Abstract
Aims
An intensified monitoring system was set up to identify drug related hospital admissions and estimate population-based incidences for commonly prescribed medications.
Methods
Pharmacovigilance-centres systematically screened nonelective admissions to emergency rooms or departments of internal medicine for drug related hospitalizations (DRH). Clinical pharmacologists used standardized causality assessment. Service areas of each acute care hospital were defined by 5 digit postal codes that covered 60% of all admissions. Drug dispensing information was available through claims processed by regional pharmacy computing centres. Quarterly incidences were estimated by dividing the number of events by the number of treated patients.
Results
435 DRHs were reported during five quarters. The incidence of ADRs leading to admissions varied for specific drug groups from 1.5/10 000 treated patients to 24/10 000. Quarterly variation of incidences was moderate except for insulin and calcium antagonists. 95% confidence intervals overlap for all quarters within each group. Incidences are sensitive to changes in the definition of the source population.
Conclusions
Our pharmacovigilance monitoring system allows comparisons of population-based incidences of drug-related hospitalizations among drugs and over time. It provides important information for risk management and monitoring outcomes of pharmaceutical quality management programmes.
Keywords: adverse drug reaction, drug related hospitalization, monitoring, pharmacovigilance, population-based
Introduction
Although valuable, spontaneous reporting suffers from high underreporting of ADRs in hospitals, frequently misses important information on the nature and chronological sequence of events, and lacks a defined source population [1]. Meta-analyses estimated that the proportion of drug related hospitalizations (DRH) varies between 2.4% and 6.2% [2, 3]. Monitoring the frequency of DRHs is necessary to evaluate interventions on better prescribing. For valid population-based incidence estimation many requirements must be fulfilled, including complete event documentation, a sensitive yet specific screening tool, and a defined source population. The objective of this paper is to describe a monitoring system and incidence estimation for drug related hospitalizations.
Methods
The Departments of Clinical Pharmacology in Jena, Rostock, and Dresden established an intensified Pharmacovigilance program from October 1, 1997 to December 31, 1998. Each centre systematically screened nonelective admissions to emergency rooms or departments of general internal medicine of all hospitals in the surrounding urban areas. Dresden has 5 acute care hospitals, Rostock 2, and Jena 1.
DRHs were identified 1) by systematic and prospective screening of admissions to emergency rooms and departments of general internal medicine with regard to characteristic symptoms/diagnoses of known ADRs, and 2) by spontaneous reports from the screened hospitals. Clinical pharmacologists screened admission protocols according to a defined list of trigger symptoms and followed up patients in case of suspicious symptoms. Drug exposure was assessed by a combination of chart review and patient interview. A standardized assessment of causality of each medication was made by trained clinical pharmacologists [4]. The evaluation included the chronologic sequence, clinical symptoms, absence of other causes, and laboratory results. Only drugs which possibly, likely, or very likely caused the hospital admission (I2-I4) [4] were used for incidence calculations. The inclusion of patients was independent of the severity of symptoms.
Suspicious cases were entered into a database and reported. The centre specific databases allowed to enter additional patient information as it became available until a final decision about an event was reached. Cases that remained unclear were discussed by clinical pharmacologists of all three centres.
Since Dresden had limited coverage of all hospital admissions in the first year their numbers were excluded from incidence estimation. The urban areas of Jena and Rostock comprise about 520 000 people. For valid incidence estimation the correct source population would be all people who would have been admitted to the urban hospitals had they developed an ADR serious enough so seek medical assistance in a nearby hospital. According to this definition the likelihood of being part of the source population decreases with the distance to the study hospitals. Therefore, we decided to exclude people living in more distant areas. We sorted the residential postal codes of all patients admitted to medical wards in the year 1997 by their frequency. The source population was then defined as those medication users living in the postal code areas that contribute to the first 60 cumulative percentage of all admissions. The remaining 40% were spread over a wide area surrounding the urban area. We defined a secondary hospital service area consisting of 70% cumulative admissions.
Pharmacy computing centres pay pharmacies each quarter for all reimbursable prescription medications on behalf of the sickness funds covering 90% of the population. Considering the low number of events, quarterly intervals were not divided into shorter periods. Prescriptions from individual patients were linked together through their health insurance number and the number of treated patients was defined as having received at least one pack during that quarter. For this analysis, data were restricted to the 45 most commonly prescribed substances in 1997. Data were adjusted for the proportion of pharmacies that did not work with pharmacy computing centres.
Incidences were estimated as events per 10 000 treated patients per quarter. The number of treated patients was chosen as the denominator because it is ‘the most adequate denominator for quantifying a risk, comparing several treatments with respect to safety or making public health decisions’ [5].
Results
During the study period 435 DRHs were reported. 71.6% were possibly caused by a drug, 24.4% likely, and 4% very likely. Gastroduodenal lesions, including gastritis and ulcer, were most frequently reported (38% of all reports).
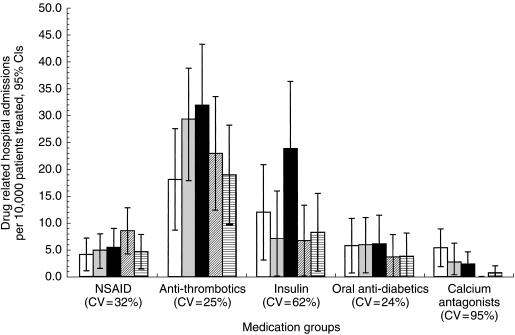
Anti-thrombotics showed the highest incidence with 24.3 drug related admissions per 10 000 treated patients pooled over five quarters (left column of Table 1). Some quarterly variability of incidences was observed depending on therapeutic group (Figure 1). The 95% confidence intervals of incidences overlap for all quarters within each group. Only for calcium antagonists we observed a decreasing linear trend (P < 0.05).
Table 1.
Incidence estimates of drug related hospital admissions combined over 5 quarters and their sensitivity to a larger source population defined by 70% of cumulative admissions.
| Source population defined by 60% cumulative admissions (25 postal codes) | Source population defined by 70% cumulative admissions (44 postal codes) | ||||||
|---|---|---|---|---|---|---|---|
| Medication group | Number ADRs | Treated patients | Incidence⋆ | 95% Cl⋆⋆ | Treated patients | Incidence | 95% Cl |
| NSAIDs | 47 | 85030 | 5.53 | 3.94, 7.11 | 146416 | 3.21 | 2.29, 4.13 |
| Antithrombotics | 98 | 40338 | 24.30 | 19.5, 29.1 | 64071 | 15.3 | 12.3, 18.3 |
| Systemic corticosteroids | 19 | 14989 | 12.68 | 6.10, 18.4 | 24624 | 7.72 | 4.25, 11.2 |
| ACE inhibitors | 14 | 60875 | 2.30 | 1.10, 3.50 | 102288 | 1.37 | 0.65, 2.08 |
| Diuretics | 7 | 37901 | 1.85 | 0.47, 3.22 | 66041 | 1.06 | 0.27, 1.85 |
| Digitalis | 15 | 62055 | 2.42 | 1.19, 3.64 | 100153 | 1.50 | 0.74, 2.26 |
| Calcium antagonists | 19 | 84097 | 2.26 | 1.24, 3.28 | 134692 | 1.41 | 0.78, 2.05 |
| β-adrenoceptor blockers | 11 | 55857 | 1.97 | 0.81, 3.13 | 91822 | 1.20 | 0.49, 1.91 |
| Nitrates | 13 | 84612 | 1.54 | 0.70, 2.37 | 144420 | 0.90 | 0.41, 1.39 |
| Insulin | 34 | 29327 | 11.6 | 7.70, 15.5 | 47730 | 7.12 | 4.73, 9.52 |
| Oral antidiabetics | 21 | 41264 | 5.09 | 2.91, 7.27 | 72429 | 2.90 | 1.66, 4.14 |
Incidence of drug related hospital admissions per 10 000 treated patients.
Cl = confidence interval assuming Poisson distributed errors.
Figure 1.
Changes in incidence of drug related hospital admissions for selected medication groups over 5 quarters with 95% confidence intervals (□ Q4/97,  Q1/98, ▪ Q2/98,
Q1/98, ▪ Q2/98,  Q3/98,
Q3/98,  Q4/98). Variability of incidence estimates among quarters is presented as the coefficient of variation (CV). CV=standard deviation of 5 quarterly incidence estimates divided by the mean to allow comparison of variation between groups.
Q4/98). Variability of incidence estimates among quarters is presented as the coefficient of variation (CV). CV=standard deviation of 5 quarterly incidence estimates divided by the mean to allow comparison of variation between groups.
The source population defined by the cumulative 60% postal codes of all admissions during 1 year comprises 12 postal codes in Rostock and 13 in Jena. Increasing the cumulative percentage of admission by only one sixth to 70% doubled the number of postal codes in Jena (26) and increased them by a third in Rostock (18). This expanded the exposed source population 1.6 times (right column of Table 1). The incidence of DRHs decreased on average by 39% in all medication groups. The 95% confidence intervals between both source population definitions overlapped.
Discussion
Several points are critical to the interpretation of population-based incidence estimation of DRHs: 1) The monitoring system, which is prospectively screening hospital admissions might not be sensitive enough compared with a complete record abstraction of all admissions. However, for NSAIDs we computed the proportion of gastro-duodenal lesions and bleedings that were attributed to NSAIDs and found a proportion of 39%. This high proportion does not support the hypothesis of underreporting. 2) Depending on the substances that are grouped together we may observe differences in incidences to the extent that medications differ in their underlying incidences of adverse reactions. More importantly, regional patterns of comedication might be vastly different. Since polypharmacy was found to a considerable extent, comedication patterns should be considered in the comparison of ADR incidences. 3) Results depend on the definitions of drug related hospitalizations. Our system screened only departments of medicine and emergency rooms and deliberately excluded intentional overdosing as well as cytostatic medications and severe dermatological reactions. 4) Critical to any incidence estimation that combines numerator from high quality monitoring data with a secondarily defined denominator is the extent to which the exposure of the operational source population reflects the true denominator. Our sensitivity analysis showed that sleight changes in the source population definition might have some impact on incidence estimates. Therefore, a more refined definition of the source population beyond crude administrative boundaries of an urban region is recommended. Admission data from the surrounding hospitals would be required to adjust for the proportion of patients seeking help in areas more distant from the urban centre [6]. Therefore, longitudinal comparisons of incidences with the above factors held constant are valid before detailed research may confirm the exact source population.
We could demonstrate that incidences are relatively stable over time especially in medication groups with many events, e.g. NSAIDs or antithrombotics. Other medication groups like Insulin with an almost twice the average incidence in the second quarter of 1998, and calcium antagonists with constantly declining incidence showed unexpected changes in time trends thus generating a signal that requires more detailed analyses. The analysis of time trend changes may be supplemented by comparisons between substances within a group. The capacity of our system to detect differences is still limited by the low number of events per substance, which may require a time series of more than 5 quarters or more regions.
The single case causality assessments prepared by clinical pharmacologists and transmitted to the regulatory authority are of considerably better quality than average reports from the existing spontaneous reporting system. All cases with only doubtful causal relations to a prescription medication were excluded to increase the specificity of the case definition. Only 4% of events were categorized as very likely but 71.6% as possibly related to a medication, illustrating the well-described range of uncertainty of single case causality assessment. Despite the use of a standardized algorithm some undesirable events that have no bearing on the risks associated with the appropriate use of drugs may be counted as drug related admissions.
In conclusion the monitoring system provides a series of quarterly incidence estimates of adverse drug reactions focused on reactions that are serious enough to lead to hospital admissions and cause considerable morbidity and costs. The method has its strength in longitudinal monitoring of incidences supplemented by comparison of incidences between specific drugs. A hospital based drug event monitoring is useful for supplementing existing spontaneous reporting systems to support risk management decisions and for monitoring outcomes of pharmaceutical quality management programs.
Acknowledgments
This study and the monitoring system was funded by the Federal Institute for Drugs and Medical Devices (BfArM) of Germany (Fo 2.1–68502–201). Dr Schneeweiss was supported by the Deutsche Forschungsgemeinschaft (SCHN 527/3 & SCHN 527/4) and the Pharmacoepidemiology Teaching and Research Fund of the Harvard University School of Public Health.
Appendix
Principal investigators
Prof. Dr J. Hasford, Universität München
Dr S. Schneeweiss, Universität München and Harvard Medical School
Data coordinating and data quality centre
Institut für Medizinische Informationsverarbeitung, Biometrie und Epidemiologie der Universität München (Dr M. Göttler, Dr W. Swoboda)
Participating pharmacovigilance centres and investigators
Universität Rostock: PD Dr A.-K. Riethling, Dr P. Gordalla
Universität Jena: Prof. Dr A. Hoffmann, PD Dr A Hippius, Dr T. Sicker, B. Humaid, C. Stahr
Universität Dresden: Dr J. Krappweis, Dr U. Schwarz, Prof. Dr Dr W. Kirch
Pharmacy computing centres
Gesellschaft für Datenverarbeitung GmbH/Health Care Consulting (A. Liesenhoff, R. Webersinke)
Verrechnungsstelle der Süddeutschen Apotheken GmbH (VSA) (Dr A. Lacher)
Norddeutsches Apotheken-Rechenzentrum e.V. (NARZ) (H. Helmker, Hr. Ruhdorfer)
Apotheken-Rechen-Zentrum Darmstadt (P. Milius, Hr. Balzter)
Bundesverband Deutscher Apothekerverbände (ABDA), Arzneimittelinformationsstelle (Dr M. Schulz)
References
- 1.Smith CC, Bennett PM, Pearce HM, et al. Adverse drug reactions in a hospital general medical unit meriting notification to the Committee on Safety of Medicines. Br J Clin Pharmacol. 1996;42:423–429. doi: 10.1046/j.1365-2125.1996.04376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhlberger N, Schneeweiss S, Hasford J. Adverse drug reaction monitoring-cost and benefit considerations. Part 1: Frequency of ADRs leading to hospital admissions. Pharmacoepidemiol Drug Safety, 6 S. 1997;3:71–77. doi: 10.1002/(sici)1099-1557(199710)6:3+<s71::aid-pds282>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Roughead EE, Gilbert AL, Primrose JG, et al. Drug-related hospital admissions: a review of Australian studies published 1988–1996. Med J Aust. 1998;165:405–408. doi: 10.5694/j.1326-5377.1998.tb138996.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore N, Paux G, Begaud B, et al. Adverse drug reaction monitoring: doing it the French way. Lancet. 1985;ii:1058. doi: 10.1016/s0140-6736(85)90918-3. [DOI] [PubMed] [Google Scholar]
- 5.Begaud B, Pere J-C, Miremont G. Estimation of the denominator in spontaneous reporting. Postmarketing Surveillance. 1993;7:51–70. [Google Scholar]
- 6.Senn SJ, Samson WB. Estimating hospital catchment populations. The Statistician. 1982;31:81–96. [Google Scholar]