Abstract
Aims
Endothelin-1 (ET-1) is a potent vasoconstrictor produced by the vascular endothelium. The interactions of ET with the mediators of the sympathetic nervous system and the renin-angiotensin-system in humans are unclear.
Methods
We studied the effects of the ETA-selective antagonist BQ-123 and the ETB-selective antagonist BQ-788 (both 10−10−10−8 m) on ET-1 (10−16−10−10 m), angiotensin II (AT, 10−16−10−10 m) and noradrenaline (NA, 10−16−10−10 m) induced vasoconstriction in the human skin microcirculation in vivo in 25 healthy male volunteers using laser Doppler flowmetry and double injection technique.
Results
BQ-123 caused a dose-dependent vasodilatation (maximum effect: + 949 ± 84 AUC-PU, P < 0.001), whereas BQ-788 induced mild vasoconstriction (maximum effect: −388 ± 96 AUC-PU, P < 0.01). In the presence of BQ-123, but not BQ-788, ET-1, AT and NA caused markedly less vasoconstriction at any tested agonist dose; the effect was most pronounced on ET-1 (maximum effect at 10−14 m: + 814 ± 93 AUC-PU vs ET alone, P < 0.001), followed by noradrenaline (maximim effect at 10−16 m: + 580 ± 107 AUC-PU vs NA alone, P < 0.01) and angiotensin II (maximim effect at 10−14 m: + 493 ± 111 AUC-PU vs AT alone, P < 0.001).
Conclusions
ETA-selective antagonism inhibits vasoconstriction to AT and NA in vivo in healthy subjects. This beneficial effect may be useful for the treatment of patients with cardiovascular disease including hypertension especially in combination therapy with sympatholytic agents and inhibitors of the renin-angiotensin system.
Keywords: angiotensin II, BQ-123, BQ-788, endothelin antagonist, noradrenaline, skin microcirculation
Introduction
Regulation of cardiovascular homeostasis involves different systems, the sympathetic nervous system (SNS), the renin-angiotensin-system (RAS) and the vascular endothelium, which produces endothelin (ET) [1]. Although three isoforms of ET have been described, in man mainly ET-1 is present [1]. Different ET receptors have been cloned, ETA-, ETB-and ETC-receptors [2]. Under physiological conditions, the constrictor effects of ET-1 are mediated via ETA-receptors, which are located on vascular smooth muscle cells [3]. On the other hand, ETB-receptors are located on vascular endothelial cells and lead to the release of nitric oxide and prostacyclin [4]. However, in some vessels and under pathophysiological conditions (i.e. atherosclerosis), functional constrictive ETB-receptors on vascular smooth muscle cells have been described [5]. However, there is still controversy whether functional constrictive ETB-receptors play a role under physiological and pathophysiological conditions; in healthy subjects, systemic inhibition of ETB-receptors leads to vasoconstriction [6]. The effect of ETB-receptor blockade in patients with impaired endothelial function, where an upregulation of ETB-receptors has been described, has not been extensively studied; however, in hypertensive patients, combined blockade of both ETA-and ETB-receptors induced a greater forearm dilatation than an ETA-selective antagonist alone [7, 8].
In the past decade, several endothelin antagonists have been synthesized, which inhibit endothelin-induced vascular effects both in vitro and in vivo (see review [2]). Some of these ET-antagonists selectively inhibit ETA-receptors whereas others block both ETA-and ETB-receptors [2]. Furthermore, newer, nonpeptidic orally available molecules are in clinical evaluation and have already been studied in patients with hypertension, coronary artery disease and heart failure [9–11].
The pressor systems, i.e. SNS, RAS and endothelin system (ETS) have important interactions under experimental conditions. The interactions between the SNS and the RAS are well studied, while data on the effects of the ETS on SNS and RAS are lacking; in vitro, ET synthesis is stimulated by angiotensin II and ET itself, whereas nitric oxide (NO) inhibits ET production [12]. In vivo, intracisternal injection of ET stimulates central SNS activity [13]. ET-antagonists allow study of the interactions between endogenous ET and the SNS/RAS. Indeed, in vitro ETA-selective antagonism with BQ-123 attenuates the constrictor effects of angiotensin II, but not noradrenaline [14]. Furthermore, ET mediates part of the vasoconstriction to angiotensin II in smaller vessels, an effect that can be blocked with the ETA-selective antagonist BQ-123 [15]. No data exist so far in the human circulation under in vivo conditions.
These potential interactions could be relevant for the forthcoming clinical use of the nonpeptidic ET-antagonists; if mutual potentiation or synergism of the ETS with the SNS or RAS appear to be clinically relevant, this would have therapeutic consequences for the use of these drugs in combination therapy.
Therefore, we studied the effects of the ETA-selective ET-antagonist BQ-123 and ETB-selective ET-antagonist BQ-788 on angiotensin II and noradrenaline induced vasoconstriction in the human skin microcirculation in vivo.
Methods
Subjects
Twenty-five healthy male subjects participated in this study (age 22.7 ± 0.3 years). All subjects were screened for cardiovascular and other medical history and underwent physical examination before each study began. None of the subjects took any medication. There were no statistically significant differences between the groups concerning age, height, body weight and blood pressure (NS). The ethics committee of the University Hospital Essen (Germany) approved all experimental protocols. Written informed consent had been obtained before the study began.
Experimental protocol
All subjects were studied under the same conditions, i.e. in the morning in the supine position, after a light breakfast. A Periflux laser Doppler flowmeter (Perimed®, Sweden) with a probe holder was used to assess skin blood flow [16, 17]. The resulting voltage output, expressed in arbitrary ‘perfusion units’, is an index of blood flow [17]. After 30 min of resting, baseline measurements of skin blood flow at predefined measurement points were performed. Predefined measurement-points were selected according to the homogeneous basal blood-flow on the volar surface of the forearm before injection as described [18]. Measurement points with high or highly variable blood flow (e.g. in the vicinity of superficial veins) were excluded. Room temperature was kept between 19 and 22 °C. In all subjects, stability of skin blood flow during the experiment was confirmed at a site where no injection was made by measuring basal blood flow before, after 15 min and at the end of the measurement period. We have shown previously, that under the standardized conditions of the study protocol used, variations in skin blood flow were negligible compared with the pharmacological effects observed and interday-reproducibility was high [18]. Injections of the substances under examination were performed in a randomized manner using the double injection technique as described [18–20]. First, either saline or the ET-antagonist BQ-123 or BQ-788 (10−8 m) was injected followed by endothelin-1 (10−16−10−10 m), angiotensin II (10−16−10−10 m) or noradrenaline (10−16−10−10 m). The highest dose of endothelin-1 (10−10 m) was only applied in n = 11 healthy volunteers because there was no additional effect on blood flow when compared with the lower dose (10−12 m); however, local side-effects (burning pruritus) were more pronounced. To study the effects of the ET-antagonists alone, dose–response curves for BQ-123 and BQ-788 were performed (10−10−10−8 m). Control injections were made with saline. To assess the effects of an endothelin-independent vasodilator, the effects of nitroprusside (10−10 m) on angiotensin II (10−16−10−12 m) and noradrenaline (10−16−10−12 m) were assessed in a subgroup of six healthy volunteers.
Injections had to be strictly intradermal, producing a symmetrical wheel without visible spreading outside the wheel. If these criteria were not fulfilled, the injection site was excluded from analysis [18]. Agents were injected with 0.4-mm needles (Omnikan®30, B. Braun, Melsungen/FRG). No more than five injections per forearm were allowed. Blood flow was measured after 2, 5, 8, 10, 15, 20, 25 and 30 min. Each healthy volunteer was examined on 3 days to perform the whole study protocol under the same environmental conditions. The subjects were blinded for the substance injected. A high interday reproducibility of the values obtained has been shown previously [18].
Drugs
Endothelin-1, angiotensin II, BQ-123 and BQ-788 were provided by Clinalfa, Laufelfingen, Switzerland). Noradrenaline, nitroprusside and saline (0.9% NaCl solution) were provided by the pharmacy of our hospital (University Hospital, Essen, Germany). Solutions were prepared immediately before use to avoid loss of efficacy using saline to dilute the drugs to the corresponding concentration [18].
Data analysis
The mean between minimum and maximum value of a 20 s reading was calculated. Results are expressed as difThe mean between minimum and maximum value of a 20 s reading was calculated. Results are expressed as differences from baseline and control (Δ) in mean ± s.e. mean; the area under the time–response curve was calculated (AUC). The significance of differences was calculated by multiple measures analyses of variance (ANOVA) with the factors drug and time (95% confidence intervals). A Bonferroni posthoc test with correction for multiple comparisons was used to identify the statistical differences of the drug used.
Results
Effect of ET-antagonists
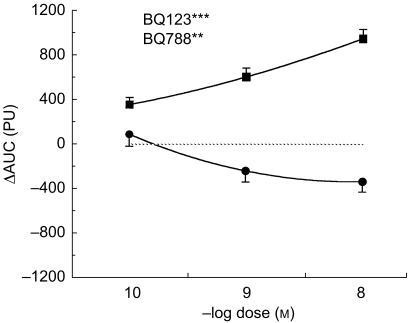
The ETA-selective antagonist BQ-123 led to a dose-dependent vasodilatation (P < 0.001 vs saline, Figure 1). In contrast, the ETB-selective antagonist BQ-788 at higher doses led to a mild vasoconstriction (P < 0.01 vs saline, Figure 1).
Figure 1.
Dose–response curve of the ETA-selective antagonist BQ-123 (▪)and the ETB-selective antagonist BQ-788 (•) in the human skin microcirculation in vivo. ⋆⋆/⋆⋆⋆ P < 0.01/0.001 vs control. ΔAUC: Area under the curve of the time-response to each constrictor. PU: perfusion units.
Effect of exogenous vasoconstrictors
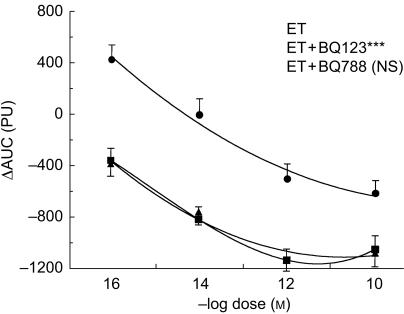
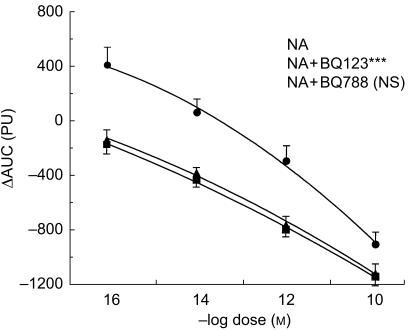
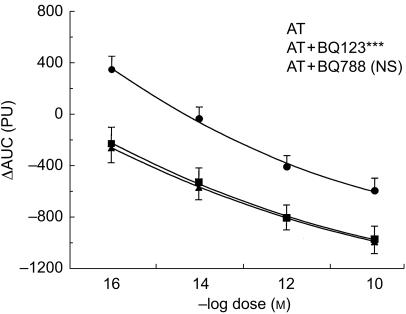
Angiotensin II, noradrenaline and endothelin-1 led to a dose dependent vasoconstriction (Figures 2–4). The constriction to endothelin-1 was significantly greater at the lower doses (10−14 and 10−12 m) when compared with noradrenaline (P < 0.01) and angiotensin II (P < 0.01). The highest dose of endothelin-1 (10−10 m) had no additional effect when compared with the lower dose (10−12 m), but induced a marked local burning. There was no statistically significant difference in the constriction to noradrenaline and angiotensin II, although at low doses the constriction to noradrenaline tended to be less pronounced (Figures 2–4).
Figure 2.
Dose–response curve of endothelin-1 (ET) alone (▪) and in the presence the ETA-selective antagonist BQ-123 (ET + BQ-123, •) and the ETB-selective antagonist BQ-788 (ET + BQ-788, ▴) in the human skin microcirculation. ⋆⋆⋆ P < 0.001 vs ET; NS not significant. ΔAUC: Area under the curve of the time-response to each constrictor. PU: perfusion units.
Figure 4.
Dose-response curve of noradrenaline (NA) alone (▪) and in the presence of the ETA-selective antagonist BQ-123 (NA + BQ-123, •) and the ETB-selective antagonist BQ-788 (NA + BQ 788, ▴) in the human microcirculation. ⋆⋆ P < 0.01 vs NA; NS not significant. ΔAUC: Area under the curve of the time-response to each constrictor. PU: perfusion units.
Effect of BQ-123 and BQ-788 on exogenous vasoconstrictors
The ETA-selective antagonist BQ-123 (10−8 m) significantly inhibited endothelin-1 induced vasoconstriction and shifted the dose–response curve to endothelin-1 to the right (P < 0.001, Figure 2). In contrast, the ETB-selective antagonist BQ-788 (10−8 m) had no effect on endothelin-1 induced vasoconstriction (NS, Figure 2).
The ETA-selective antagonist BQ-123 (10−8 m) significantly inhibited the constriction to angiotensin II (P < 0.001, Figure 3). In contrast, the ETB-selective antagonist BQ-788 (10−8 m) had no effect on angiotensin II induced vasoconstriction (NS, Figure 3).
Figure 3.
Dose–response curve of angiotensin II (AT) alone (▪) and in the presence the ETA-selective antagonist BQ-123 (AT + BQ-123, •) and the ETB-selective antagonist BQ-788 (AT + BQ-788, ▴) in the human skin microcirculation. ⋆⋆⋆ P < 0.001 vs ET; NS not significant. ΔAUC: Area under the curve of the time-response to each constrictor. PU: perfusion units.
Finally, the ETA-selective antagonist BQ-123 (10−8 m) inhibited the constriction to noradrenaline (P < 0.001, Figure 4). Again, the ETB-selective antagonist BQ-788 (10−8 m) had no effect on noradrenaline-induced vasoconstriction (NS, Figure 4).
Control experiments using the endothelin-independent vasodilator nitroprusside (10−10 m) showed a mild attenuation of angiotensin II induced vasoconstriction (mean effect: + 170 ± 150 ΔPU vs angiotensin II alone; NS). Effects of nitroprusside on noradrenaline-induced vasoconstriction were more pronounced; however, the effect was not statistically significant (mean effect: + 400 ± 70 ΔPU vs noradrenaline alone; NS).
Discussion
In the present study, we describe for the first time under in vivo conditions, that in the human skin microcirculation, inhibition of ETA-receptors attenuated not only the vasoconstriction to ET, but also to angiotensin II and noradrenaline. The ETA-selective antagonist BQ-123, but not the ETB-selective antagonist BQ-788 shifted the dose–response curve to angiotensin II and noradrenaline to the right. Furthermore, the ETA-selective antagonist BQ-123 itself induced vasodilatation as shown previously, whereas the ETB-selective antagonist BQ-788 caused mild vasoconstriction.
The effects of endothelins in the human skin microcirculation are well studied. We have previously shown that ET-1 acts mainly via ETA-receptors leading to profound and long lasting vasoconstriction [18]. These data have been confirmed in a recent study where ET-1 was infused intra-arterially in isolated perfused human skin flaps [21]. In other areas of the human circulation, i.e. the forearm and the coronary circulation, ET-induced vasoconstriction is mainly mediated via ETA-receptors, too [22–24]. However, a discrete contribution of functionally contractile ETB-receptors is still under controversial discussion, especially under pathophysiological conditions, i.e. in atherosclerosis [7, 22–25].
For a given vasoconstriction, a 10-fold higher dose of noradrenaline and angiotensin II was needed compared with ET. The effect of the three vasoconstrictors was dose-dependent. These findings are in line with the known effects of ET in other areas of the circulation [23, 26]. The highest dose of ET-1 (10−10 m) had no further effect showing that the maximal effect has already been achieved at the lower dose (10−12 m).
We found a dose-dependent vasodilation after injection of the ETA-selective antagonist BQ-123. This is consistent with previous studies using ETA-selective antagonists in the skin microcirculation, in the human forearm and in the systemic circulation [18, 21, 23, 24]. Most likely, vasodilation after ETA-receptor blockade is due to inhibition of endogenous ET, which may unmask the basal vasodilator tone due to the presence of nitric oxide and other vasodilators [24]. BQ-123 also potently inhibited vasoconstriction to exogenous ET-1, which has been previously shown in several other studies [18, 19, 21, 23].
In our study, the ETB-selective antagonist BQ-788 induced a mild vasoconstriction. This confirms in vitro and in vivo studies showing, that selective blockade of ETB-receptors induces vasoconstriction, possibly by inhibiting the release of NO and/or prostacyclin via stimulation of endothelial ETB-receptors [6, 21, 22, 27]. Functionally distinct constrictive ETB-receptors on vascular smooth muscle cells are present at least in experimental studies and depending on the vessel under investigation [3, 25]. However, at least in healthy men, the functional contribution of these receptors seems to be negligible, as systemic selective ETB-receptor blockade induces net vasoconstriction; thus, the overall balance of the ETB-receptor-mediated effects of endogenous endothelin seem to favour vasodilation [6]. In the present study, we did not use L-NMMA to assess, whether ET mediated vasoconstriction is potentiated by NO-inhibition.
The ETB-selective antagonist BQ-788 had no effect on vasoconstriction to exogenous ET-1. This finding supports results from previous studies in the human skin microcirculation and underscores, that ET mediated vasoconstriction under physiological conditions is mainly mediated by ETA-receptors [18, 28].
Experimental data show important interactions between the SNS, the RAS and the endothelin system (ETS) at the level of release, and at the effector organ. Indeed, ET activates central sympathetic activity after intracisternal injection [13], hypoxia stimulates both the ETS and the SNS [29]. At the level of vascular smooth muscle cells, ET, angiotensin II and noradrenaline seem to potentiate each other as judged from in vitro studies [15, 30, 31]. These findings are supported by the fact that the ETA-selective antagonist BQ-123 blunts the vasoconstriction to angiotensin II, whereas the constriction to noradrenaline was not affected; the effect of BQ-123 on angiotensin II induced constriction was mainly observed in small vessels [14, 15].
It could be possible that the local vasodilation brought about by any vasodilator including BQ-123 might also influence the vasoconstriction to the substances under investigation. However, the control experiments using a pure endothelin-independent vasodilator, i.e. nitroprusside show that the effect of nitroprusside on angiotensin II and noradrenaline-induced vasoconstriction is much weaker when compared with the effect of the ETA-receptor antagonist BQ-123, although nitroprusside when given alone exerts a marked vasodilation. Several in vitro studies also show high specificity for the endothelin-receptor of the ET-antagonists (i.e. BQ-123 and BQ-788) used. The effect of a specific inhibitor of endothelin (i.e. BQ-123) on angiotensin II and noradrenaline suggests that an endothelin-related mechanism must be involved. It can, however, not be clarified from our in vivo study, whether the main effect of the ETA-antagonist on noradrenaline and angiotensin II is due to inhibition of endogenous endothelin or competition at the receptor-level (see below).
Besides its vasoactive properties, ET acts as (co)mitogen and promotes vascular proliferation [32–34]. This effect is mainly mediated by ETA-receptors, too [34]. The importance of the interactions between the ETS with the RAS is underlined by the fact that in experimental angiotensin II-induced hypertension, vascular proliferation can be reversed with an ETA-selective antagonist [33].
The main finding in our study was that angiotensin II- and noradrenaline-induced vasoconstriction was significantly attenuated with the ETA-selective antagonist BQ-123, but not with BQ-788. It is likely, that endogenous ET potentiates angiotensin II and noradrenaline induced constriction, as experimental studies suggest [14, 30]. Indeed, inhibition of ETA-receptors in vitro leads to an inhibition of angiotensin II induced contraction [14]. However, this in vivo study cannot rule out other mechanisms, e.g. inhibition of receptors binding to angiotensin II or noradrenaline by BQ-123, may have contributed to this effect. Indeed, recently a dual endothelin-1/angiotensin-receptor has been characterized; functional analysis demonstrated endothelin-1- and angiotensin II-specific binding as well as endothelin-1- and angiotensin II-induced coupling to a Ca2+ mobilizing transduction system [35]. The effects of BQ-123 on noradrenaline induced constriction may indeed be due to endothelin induced potentiation, as in vitro data suggest [30]. The fact, that very low doses of AT and NA lead to a mild vasodilatation in the presence of BQ-123 may be explained by inhibition of endogenous endothelin-1.
In clinical hypertension, the antihypertensive effects of the endothelin-antagonist bosentan were comparable with the ACE-inhibitor enalapril [9]. Furthermore, endothelin antagonists have beneficial effects in patients with CHF, where they improve systemic and pulmonary haemodynamics and hospitalization, although a recent chronic study with the mixed antagonist bosentan had to be discontinued because of increased liver enzymes [11, 36].
Studies in other areas of the human circulation confirming the results found in our trial in the skin microcirculation are needed. If endothelin antagonists influence also the SNS and the RAS in a clinically significant way, they could have synergistic effects with inhibitors of the SNS and the RAS, i.e. sympatholytic agents and ACE-inhibitors or angiotensin II receptor antagonists. Thus, endothelin antagonists in combination with blockers of the RAS and the SNS may lead to a more complete vasodilation without reflex increases in renin, angiotensin II and noradrenaline and may potentiate the inhibitory effects of these drugs on vascular and myocardial hypertrophy.
Acknowledgments
The study was supported by a grant of the German Research Association (DFG, WE 1772/3–1) and the OERTEL-Foundation.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:151–157. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RR, Czyborra PL, Luscher TF, Philipp T. Endothelin in cardiovascular control: The role of endothelin antagonists. Curr Hypertension Rep. 1999;1:79–87. doi: 10.1007/s11906-999-0077-7. [DOI] [PubMed] [Google Scholar]
- 3.Seo BL, Luscher TF. ETA and ETB receptors mediate contraction to endothelin-1 in renal artery of aging SHR. Effects of FR139317 and bosentan. Hypertension. 1995;25:501–506. doi: 10.1161/01.hyp.25.4.501. [DOI] [PubMed] [Google Scholar]
- 4.Clozel M, Gray GA, Breu VL, et al. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186:867–873. doi: 10.1016/0006-291x(92)90826-7. [DOI] [PubMed] [Google Scholar]
- 5.Seo B, Oemar BS, Siebenmann R, von Segesser LL, Luscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 6.Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, Webb DJ. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33:581–585. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- 7.Dagassan PH, Breu V, Clozel MK, et al. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol. 1996;27:147–153. doi: 10.1097/00005344-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO 3rd, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 9.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel RR, Fleisch M, Shaw S, et al. Hemodynamic and Coronary Effects of the Endothelin Antagonist Bosentan in Patients with Coronary Artery Disease. Circulation. 1998;98:2235–2240. doi: 10.1161/01.cir.98.21.2235. [DOI] [PubMed] [Google Scholar]
- 11.Kiowski W, Sutsch G, Hunziker P, et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 12.Boulanger CL, Luscher TF. Release of endothelin from the porcine aorta. Inhibition of endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosqueda-Garcia R, Inagami T, Appalsamy M, Sugiura M, Robertson RM. Endothelin as a neuropeptide. Cardiovascular effects in the brainstem of normotensive rats. Circ Res. 1993;72:20–35. doi: 10.1161/01.res.72.1.20. [DOI] [PubMed] [Google Scholar]
- 14.Webb ML, Dickinson KE, Delaney CL, et al. The endothelin receptor antagonist, BQ-123, inhibits angiotensin II-induced contractions in rabbit aorta. Biochem Biophys Res Commun. 1992;185:887–892. doi: 10.1016/0006-291x(92)91710-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, McNeill JR, Wilson TW, Gopalakrishnan V. Heterogeneity in vascular smooth muscle responsiveness to angiotensin II. Role of endothelin. Hypertension. 1995;26:83–88. doi: 10.1161/01.hyp.26.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254:56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson GE, Tenland T, Oberg PA. A new instrument for continuous measurement of tissue blood flow by light beating spectroscopy. IEEE Trans Biom Eng. 1980;1:12–19. doi: 10.1109/TBME.1980.326686. BME-27. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel RR, Noll GL, Luscher TF. Endothelin receptor antagonists inhibit endothelin in human skin microcirculation. Hypertension. 1994;23:581–586. doi: 10.1161/01.hyp.23.5.581. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel RR, Duthiers N, Noll G, Bucher J, Kaufmann UL, Luscher TF. Endothelin and calcium antagonists in the skin microcirculation of patients with coronary artery disease. Circulation. 1996;94:316–322. doi: 10.1161/01.cir.94.3.316. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel RR. Minimal invasive in vivo pharmacology: news from a new method holding promise in nephrology-related research. Nephrol Dial Transplant. 1997;12:649–651. doi: 10.1093/ndt/12.4.649. 10.1093/ndt/12.4.649. [DOI] [PubMed] [Google Scholar]
- 21.Lipa JE, Neligan PC, Perreault TM, et al. Vasoconstrictor effect of endothelin-1 in human skin: role of ETA and ETB receptors. Am J Physiol. 1999;276:H359–H367. doi: 10.1152/ajpheart.1999.276.2.H359. [DOI] [PubMed] [Google Scholar]
- 22.Pierre LN, Davenport AP. Relative contribution of endothelin A and endothelin B receptors to vasoconstriction in small arteries from human heart and brain. J Cardiovasc Pharmacol. 1998;31:S74–S76. doi: 10.1097/00005344-199800001-00024. [DOI] [PubMed] [Google Scholar]
- 23.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 24.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 25.Teerlink JR, Breu V, Sprecher U, Clozel M, Clozel JP. Potent vasoconstriction mediated by endothelin ETB receptors in canine coronary arteries. Circ Res. 1994;74:105–114. doi: 10.1161/01.res.74.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Kiowski WL, Luscher TF, Linder LB, Buhler FR. Endothelin-1-induced vasoconstriction in humans. Reversal by calcium channel blockade but not by nitrovasodilators or endothelium-derived relaxing factor. Circulation. 1991;83:469–475. doi: 10.1161/01.cir.83.2.469. [DOI] [PubMed] [Google Scholar]
- 27.Shetty SS, Okada T, Webb RL, Del Grande D, Lappe RW. Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochem Biophys Res Commun. 1993;191:459–464. doi: 10.1006/bbrc.1993.1240. 10.1006/bbrc.1993.1240. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel RR, Zbinden S, Noll G, Meier BL, Luscher TF. Endothelin-1 induces vasodilation in human skin by nociceptor fibres and release of nitric oxide. Br J Clin Pharmacol. 1998;45:441–446. doi: 10.1046/j.1365-2125.1998.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noll G, Wenzel RR, Schneider M, et al. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation. 1996;93:866–869. doi: 10.1161/01.cir.93.5.866. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Von Richard VSL, Bauer E, Stulz P, Turina ML, Luscher TF. Threshold concentrations of endothelin-1 potentiate contractions to norepinephrine and serotonin in human arteries. A new mechanism of vasospasm? Circulation. 1990;82:188–195. doi: 10.1161/01.cir.82.1.188. [DOI] [PubMed] [Google Scholar]
- 31.Nally JE, Clayton RA, Wakelam MJ, Thomson NC, McGrath JC. Angiotensin II enhances responses to endothelin-1 in bovine bronchial smooth muscle. Pulm Pharmacol. 1994;7:409–413. doi: 10.1006/pulp.1994.1048. 10.1006/pulp.1994.1048. [DOI] [PubMed] [Google Scholar]
- 32.Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. Febs Lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 33.Moreau P, d'Uscio LV, Shaw S, Takase H, Barton ML, Luscher TF. Angiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET (A) -receptor antagonist. Circulation. 1997;96:1593–1597. doi: 10.1161/01.cir.96.5.1593. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Krasnici NL, Luscher TF. Endothelin-1 potentiates human smooth muscle cell growth to PDGF. effects of ETA and ETB receptor blockade. Circulation. 1999;100:5–8. doi: 10.1161/01.cir.100.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VL. Molecular characterization of a dual endothelin-1/Angiotensin II receptor. Mol Med. 1998;4:96–108. [PMC free article] [PubMed] [Google Scholar]
- 36.Packer M, Caspi A, Charlton V, et al. Multicenter double-blind, placebo-controlled study of long-term endothelin blockade with bosentan in chronic heart failure – Results from the REACH-1 trial. Circulation. 1998;21 Abstract. [Google Scholar]