent in human liver microsomes from hetero- and homozygotes of CYP2D6*10.
Keywords: CYP2D6*10, human liver microsomes, mexiletine
Introduction
Mexiletine is a class 1b antiarrhythmic agent used for the treatment and prophylaxis of ventricular dysrhythmias [1]. Hydroxylation is a major route of metabolism for mexiletine in humans resulting in the formation of p-hydroxymexiletine (PHM) and hydroxymethylmexiletine (HMM), both of which lack significant antiarrhythmic activity [1]. Several in vivo studies [2, 3] have suggested that the formation of PHM and HMM is mediated by CYP2D6, an isoform of cytochrome P450 showing genetic polymorphism. There are more than 30 mutant alleles of CYP2D6 which are associated with deficient, decreased or increased activity of the enzyme [http://www.imm.ki.se/CYPalleles/cyp2d6.htm].
CYP2D6*10 is the most frequently seen mutant allele in Oriental populations [4, 5] and is associated with a decreased metabolic clearance of CYP2D6 substrates [5–7]. However there is little information regarding the influence of CYP2D6*10 on the metabolism of mexiletine. In the present study, we examined the influence of CYP2D6*10 on the formation rates of PHM and HMM using microsomes prepared from the liver possessing CYP2D6*1/*1 (wild-type), *1/*10 and *10/*10 genotypes.
Methods
Human liver samples were obtained from Japanese patients undergoing partial hepatectomy for the treatment of metastatic tumours. These patients did not take any known inducers or inhibitors of cytochrome P450 and did not have a history of alcohol intake or drug abuse. Livers were immediately frozen in liquid nitrogen. Informed consent for the use of tissue was obtained from each patient. Genomic DNA was isolated from liver tissue using DNAZOL Reagent (GibcoBRL, ML, USA) and genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) with mismatch-PCR or long-PCR analysis using allele-specific primers as reported previously [5]. Livers with the genotypes of CYP2D6*1/*1 (n = 5), *1/*10 (n = 6) and *10/*10 (n = 6) were selected for this study and the microsomes were prepared by differential ultracentrifugation [8]. A microsomal sample of CYP2D6*5/*5, which is a defective allele of CYP2D6, was also included in the study as a negative control. Protein concentration was measured with a Protein Kit (Bio-Rad, Hercules, USA). A typical incubation mixture (0.25 ml total volume) contained 100 mm potassium phosphate buffer (pH 7.4), an NADPH-generating system (0.5 mm NADP+, 2 mm glucose 6-phosphate, 4 mm MgCl2 and 1 unit ml−1 glucose 6-phosphate dehydrogenase), 0.1 mm EDTA, 0.2 or 0.5 mg ml−1 of microsomal protein and mexiletine as the substrate [9]. After preincubation at 37 °C for 5 min, the reaction was initiated by addition of the NADPH-generating system and the incubation was continued for 20 min at 37 °C. The reaction was terminated by the addition of 0.2 ml of 0.3 m H3PO4. All incubations were prepared in duplicate. After the addition of an internal standard (lignocaine 250 ng), the reaction mixture was extracted with diethylether and metabolites were back-extracted into 0.2 ml of 0.01 n HCl.
The hydroxylated metabolites of mexiletine in 0.1 ml of the extract were analysed by high-performance liquid chromatography on a system equipped with an analytical STR ODS-II column (4.6 mm i.d. × 150 mm, 5 µm, Shimadzu, Kyoto, Japan), a type-550 fluorescence detector (Shimadzu, Kyoto, Japan) and a type-486 u.v. detector (Waters, Milford, USA). Fluorescence conditions were set at 270 nm for excitation and at 312 nm for emission to detect PHM and HMM, and the u.v. detector was set at 210 nm to detect the internal standard. A gradient system was used for the separation of the analytes. The composition of the mobile phase was as follows: the initial mobile phase consisted of 150 ml l−1 acetonitrile−850 ml l−1 44 mm phosphate buffer containing 0.5% triethylamine (pH 2.6). The composition was changed to 250 ml l−1 acetonitrile−750 ml l−1 44 mm phosphate buffer containing 0.5% triethylamine (pH 2.6) using a linear gradient over 6 min, and was maintained for 4 min. Thereafter, the acetonitrile content was decreased to 15% over 1 min. The equilibration time was 4 min between runs. The flow rate was 1.0 ml min−1 and the column temperature was 35 °C. Samples were analysed in duplicate and a standard curve was included in each analysis run. The lower detection limit of the PHM assay was 0.1 µm, the intra-assay coefficient of variation was 2.3% and the interassay coefficient of variation was 9.2% in the concentration range of 0.1–2.0 µm. The corresponding values were 0.1 µm, 10.0% and 13.0% for the HMM assay, respectively.
For the kinetic study, one of 5 or 6 microsomal samples of CYP2D6*1/*1, *1/*10 or *10/*10 was incubated with 5–200 µm mexiletine. To determine values of Km and Vmax, the Michaelis-Menten equation was fitted to the data by means of nonlinear regression analysis.
Statistical comparisons were made by use of Student's or Welch's t-test.
Results
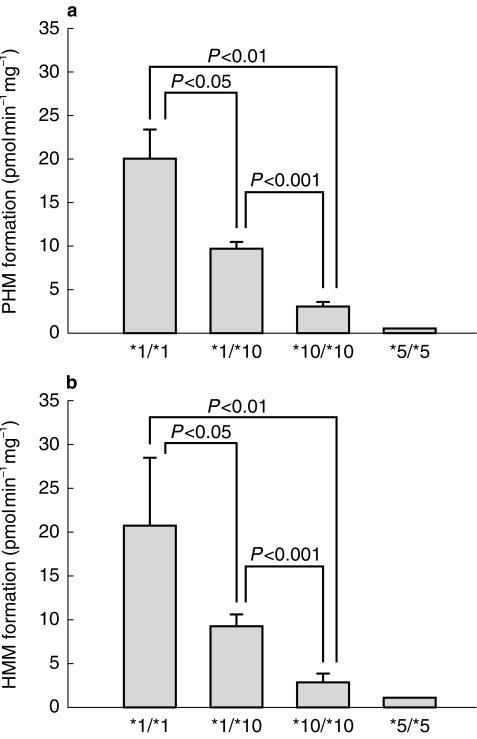
Formation rates of PHM and HMM in microsomes of livers of genotype CYP2D6*1/*1, *1/*10, *10/*10 and *5/*5 are shown in Figure 1. The mean (± s.d.) formation rates of PHM and HMM were significantly (P < 0.01 or 0.05) lower in human liver microsomes of CYP2D6*1/*10 and *10/*10 microsomes as compared with those of *1/*1 microsomes. Mean (± s.d.) rates of formation of PHM were 9.9 ± 1.8 and 3.1 ± 1.2 pmol min−1 mg−1 in CYP2D6*1/*10 and *10/*10 microsomes, which were 50% and 85% lower than those in *1/*1 microsomes (20.0 ± 7.5 pmol min−1 mg−1), respectively. Similarly, the corresponding values of HMM were 9.4 ± 1.5 and 2.8 ± 1.1 pmole min−1 mg−1 in CYP2D6*1/*10 and *10/*10, which were 53% and 87% lower than those in *1/*1 (20.8 ± 7.7 pmol min−1 mg−1), respectively. The formation rates of PHM and HMM in microsomes of CYP2D6*5/*5 microsomes were less than 5% of those from *1/*1 genotyped livers.
Figure 1.
Mexiletine hydroxylation activity in microsomes from human livers genotyped as CYP2D6*1/*1 (n = 5), *1/*10 (n = 6), *10/*10 (n = 6) and *5/*5 (n = 1). (a) PHM formation and (b) HMM formation. Each bar represents the mean value and the error bar represents s.d.
The kinetic parameters for the formation of PHM in CYP2D6*1/*1, *1/*10 and *10/*10 microsomes were: Km = 16, 40 and 127 µm, Vmax=26, 11 and 6 pmol min−1 mg−1 and Vmax/Km = 1.6, 0.3 and 0.05 ml min−1 mg−1, respectively. Corresponding values for HMM in CYP2D6*1/*1 and *1/*10 microsomes were: Km = 15 and 49 µm, Vmax=28 and 14 pmol min−1 mg−1 and Vmax/Km = 1.8 and 0.3 ml min−1 mg−1, respectively. The kinetic parameters for HMM in CYP2D6*10/*10 microsomes could not be determined due to the negligible amount of HMM formed in the incubation mixture at low substrate concentrations.
Discussion
The results of the present study showed that the formation rates of PHM and HMM from mexiletine decrease with an increase in the number of CYP2D6*10 alleles present in a human liver (Figure 1), suggesting a gene dosage effect. The decreased extent of the formation of both metabolites was more than 50% and 85% for the microsomes from CYP2D6*1/*10 and 10/*10 livers, respectively. This in vitro finding is fairly consistent with those of in vivo studies with other substrates. For example, Lai et al. [6] showed that the partial metabolic clearances of codeine to morphine, which is mediated by CYP2D6, are 48 and 77% lower in hetero- and homozygous subjects with respect to CYP2D6*10 than in the homozygotes of CYP2D6*1, respectively. Similarly, Fukuda et al. [7] reported that the area under the plasma concentration-time curves for venlafaxine, which is mainly metabolized by CYP2D6, are 2- and 6-times greater in hetero- and homozygotes with respect to CYP2D6*10 than in CYP2D6*1 homozygotes, respectively. These findings coupled with the present observation suggest that the metabolic clearance of mexiletine to form PHM and HMM decreases in hetero- and homozygotes of CYP2D6*10 to a similar extent to that found for codeine [6] or venlafaxine [7]. However, an in vivo study is required to confirm this possibility.
The results of the present study also showed that Vmax values of both metabolites tend to decrease and Km values tend to increase with an increase in the number of CYP2D6*10 alleles of the human liver. The decrease in Vmax values is consistent with the proposed mechanism that CYP2D6*10 alleles produce an unstable enzyme that leads to a decreased level of functional CYP2D6 protein [4]. However, it remains unclear whether the catalytic property of CYP2D6 protein encoded by CYP2D6*10 (CYP2D6.10) is altered or not. The present findings suggest that the affinity of CYP2D6*10 for substrates of CYP2D6 is decreased, although further studies are needed to confirm this possibility using different substrates.
The present study also showed that the PHM and HMM were formed in the microsomes from livers genotyped as CYP2D6*5/*5. This is in accordance with the finding of Nakajima et al. [10] that mexiletine is metabolized by CYP1A2 in addition to CYP2D6, although the present data suggest that the contribution of enzymes other than CYP2D6 to the formation of PHM and HMM is small in human liver microsomes.
In conclusion, the present study showed that the formation rates of PHM and HMM decrease with an increase in the number of CYP2D6*10 alleles present in human liver, suggesting that the in vivo metabolic clearance of mexiletine via these metabolic processes would be decreased in subjects who are the hetero- and homozygotes for CYP2D6*10 compared with those with the homozygous wild-type genotype.
References
- 1.Campbell NPS, Kelly JG, Adgey AAJ, Shanks RG. The clinical pharmacology of mexiletine. Br J Clin Pharmacol. 1978;6:103–108. doi: 10.1111/j.1365-2125.1978.tb00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broly F, Vandamme N, Libersa C, Lhermitte M. The metabolism of mexiletine in relation to the debrisoquine/sparteine-type polymorphism of drug oxidation. Br J Clin Pharmacol. 1991;32:459–466. doi: 10.1111/j.1365-2125.1991.tb03931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turgeon J, Fiset C, Giguere R, et al. Influence of debrisoquine phenotype and of quinidine on mexiletine disposition in man. J Pharmacol Exp Ther. 1991;259:789–798. [PubMed] [Google Scholar]
- 4.Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjoqvist F, Ingelman-Sundberg M. Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452–459. [PubMed] [Google Scholar]
- 5.Kubota T, Yamaura Y, Ohkawa N, Hara H, Chiba K. Frequencies of CYP2D6 mutant alleles in a normal Japanese population and metabolic activity of dextromethorphan O-demethylation in different CYP2D6 genotypes. Br J Clin Pharmacol. 2000;50:31–34. doi: 10.1046/j.1365-2125.2000.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai ML, Wang SL, Lai MD, Lin ET, Tse M, Huang JD. Propranolol disposition in Chinese subjects of different CYP2D6 genotypes. Clin Pharmacol Ther. 1995;58:264–268. doi: 10.1016/0009-9236(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda T, Yamamoto I, Nishida Y, et al. Effect of the CYP2D6*10 genotype on venlafaxine pharmacokinetics in healthy adult volunteers. Br J Clin Pharmacol. 1999;47:450–453. doi: 10.1046/j.1365-2125.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiba K, Kobayashi K, Tani M, Manabe K, Ishizaki T. The kinetic characterization of S-mephenytoin 4-hydroxylase (P-450IICMP) activity in human liver samples obtained as surgical waste from Japanese patients. Drug Metab Dispos. 1993;21:747–749. [PubMed] [Google Scholar]
- 9.Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T. Oxidative metabolism of omeprazole in human liver microsomes: cosegregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther. 1993;266:52–59. [PubMed] [Google Scholar]
- 10.Nakajima M, Kobayashi K, Shimada N, Tokudome S, Yamamoto T, Kuroiwa Y. Involvement of CYP1A2 in mexiletine metabolism. Br J Clin Pharmacol. 1998;46:55–62. doi: 10.1046/j.1365-2125.1998.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]