Abstract
Aims
To determine (i) which factors, including metformin, are associated with the fasting plasma lactate concentration in type 2 diabetes, and (ii) whether plasma lactate is associated with haemodynamic and metabolic effects.
Methods
We measured fasting plasma lactate in 272 well-characterized diabetic patients from a community-based sample, 181 (67%) of whom were taking metformin with or without other therapies. Linear regression analysis was used to identify predictors, including metformin therapy, of the plasma lactate, and to investigate associations between plasma lactate and resting pulse rate and serum bicarbonate. Factor analysis assessed independent relationships between groups of cosegregating variables.
Results
Metformin-treated patients had higher plasma lactate concentrations than nonmetformin-treated subjects (geometric mean [s.d. range] 1.86 [1.34–2.59] vs 1.58 [1.09–2.30] mmol l−1, respectively; P < 0.001). In a linear regression model, plasma glucose, BMI and metformin use (but not dose) were independently associated with plasma lactate (P ≤ 0.028); after adjustment for the former two variables, metformin-treated patients had a mean plasma lactate 0.16 mmol l−1 greater than in subjects not taking the drug. Factor analysis revealed that plasma lactate, plasma glucose, BMI and pulse rate cosegregated but serum bicarbonate was not in this grouping.
Conclusions
The present results show that metformin therapy increases the fasting plasma lactate in ambulant patients with type 2 diabetes from a community-based cohort. From associations in the data we hypothesize that this increase reflects (i) increased sympathetic activity in patients with the metabolic syndrome (ii) increased substrate (glucose) availability and (iii) a direct metformin effect.
Keywords: factor analysis, metformin, plasma lactate, type 2 diabetes
Introduction
The efficacy and benefits of metformin treatment in type 2 diabetes have been confirmed by recent large-scale studies [1, 2]. Although the most serious adverse effect associated with metformin is acknowledged to be lactic acidosis [3], this association has been called into question. Most metformin-treated diabetic patients developing lactic acidosis have other conditions, such as heart failure and renal insufficiency, that can lead independently to lactic acidosis [4–7]. In addition, the rate of lactic acidosis in type 2 diabetes in the community does not appear to be influenced by the availability of metformin [8]. Although up to 50% of patients receiving metformin in the UK have at least one contra-indication to its use [9, 10], the incidence of lactic acidosis in these patients appears very low [10]. Both nonsteroidal anti-inflammatory drugs (NSAIDs) [11] and angiotensin converting enzyme (ACE) inhibitors [12] may increase the risk of lactic acidosis in nephropathic patients treated with metformin, but reports of these interactions have been confined to single cases.
Lactic acidosis is defined conventionally as a plasma lactate concentration > 5.0 mmol l−1 together with an arterial blood pH < 7.35 [13]. In diabetic patients receiving metformin, it has been recommended that the measurement of blood lactate be used to assess whether a change in symptoms reflects incipient acidosis (Glucophage® Product Information, Bristol-Myers Squibb Company, Princeton, NJ, USA) or as a guide to whether the drug should be stopped during acute intercurrent illness [14]. Interpretation of measurements in these situations requires knowledge of reference blood lactate concentrations in asymptomatic patients taking regular metformin. Available data obtained mainly from subjects participating in comparative efficacy trials suggest that metformin has either a neutral effect on the fasting plasma lactate [15, 16] or increases it by an average of up to 0.4 mmol l−1 [17, 18]. These latter studies are consistent with reports that metformin is associated with higher blood lactate levels than either diet [19] or sulphonylurea [20] treatment. A positive relationship between plasma lactate and serum creatinine has been described in patients with type 2 diabetes taking metformin but not with other blood-glucose lowering regimens [21].
Factors not directly related to diabetes treatment such as obesity [22], hypertension [23] and hyperglycaemia itself [24, 25] have also been associated with raised plasma lactate in diabetic patients. It is therefore likely that the plasma lactate is influenced by a range of potentially interacting variables in type 2 diabetes including body weight, glycaemia, blood pressure and renal function, as well as blood glucose lowering therapy per se. Without knowledge of the relative importance of these factors, the interpretation of a fasting plasma lactate concentration is difficult.
The aims of the present study were to (i) measure fasting plasma lactate concentrations in a community-based sample of patients with type 2 diabetes who were taking metformin and/or other blood glucose lowering therapy (ii) identify which factors, including metformin treatment, are independent predictors of plasma lactate, and (iii) determine whether plasma lactate is associated with significant haemodynamic and metabolic effects in ambulant patients on established therapy for diabetes.
Methods
Patients
We studied 272 patients with type 2 diabetes. All were participants in the Fremantle Diabetes Study (FDS), a prospective observational study of diabetes in a postcode-defined region of 120 097 people surrounding the port of Fremantle in Western Australia. The FDS protocol was approved by the Human Rights Committee, Fremantle Hospital and all patients gave informed consent. Patient identification was through hospital records, general practitioners, specialists, allied health services, advertisements and word of mouth. Recruitment criteria included diagnosis of diabetes by a medical practitioner and residence in the catchment area. From 1993 to 1996, 2277 diabetic subjects were identified and 1426 (64%) recruited. Of these, 1294 (91%) had type 2 diabetes which was defined as that (i) treated with diet and/or oral hypoglycaemic agents irrespective of age at diagnosis, (ii) in patients aged ≥ 60 years at diagnosis whatever the treatment history and (iii) diagnosed between 40 and 60 years of age, treated with insulin at study entry but not at diagnosis, and associated with a body mass index (BMI) > 30 kg m−2. Subjects with secondary diabetes were excluded. Where classification required other data, case records were consulted for evidence of ketosis, islet cell autoantibody levels and serum insulin/C-peptide concentrations.
Patients were eligible for recruitment to the present study if they attended for an annual FDS assessment during a 12 month period from September 1998. All metformin-treated patients were invited to participate. A second group of patients on diet alone with or without sulphonylurea therapy was also recruited. This second group was matched in a ratio of 1 : 2, and as closely as possible for age and sex, with the metformin-treated patients. The present combined sample represented 21% of the patients with type 2 diabetes in the FDS cohort and 13% of all identified in the study catchment area through active case detection.
Clinical assessment
At each annual visit, all FDS subjects have a comprehensive medical history taken (including full details of therapy for diabetes) and undergo physical examination, and provide fasting blood and urine samples for automated biochemical analyses. Subjects in the present study provided an additional blood sample taken into fluoride-oxalate for plasma lactate assay. All lactate assays were performed using the Monotest Lactate® kit (Roche Diagnostics, Castle Hill, NSW, Australia) and the Cobas Fara centrifugal analyser (Roche Diagnostics) with calibration by lithium lactate standard. The reference range for venous plasma was 0.6–2.0 mmol l−1. Within-run precision was 1.0% at 4.3 mmol l−1 and between-run precision was 1.8% at 5.2 mmol l−1. Patients were classified as having coronary heart disease (CHD) if there was a self-reported history of myocardial infarction, angina, coronary artery bypass grafting, angioplasty and/or definite or probable ischemic changes on Minnesota coding of a resting 12-lead electrocardiogram.
Statistical analysis
SPSS for Windows (SPSS Inc., Chicago, Illinois, USA) was used for all data analyses. Data are presented as mean ± s.d. or, in the case of variables (including plasma glucose and lactate, and glycosylated haemoglobin (HbA1c)) which did not conform to a normal distribution by Kolmogorov-Smirnov test, geometric mean and [s.d. range]. Two-sample comparisons were by Student's t-tests. Comparison of proportions was by Chi-square test and of multiple means by analysis of variance (anova) and the Bonferroni post hoc test. Associations between variables were assessed using multiple linear regression analysis with a stepwise forward selection procedure. A two-tailed level of significance of 0.05 was used throughout.
To further investigate relationships between correlated metabolic and haemodynamic variables of interest, we performed factor analysis using principal component analysis (PCA) and varimax orthogonal rotation [26]. This process identifies the minimum number of factors that are presumed to underlie variables that cosegregate and which might, for example, represent distinct pathophysiological processes. After extraction of initial components using PCA and the Scree test, orthogonal rotation is used to transform the original components to independent clusters or domains that can then be interpreted. Based on the number of subjects in the present study, a loading of ± 0.34 was considered significant on any one factor [26].
Results
Subject characteristics
Of the 272 patients recruited to the present study, 181 (67%) were taking metformin. Details of the patients grouped by diabetes treatment are summarized in Table 1. Patients taking more than one blood glucose lowering drug had a significantly longer duration of diabetes than patients in the other treatment groups (P < 0.001 by anova) and also had the highest mean HbA1c levels (P < 0.001). Patients on diet alone had the lowest fasting plasma glucose, HbA1c and resting pulse rate (P < 0.01 in each case; see Table 1).
Table 1.
Details of patients grouped by diabetes treatment. Data are mean ± s.d. or geometric mean and (s.d. range).
| Diet alone | Sulphonylurea alone | Metformin alone | Metformin+sulphonylurea | Metformin+insulin±sulphonylurea | |
|---|---|---|---|---|---|
| Number | 57 | 34 | 57 | 109 | 15 |
| Age (years) | 66.6 ± 9.2 | 67.7 ± 8.1 | 65.9 ± 10.3 | 66.4 ± 8.6 | 66.0 ± 10.8 |
| Sex (% males) | 50.9 | 76.5 | 43.9 | 58.7 | 60.0 |
| Diabetes duration (years) | 7.0 ± 4.2 | 7.7 ± 3.5 | 8.4 ± 5.4 | 11.2 ± 5.7abc | 16.9 ± 8.2abcd |
| Body mass index (kg m−2) | 28.7 ± 5.1 | 28.0 ± 3.6 | 30.1 ± 4.6 | 30.0 ± 5.1 | 30.9 ± 3.9 |
| Resting pulse rate (beats min−1) | 64 ± 11 | 69 ± 14 | 72 ± 13a | 71 ± 12a | 67 ± 11 |
| Supine systolic blood pressure (mmHg) | 142 ± 21 | 150 ± 22 | 146 ± 24 | 149 ± 25 | 150 ± 23 |
| Supine diastolic blood pressure (mmHg) | 70 ± 10 | 74 ± 10 | 73 ± 11 | 75 ± 12 | 74 ± 10 |
| Fasting plasma glucose (mmol l−1) | 7.5 (5.7–9.8) | 8.6 (6.8–11.0) | 8.7 (7.1–10.5)a | 9.3 (7.1–12.0)a | 7.9 (5.9–10.6) |
| HbA1c (%) | 6.5 (5.6–7.6) | 7.2 (6.3–8.1)a | 7.1 (6.1–8.4)a | 7.7 (6.7–8.9)ac | 7.9 (7.0–8.9)a |
| Fasting plasma lactate (mmol l−1) | 1.57 (1.06–2.32) | 1.58 (1.10–2.27) | 1.87 (1.28–2.73)a | 1.86 (1.39–2.51)a | 1.81 (1.22–2.67) |
P < 0.05 vs diet alone
P < 0.05 vs sulphonylurea alone
P < 0.05 vs metformin alone
P < 0.05 vs metformin+sulphonylurea.
Fasting plasma lactate
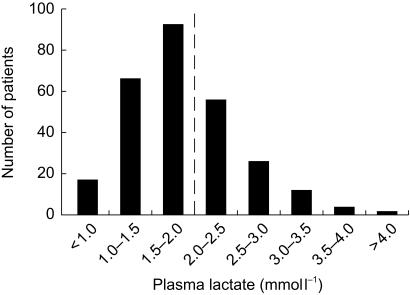
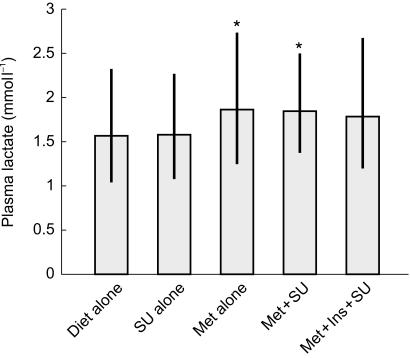
The distribution of fasting plasma lactate concentrations in the total sample is shown in Figure 1. In 35.7% of cases, the plasma lactate was above the upper limit of the reference range. The highest value was 6.4 mmol l−1. Plasma lactate concentrations in the five treatment groups are summarized in Figure 2. There were significant differences between the groups (P = 0.008), with geometric mean [s.d. range] concentrations in patients on diet or sulphonylurea alone being similar but both significantly lower than those in patients on metformin alone or taking metformin combined with a sulphonylurea drug (P < 0.05 in each case; see Table 1).
Figure 1.
Frequency histogram showing distribution of plasma lactate concentrations in the present study. The upper limit of the reference range (2 mmol l−1) is indicated by a vertical dashed line.
Figure 2.
Geometric mean (bars) and standard deviation range (vertical lines) for patients grouped according to type of treatment for diabetes. Numbers of patients in each group are as in Table 1. SU = sulphonylurea. Met = metformin. Ins = insulin. ⋆ indicates significantly different from both diet alone and SU alone.
Because of the similarities between mean plasma lactate concentrations in the five treatment groups when considered according to whether or not patients were taking metformin, subsequent analyses were based on this simplified binary classification (see Table 2). Metformin-treated patients had significantly longer duration of disease, a greater body mass index (BMI), and a higher pulse rate, fasting plasma glucose and HbA1c compared with those not receiving metformin (P ≤ 0.003). Although the differences between the group means were small, patients on metformin had significantly lower serum bicarbonate and higher serum potassium concentrations than those not on the drug (P < 0.04), and more were treated with an ACE inhibitor (P = 0.009).
Table 2.
Details of patients classified by whether or not they were taking metformin when studied. Data are mean ± s.d. or geometric mean and (s.d. range).
| Not taking metformin | Taking metformin | P value | |
|---|---|---|---|
| Number | 91 | 181 | – |
| Age (years) | 66.7 ± 8.8 | 66.2 ± 9.3 | 0.67 |
| Sex (% males) | 60.4 | 54.1 | 0.31 |
| Diabetes duration (years) | 7.6 ± 4.3 | 10.8 ± 6.2 | < 0.001 |
| Daily metformin dose (g) | – | 1.5[1.0–2.0]¶ | – |
| Body mass index (kg m−2) | 28.5 ± 4.5 | 30.1 ± 4.9 | 0.006 |
| Resting pulse rate (beats min−1) | 66 ± 13 | 71 ± 12 | 0.003 |
| Supine systolic blood pressure (mmHg) | 145 ± 23 | 148 ± 24 | 0.42 |
| Supine diastolic blood pressure (mmHg) | 72 ± 10 | 74 ± 11 | 0.09 |
| Fasting plasma glucose (mmol l−1) | 8.0 (6.1–10.4) | 8.9 (7.0–11.5) | < 0.001 |
| HbA1c (%) | 6.8 (5.8–8.0) | 7.6 (6.5–8.8) | < 0.001 |
| Fasting plasma lactate (mmol l−1) | 1.58 (1.09–2.30) | 1.86 (1.34–2.59) | < 0.001 |
| Serum creatinine (µmol l−1) | 94 ± 19 | 100 ± 58 | 0.33 |
| Serum bicarbonate (mmol l−1) | 26.8 ± 2.4 | 26.1 ± 2.4 | 0.032 |
| Serum potassium (mmol l−1) | 4.4 ± 0.3 | 4.6 ± 0.4 | < 0.001 |
| Ischaemic heart disease (%) | 30.9 | 26.5 | 0.48 |
| β-adrenoceptor blockers (%) | 13.8 | 11.6 | 0.70 |
| Angiotensin converting enzyme inhibitors (%) | 16.0 | 30.7 | 0.009 |
| Non-steroidal anti-inflammatory drugs (%) | 6.4 | 2.8 | 0.19 |
Median and [interquartile range].
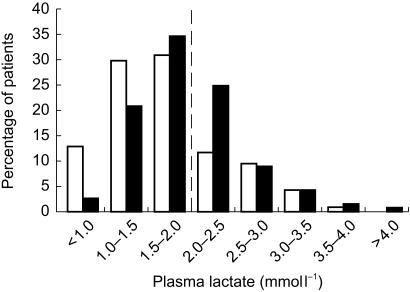
The fasting plasma lactate concentration in metformin-treated patients was, on average, 0.28 mmol l−1 higher than that of patients on diet with or without a sulphonylurea drug (P ≤ 0.001; see Table 2). The distribution of plasma lactate concentrations in the two groups is shown in Figure 3. Of those patients not taking metformin, 27% had an elevated fasting plasma lactate compared with 42% of those taking metformin (P = 0.027 by χ2 test).
Figure 3.
Frequency histogram showing distribution by percentage of plasma lactate in nonmetformin-treated patients (light bars) and metformin-treated patients (dark bars). The upper limit of the reference range (2 mmol l−1) is indicated by a vertical dashed line.
Determinants of the fasting plasma lactate
In a linear regression analysis with the logarithm of the fasting plasma lactate as dependent variable, there were strong positive associations with both the logarithm of the simultaneous plasma glucose and BMI (P < 0.001), while an independent association with metformin treatment was also present (P = 0.028). After adjusting for both plasma glucose and BMI (adjusted means [95% confidence intervals] 1.66 [1.55, 1.78] and 1.82 [1.74, 1.92] mmol l−1. in patients not receiving and receiving metformin, respectively), the difference between the mean plasma lactate in the two groups fell from 0.28 mmol l−1 to 0.16 mmol l−1. Variables entered into, but excluded from, the model included age, sex, diabetes duration, the logarithm of the HbA1c, systolic and diastolic blood pressure, serum creatinine, a history of CHD, and ACE inhibitor and NSAID use (P > 0.1 in each case). When the metformin-treated patients were considered separately, there was no correlation between plasma lactate and the daily dose of metformin in the same model (P = 0.33).
Haemodynamic and metabolic effects in metformin-treated patients
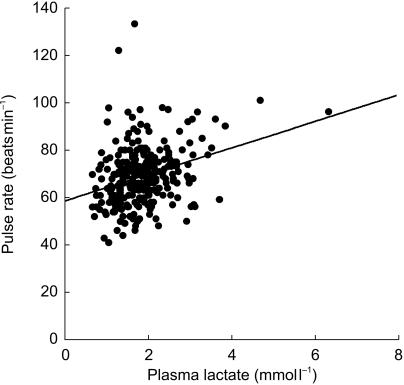
Factors associated with the resting pulse rate were also assessed in a linear regression model. The logarithm of the fasting plasma lactate (P < 0.001), diastolic blood pressure (P < 0.001) and female sex (P = 0.004) were strong independent and positive predictors, while use of β-adrenoceptor blocker therapy (P = 0.005) and systolic blood pressure (P = 0.006) were inversely associated. The bivariate association between plasma lactate and pulse rate is shown in Figure 4. Age, diabetes duration, BMI, the logarithm of both fasting plasma glucose and HbA1c, and metformin use were variables that were entered into, but excluded from, the analysis (P > 0.09).
Figure 4.
Scattergram showing the association between plasma lactate and resting pulse rate in all patients in the present study. The line of best fit is also shown.
In the case of the serum bicarbonate, both BMI and ACE inhibitor use were inversely associated (P ≤ 0.04), but age, sex, serum creatinine, the logarithm of fasting plasma glucose and lactate, the logarithm of the HbA1c, and metformin and β-adrenoceptor blocker use were the excluded independent variables (P > 0.1). In a similar model, metformin (P ≤ 0.001) and β-adrenoceptor blocker (P = 0.019) use were independently and positively predictive of the serum potassium while all the other variables were excluded (P > 0.1).
Factor analysis
The correlation matrix between variables that could influence, or be influenced by, the plasma lactate is shown in Table 3 and factor loadings after varimax rotation of principal components of the four factors identified in Table 4. In factor 1, plasma lactate and glucose, metformin treatment and pulse rate are clustered. In factor 2, age and body mass index cosegregate inversely. In factor 3, use of a β-adrenoceptor blocker is grouped with serum bicarbonate but inversely with pulse rate. Factor 4 is a cluster containing male sex and the serum creatinine as its significant loadings. When considered cumulatively, 56.7% of the variance in the data is explained by the four factors identified.
Table 3.
Pearson's correlation coefficient matrix for factors associated with plasma lactate in the present study.
| Age | Sex | Body mass index | Taking metformin | Serum creatinine | Serum bicarbonate | Pulse rate | Plasma glucose | Plasma lactate | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | −0.04 | ||||||||
| Body mass index | −0.35 | −0.04 | |||||||
| Taking metformin | −0.03 | −0.06 | 0.14⋆⋆ | ||||||
| Serum creatinine | 0.21⋆⋆ | 0.31⋆⋆ | −0.03 | 0.05 | |||||
| Serum bicarbonate | 0.11 | 0.02 | −0.16⋆⋆ | −0.13⋆ | −0.04 | ||||
| Pulse rate | −0.03 | −0.12⋆ | 0.18⋆⋆ | 0.18⋆⋆ | −0.01 | −0.16⋆⋆ | |||
| Plasma glucose | −0.07 | 0.07 | 0.12⋆ | 0.21⋆⋆ | −0.05 | −0.11⋆ | 0.08 | ||
| Plasma lactate | −0.14 | −0.06 | 0.25⋆⋆ | 0.20⋆⋆ | −0.01 | −0.13⋆ | 0.29⋆⋆ | 0.28⋆⋆ | |
| Taking β-adrenoceptor blocker | 0.09 | 0.02 | −0.09 | −0.04 | 0.05 | 0.10⋆ | −0.15⋆⋆ | 0.02 | 0.03 |
P < 0.05
P < 0.01.
Table 4.
Loadings for factors identified after varimax rotation of the results of principal component analysis.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| Age | −0.01 | 0.86⋆ | 0.02 | 0.06 |
| Sex | −0.06 | −0.17 | 0.14 | 0.83⋆ |
| Body mass index | 0.26 | −0.63⋆ | −0.26 | 0.01 |
| Taking metformin | 0.59⋆ | 0.10 | −0.24 | −0.01 |
| Pulse rate | 0.36⋆ | 0.08 | −0.62⋆ | −0.17 |
| Serum creatinine | 0.06 | 0.32 | −0.16 | 0.77⋆ |
| Serum bicarbonate | −0.19 | 0.15 | 0.51⋆ | −0.11 |
| Fasting plasma glucose | 0.66⋆ | −0.18 | 0.16 | 0.08 |
| Fasting plasma lactate | 0.70⋆ | −0.18 | −0.12 | −0.07 |
| Taking β-adrenoceptor blocker | 0.30 | 0.17 | 0.69⋆ | 0.02 |
| Variance explained (%) | 20.3 | 13.5 | 11.7 | 11.1 |
Loading > ± 0.34.
Discussion
The present results show that metformin therapy is associated with an increase in the fasting plasma lactate concentration in ambulant patients with type 2 diabetes from a community-based cohort. The adjusted 0.16 mmol l−1 difference between mean plasma lactate concentrations in our two main groups of subjects is consistent with previously published reports [17, 18]. In addition, its relatively small magnitude might also explain why it was not evident in other studies with limited subject numbers [15]. In a larger scale study by De Fronzo and coworkers [16], a nonsignificant mean increase in fasting plasma lactate of 0.04–0.08 mmol l−1 was observed in three groups of metformin-treated patients while, in those allocated placebo or glibenclamide, the mean lactate concentrations before and after 6 months of therapy were virtually identical [16]. Taken together, available data suggest that metformin per se has a measurable but limited effect on lactate metabolism in type 2 diabetes.
Other variables that were independently associated with fasting plasma lactate in regression analysis were the simultaneous plasma glucose and BMI. Similar associations have been found previously in type 2 diabetes [22, 24, 25]. We did not, in contrast to a previous study of type 2 diabetic patients in which systolic blood pressure and plasma lactate concentration correlated significantly [23], find a relationship between blood pressure and plasma lactate, nor did we observe independent associations with CHD, renal function, or use of either ACE inhibitors or NSAIDs. These findings, although reassuring, do not rule out the possibility that metformin treatment may, in certain clinical states such as cardiac failure and renal insufficiency [6], increase the risk of lactic acidosis, especially in the presence of potentially nephrotoxic drugs [11, 12]. However, available epidemiological evidence suggests that such situations are relatively rare [6–8].
Although the effect of metformin on plasma lactate was limited in our patients, more than one third of the total sample had a raised fasting plasma lactate concentration (> 2.0 mmol l−1). Probably because metformin is used commonly in overweight patients and in those who have had unacceptable glycaemic control on sulphonylurea therapy, metformin-treated subjects were over-represented amongst such cases. Nevertheless, our data show that a single raised plasma lactate may, on its own, have little practical use in assessing the risk of lactic acidosis in a metformin-treated patient. An argument could be made for using a cut-off of 2.7 mmol l−1 for reducing or stopping metformin [6, 16], but 1 in 11 of our ambulant patients had a plasma lactate in this range irrespective of treatment. Furthermore, in those on metformin, there was no association between plasma lactate and daily dose, a finding that is consistent with previous studies in acidotic patients [5] and one that argues for complete cessation of the drug rather than dose reduction if there are significant metabolic concerns.
We used the serum bicarbonate as a simple index of acid-base status in our patients but found no association with plasma lactate or metformin therapy. However, even in injury and sepsis, the plasma lactate may be an unreliable marker of tissue hypoxia and acidosis. It has been hypothesized that adrenaline-stimulated Na+, K+-ATPase activity accelerates glycolysis in well-oxygenated skeletal muscle and that this can, on its own, elevate plasma lactate concentrations in stress states [27]. Sympathetic hyperactivity is also associated with the metabolic syndrome and can be reduced by dietary control [28].
If this situation applied to our patients with type 2 diabetes, it might help to explain the associations between glycaemia, BMI, plasma lactate and resting pulse rate we observed. Sympathetic overactivity that augmented circulating adrenaline concentrations would increase plasma lactate and pulse rate. There was a significant and positive association between these two variables in our total series that was independent of other variables. Although plasma catecholamine measurements were beyond the scope of the present study, we hypothesize that sympathetic overactivity associated with overweight can increase the plasma lactate in type 2 diabetes and that this can be exacerbated by increased substrate supply in hyperglycaemic patients. Our regression analyses suggest that metformin does not play a role in this scheme, especially since there was a positive association with serum potassium. The serum potassium would be expected to fall if Na+, K+-ATPase activity were increased by the drug, thus increasing the flux of potassium into cells. In addition, the introduction of metformin does not alter plasma catecholamine concentrations in humans [29].
Factor analysis confirmed that plasma lactate, plasma glucose and metformin therapy cosegregated with the resting pulse rate in factor 1. Although the positive loading for BMI was not significant, the simplest interpretation for this strong clustering is that metformin treatment is given to hyperglycaemic patients with other features of the metabolic syndrome. This combination leads to hyperlactaemia through (i) increased sympathetic activity, (ii) increased substrate (glucose) availability and (iii) a direct metformin effect. Consistent with the fact that raised plasma lactate concentrations may not be associated with tissue hypoxia and incipient acidosis, factor 3 shows that the serum bicarbonate does not segregate with plasma lactate but rather with the expected reciprocal relationship between the use of β-adrenoceptor blockers and resting pulse rate. Factors 2 and 4, namely those relating to the reciprocal clustering of age and BMI, and to the cosegregation of male sex and serum creatinine, respectively, are also in accord with known population associations [30, 31], which adds support to the validity of the analysis.
Our data, from a community-based sample of ambulant patients on stable therapy for type 2 diabetes, confirm that metformin is associated with a small but significant increase in the fasting plasma lactate concentration. This increase is unrelated to dose. Both hyperglycaemia and overweight have a stronger association with plasma lactate, and our data provide some indirect evidence that sympathetic overactivity may play a role in this relationship. The possible mechanisms underlying the effect of metformin on lactate metabolism remain incompletely understood [32] but our data suggest that it does not act through increased adrenaline secretion.
Although limited by their cross-sectional nature, the present data suggest that the plasma lactate alone is insufficient to monitor effectively patients at risk of lactic acidosis whether they are metformin-treated or not. Measures of acid-base status should also be obtained in each case. If there is evidence of an acidosis and the patient is on metformin, the drug should be stopped rather than be given in a reduced dose. If there is no evidence of an acidosis but the risk of acidosis persists, the plasma lactate and acid-base status should be assessed serially. Given levels of plasma lactate that have been proposed as clinically significant [6, 13, 16] and the present data, we suggest that such monitoring is intensified in metformin-treated patients when (i) the plasma lactate is > 5.0 mmol l−1, or (ii) the plasma lactate is > 2.7 mmol l−1 and the patient does not have obesity or poor glycaemic control. Although metformin remains a relatively safe drug for the treatment of diabetes, any concerns raised by the results of such monitoring should also prompt its complete cessation.
Acknowledgments
We are grateful to Giovanna Stuccio and Mary Balme for help with collecting clinical information. We thank staff of the Biochemistry Department at Fremantle Hospital for performing routine laboratory tests and lactate assays. The present study was funded by the Novo Nordisk Regional Grants Scheme. The Fremantle Diabetes Study has been supported by the Raine/University of Western Australia Research Foundation and the Health Department of Western Australia.
References
- 1.UKPDS Group. UKPDS 28: a randomised trial of efficacy of early addition of metformin in sulphonylurea-treated non-insulin dependent diabetes. Diabetes Care. 1998;21:87–92. doi: 10.2337/diacare.21.1.87. [DOI] [PubMed] [Google Scholar]
- 2.UKPDS Group. UKPDS 34: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 3.Howlett HC, Bailey CJ. A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug Safety. 1999;20:489–503. doi: 10.2165/00002018-199920060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wiholm BE, Myrhed M. Metformin-associated lactic acidosis in Sweden 1977-1991. Eur J Clin Pharmacol. 1993;44:589–591. doi: 10.1007/BF02440866. [DOI] [PubMed] [Google Scholar]
- 5.Lalau JD, Lacroix C, Compagnon P, et al. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes Care. 1995;18:779–784. doi: 10.2337/diacare.18.6.779. [DOI] [PubMed] [Google Scholar]
- 6.Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338:265–266. doi: 10.1056/NEJM199801223380415. [DOI] [PubMed] [Google Scholar]
- 7.Campbell IW. Metformin and the sulphonylureas: the comparative risk. Horm Metab Res. 1985;15(Suppl):105–111. [PubMed] [Google Scholar]
- 8.Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659–1663. doi: 10.2337/diacare.21.10.1659. [DOI] [PubMed] [Google Scholar]
- 9.Sulkin TV, Bosman D, Krentz AJ. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care. 1997;20:925–928. doi: 10.2337/diacare.20.6.925. [DOI] [PubMed] [Google Scholar]
- 10.Emslie-Smith A, Boyle DIR, Evans JMM, et al. Contra-indications to metformin therapy in patients with diabetes: a population-based study. Diabetologia. 1999;42(Suppl1):A3. (Abstract) [Google Scholar]
- 11.Chan NN, Fauvel NJ, Feher MD. Non-steroidal anti-inflammatory drugs and metformin: a cause for concern? Lancet. 1998;352:201. doi: 10.1016/s0140-6736(05)77806-5. [DOI] [PubMed] [Google Scholar]
- 12.Franzetti I, Paolo D, Marco G, Emanuela M, Elisabetta Z, Renato U. Possible synergistic effect of metformin and enalapril on the development of hyperkaliemic lactic acidosis. Diabetes Res Clin Pract. 1997;38:173–176. doi: 10.1016/s0168-8227(97)00098-3. [DOI] [PubMed] [Google Scholar]
- 13.Stacpoole PW, Harman EM, Curry SH, Baumgartner TG, Misbin RI. Treatment of lactic acidosis with dichloroacetate. N Engl J Med. 1983;309:390–396. doi: 10.1056/NEJM198308183090702. [DOI] [PubMed] [Google Scholar]
- 14.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 15.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 17.Vigneri R, Goldfine ID. Role of metformin in treatment of diabetes mellitus. Diabetes Care. 1987;10:118–122. doi: 10.2337/diacare.10.1.118. [DOI] [PubMed] [Google Scholar]
- 18.Fery F, Plat L, Balasse E. Effects of metformin on the pathways of glucose utilization after oral glucose in non-insulin-dependent diabetes mellitus patients. Metabolism. 1997;46:227–233. doi: 10.1016/s0026-0495(97)90307-3. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti P, Gregorio F, Benzi L, et al. Diurnal pattern of plasma metformin concentrations and its relation to metabolic effects in type 2 (non-insulin-dependent) diabetic patients. Diabete Metab. 1990;16:473–478. [PubMed] [Google Scholar]
- 20.McAlpine LG, McAlpine CH, Waclawski ER, Storer AM, Kay JW, Frier BM. A comparison of treatment with metformin and gliclazide in patients with non-insulin-dependent diabetes. Eur J Clin Pharmacol. 1988;34:129–132. doi: 10.1007/BF00614548. [DOI] [PubMed] [Google Scholar]
- 21.Waters AK, Morgan DB, Wales JK. Blood lactate and pyruvate levels in diabetic patients treated with biguanides with and without sulphonylureas. Diabetologia. 1978;14:95–98. doi: 10.1007/BF01263446. [DOI] [PubMed] [Google Scholar]
- 22.Iannello S, Campione R, Belfiore F. Response of insulin, glucagon, lactate, and nonesterified fatty acids to glucose in visceral obesity with and without NIDDM. relationship to hypertension. Mol Genet Metab. 1998;63:214–223. doi: 10.1006/mgme.1997.2670. [DOI] [PubMed] [Google Scholar]
- 23.Jansson PA, Larsson A, Lonnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813–818. doi: 10.1046/j.1365-2362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 24.Gan SC, Barr J, Arieff AI, Pearl RG. Biguanide-associated lactic acidosis. Case report and review of the literature. Arch Intern Med. 1992;152:2333–2336. doi: 10.1001/archinte.152.11.2333. [DOI] [PubMed] [Google Scholar]
- 25.Vaag A, Alford F, Henriksen FL, Christopher M, Beck Nielsen H. Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycaemia of NIDDM. Diabetologia. 1995;38:326–336. doi: 10.1007/BF00400638. [DOI] [PubMed] [Google Scholar]
- 26.Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate data analysis with readings. 4. New Jersey: Prentice Hall; 1995. [Google Scholar]
- 27.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–508. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- 28.Gohler L, Hahnemann T, Michael N, et al. Reduction of plasma catecholamines in humans during clinically controlled severe underfeeding. Prev Med. 2000;30:95–102. doi: 10.1006/pmed.1999.0602. [DOI] [PubMed] [Google Scholar]
- 29.Marfella R, Acampora R, Verrazzo G, et al. Metformin improves hemodynamic and rheological responses to l-arginine in NIDDM patients. Diabetes Care. 1996;19:934–939. doi: 10.2337/diacare.19.9.934. [DOI] [PubMed] [Google Scholar]
- 30.Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet. 1979;4:200–222. doi: 10.2165/00003088-197904030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cornoni Huntley JC, Harris TB, Everett DF, et al. An overview of body weight of older persons, including the impact on mortality. The National Health and Nutrition Examination Survey I – Epidemiologic Follow-up Study. J Clin Epidemiol. 1991;44:743–753. doi: 10.1016/0895-4356(91)90126-t. [DOI] [PubMed] [Google Scholar]
- 32.Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49:721–749. doi: 10.2165/00003495-199549050-00007. [DOI] [PubMed] [Google Scholar]