Abstract
Aims
To compare the haemodynamic responses of proadrenomedullin N-terminal 20 peptide (PAMP) and adrenomedullin (ADM) in the forearm vascular bed of healthy male volunteers, and to investigate the role of neutral endopeptidase (NEP) in the metabolism of ADM.
Methods
On two separate occasions, ADM (1–30 pmol min−1) and PAMP (100–3000 pmol min−1) were infused into the brachial artery of eight male subjects, and forearm blood flow (FBF) assessed using venous occlusion plethysmography. In a second study, eight male subjects received the same doses of ADM, co-infused with either the NEP inhibitor thiorphan (30 nmol min−1) or the control vasoconstrictor noradrenaline (120 pmol min−1), on separate occasions. Both studies were conducted in a double-blind, randomized manner.
Results
ADM and PAMP produced a dose-dependent increase in FBF (P ≤ 0.002). Based on the dose producing a 50% increase in FBF, ADM was ∼60 times more potent than PAMP. Thiorphan and noradrenaline produced similar reductions in FBF of 14 ± 4% (mean ± s.e. mean) and 22 ± 6%, respectively (P = 0.4). However, the area under the dose–response curve was significantly greater during co-infusion of ADM with thiorphan than with noradrenaline (P = 0.028), as was the maximum increase in FBF ratio (2.1 ± 1.0 vs 1.2 ± 0.2; P = 0.030).
Conclusions
ADM and PAMP both produce a local dose-related vasodilatation in the human forearm, but PAMP is ∼60 times less potent than ADM. In addition, NEP inhibition potentiates the haemodynamic effects of ADM. These findings suggest that PAMP may not play a role in the physiological regulation of blood flow. However, in pathophysiological conditions such as hypertension and heart failure, NEP inhibition may exert a beneficial effect by increasing the biological activity of ADM.
Keywords: adrenomedullin, arteries, forearm blood flow, neutral endopeptidase, PAMP
Introduction
Adrenomedullin (ADM) and proadrenomedullin NH2-terminal 20 peptide (PAMP) are recently discovered hypotensive peptides encoded by a single gene [1, 2]. Transcription yields a precursor peptide, proadrenomedullin, from which ADM and PAMP are enzymatically cleaved. Both peptides have a similar widespread tissue distribution throughout the body [3], and are present in plasma in healthy subjects in concentrations of ∼5–10 pmol l−1 [4, 5]. In addition, ADM is actively synthesized and secreted by human vascular endothelial and smooth muscle cells, which also possess ADM receptors [3, 6, 7]. In animal models, ADM and PAMP are both potent vasodilators and result in sustained hypotension, although PAMP appears to be 30–100 times less potent than ADM [8–11]. Adrenomedullin also has a number of other diverse biological actions including natriuresis, diuresis, positive inotropism and antiproliferative effects [3].
We have previously shown that ADM produces a dose-dependent vasodilatation in the human forearm vascular bed [12]. Moreover, systemic administration of ADM, in healthy subjects, results in a sustained decrease in arterial pressure [13]. In contrast, data concerning the effects of PAMP in humans are limited. Our preliminary data [14] and data from a Japanese population [15] indicate that PAMP is less potent than ADM, suggesting that PAMP may not play an important physiological role in the regulation of vascular tone or blood pressure [15].
In patients with congestive cardiac failure, plasma ADM concentrations are raised, suggesting that ADM may play a role in the regulation of pressure and volume homeostasis in this condition [4, 16]. Interestingly, infusion of ADM in patients with congestive cardiac failure reduces pulmonary capillary wedge pressure, increases cardiac output, causes a natriuresis, but has little effect on peripheral arterial pressure, suggesting that potentiation of ADM may be beneficial in heart failure [17]. The pathways responsible for ADM clearance remain to be fully elucidated but recent evidence suggests that neutral endopeptidase (NEP) may be involved in the breakdown of ADM [18]. Therefore, potentiation of ADM by NEP inhibition may be clinically relevant, especially in patients with heart failure.
Based on animal studies, and data from a Japanese population, we hypothesized that PAMP would induce vasodilatation in humans in vivo but be less potent that ADM, and that inhibition of NEP would potentiate the vasodilator effect of ADM. The aim of this study was to test these hypotheses in the human forearm vascular bed using intra-arterial drug administration and venous occlusion plethysmography to assess forearm blood flow (FBF).
Methods
Subjects
Sixteen healthy, normotensive, male volunteers between 26 and 47 years of age participated in the two studies. Both investigations were approved of the Local Research Ethics Committee, and written informed consent was obtained from each subject. All subjects were non-smokers and free from medication, and abstained from alcohol and caffeine-containing products for 24 h before the study. Following an overnight fast, subjects rested supine in a quiet temperature-controlled laboratory (21–24 °C) for 30 min before the start of the study.
Drugs
All drugs were prepared aseptically from freshly opened vials, using 0.9% saline (Baxter; Norfolk; UK) as a diluent. Adrenomedullin (The Peptide Institute; Osaka, Japan) was infused intra-arterially at doses of 1, 3, 10, and 30 pmol min−1, and PAMP (The Peptide Institute) at doses of 100, 300, 1000, and 3000 pmol min−1. The doses of PAMP were based on comparative animal data and pilot studies conducted in two healthy volunteers (data not shown). Thiorphan (Sigma-Aldrich; Poole, UK) was infused at 30 nmol min−1, based on previous studies [19, 20], and noradrenaline (Levophed; Abbott, Maidenhead, UK) at 120 pmol min−1, a dose selected from the published literature to produce a similar reduction in FBF to thiorphan [21]. The total infusion rate was kept constant at 1 ml min−1 throughout each study.
Intra-arterial administration
A 27 gauge steel cannula (Cooper's Needle Works; Birmingham, UK), attached to a 16 gauge epidural catheter (Portex; Kent, UK), was inserted into the left brachial artery under local anaesthesia, using ∼1 ml of 1% lignocaine hydrochloride (Antigen Pharmaceuticals; Southport, UK). Patency was maintained by continuous infusion of 0.9% saline or drugs at a rate of 1.0 ml min−1, via a P1000 IVAC infusion pump (Alaris; Hampshire, UK).
Forearm blood flow
Blood flow was measured simultaneously in both arms using venous occlusion plethysmography, with temperature-compensated, indium/gallium-in-silastic strain gauges applied to the widest part of the forearm, as previously described [22]. The hands were excluded from the circulation during each measurement period by inflation of a wrist cuff to 220 mmHg. Upper arm cuffs were inflated intermittently to 40 mmHg for 10 in every 15 s to prevent, temporarily, venous outflow from the forearm and, thus, obtain plethysmographic recordings. Blood flows were recorded during the final 3 min of each infusion period, and the mean of the final five measurements used for analysis. Data were collected using a dual channel strain-gauge plethysmograph (Hokanson) and a computer-based, R wave triggered, system for on-line, semicontinuous monitoring of FBF, as described previously [23].
Brachial artery blood pressure
Peripheral blood pressure was measured in the brachial artery of the dominant arm using the validated Omron HEM-705CP oscillometric sphygmomanometer [24]. Mean arterial pressure (MAP) was calculated as the diastolic pressure plus one-third of the pulse pressure.
Study 1 Comparison of adrenomedullin and PAMP
Eight male volunteers, aged 26–47 years, attended on two separate occasions at least 1 week apart. Basal values of FBF were established during a 30 min period of intra-arterial infusion of saline. Adrenomedullin was then infused in a stepwise manner at doses of 1, 3, 10 and 30 pmol min−1, each for 6 min and, on another occasion, PAMP at doses of 100, 300, 1000 and 3000 pmol min−1, each for 6 min. The order of the infusions was randomized and administration was double-blind. Blood pressure and heart rate were recorded at the end of each infusion period.
Study 2 Adrenomedullin with co-infusion of thiorphan or noradrenaline
Eight healthy male volunteers, aged 26–44 years, were studied on two occasions, separated by at least 1 week. At each visit, saline was infused intra-arterially for 30 min, followed by 48 min of either thiorphan 30 nmol min−1 or noradrenaline 120 pmol min−1. The order of the infusions was randomized, and administration was double-blind. Adrenomedullin was then co-infused at doses of 1, 3, 10 and 30 pmol min−1, each dose for 6 min. Blood pressure and heart rate were recorded at the end of each infusion period.
Data analysis and statistics
The ratio of FBF in the infused to non-infused arm was calculated for each time point, as previously described [25], and expressed as the percentage change from baseline or, in the case of Study 2, as the percentage change from the last recording during infusion of thiorphan or noradrenaline alone [19]. Data are expressed as means ± s.e. mean and [95% confidence limits] for differences, and were examined using analysis of variance (anova), or paired Student's t-tests, as appropriate. The dose of peptide producing a 50% increase in the FBF ratio (Study 1), or area under the dose–response curve (AUC) and FBF ratio at the 30 pmol min−1 dose of ADM (Study 2) were used as predefined summary measure statistics. P < 0.05 was considered as significant.
Results
Study 1
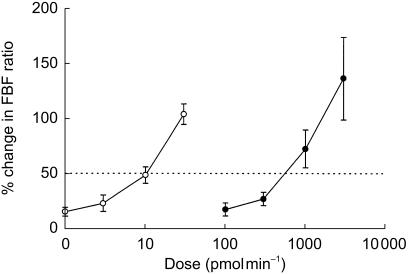
Basal MAP, heart rate and the FBF ratio were similar on the two study days, and there was no difference in blood flow between the two arms. There was no significant change in blood flow in the noninfused (control) arm, heart rate or MAP, throughout either study period (Table 1), confirming that the doses of ADM and PAMP infused did not produce systemic haemodynamic effects. Adrenomedullin and PAMP caused a dose-dependent increase in the FBF ratio (P = 0.001 and P = 0.002, respectively), as shown in Figure 1. Blood flow in the infused arm is presented in Table 1. The estimated dose required to produce a 50% increase in FBF ratio was significantly lower for ADM compared with PAMP (11 ± 2 vs 656 ± 106 pmol min−1; mean difference 645 [349, 941]; P = 0.015).
Table 1.
Mean arterial pressure, heart rate and blood flow in the infused arm during Study 1.
| ADM (pmol min−1) | PAMP (pmol min−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline | 1 | 3 | 10 | 30 | Saline | 100 | 300 | 1000 | 3000 | |
| FBF (ml 100 ml−1 min−1) | 5.2 ± 0.9 | 5.8 ± 1.0 | 5.8 ± 0.9 | 6.6 ± 1.0 | 8.7 ± 1.2 | 4.2 ± 0.7 | 3.9 ± 0.6 | 5.4 ± 0.8 | 6.8 ± 0.9 | 9.2 ± 1.2 |
| MAP (mmHg) | 86 ± 2 | 89 ± 4 | 87 ± 3 | 87 ± 3 | 92 ± 3 | 92 ± 3 | 93 ± 3 | 91 ± 3 | 90 ± 3 | 90 ± 3 |
| Heart rate (beats min−1) | 65 ± 1 | 69 ± 3 | 68 ± 2 | 64 ± 3 | 66 ± 3 | 66 ± 1 | 65 ± 3 | 65 ± 4 | 65 ± 3 | 65 ± 2 |
ADM = adrenomedullin; PAMP = proadrenomedullin NH2-terminal 20 peptide; FBF = absolute blood flow in the infused forearm; MAP = mean arterial pressure. Values are means ± s.e. mean, n = 8.
Figure 1.
Comparison of the effect of intra-arterial infusion of adrenomedullin (○) and proadrenomedullin NH2-terminal 20 peptide (•) on forearm blood flow. The dotted line represents a 50% increase in the FBF ratio, which was used as a summary measure statistic to compare the potency of the two drugs. n = 8.
Study 2
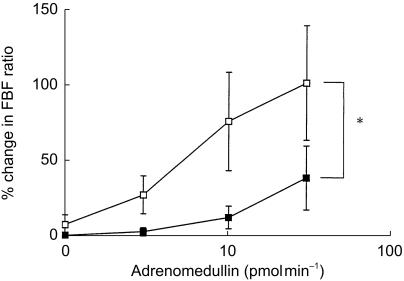
Basal MAP, heart rate and the FBF ratio were similar on the two study days, and there was no difference in blood flow between the two arms. There was no significant change in blood flow in the noninfused (control) arm, heart rate or MAP, throughout either study period (Table 2). There was a 14 ± 4% reduction in the FBF ratio following infusion of thiorphan (P = 0.030), and a 22 ± 6% reduction after noradrenaline (P = 0.018). Neither, the percentage change in the FBF ratio (mean difference 8% [−14, 35]; P = 0.4), nor the FBF ratio immediately prior to ADM infusion (1.1 ± 0.1 vs 0.9 ± 0.1; mean difference 0.2 [−0.2, 0.6]; P = 0.4), differed significantly between the two drugs. Co-infusion of 1, 3, 10 and 30 pmol min−1 ADM with noradrenaline resulted in a dose-dependent increase in the FBF ratio (P = 0.007), as did coinfusion with thiorphan (P = 0.003) (Figure 2). However, the area under the dose–response curve was significantly greater during co-infusion of ADM with thiorphan compared with noradrenaline (51 ± 8 vs 31 ± 3 AU; mean difference 20 [3, 37]; P = 0.028), as was the maximum FBF ratio (2.1 ± 1.0 vs 1.2 ± 0.2; mean difference 0.9[0.1–1.8]; P = 0.030). Compared with infusion of ADM alone in Study 1, the response to ADM was reduced during co-infusion of noradrenaline (P ≤ 0.001), but not thiorphan (P = 0.7).
Table 2.
Mean arterial pressure, heart rate and blood flow in the infused arm during Study 2.
| Thiorphan | Noradrenaline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adrenomedullin (pmol min−1) | Baseline | 1 | 3 | 10 | 30 | Baseline | 1 | 3 | 10 | 30 |
| FBF (ml 100 ml−1 min−1) | 3.9 ± 0.7 | 4.1 ± 0.7 | 4.5 ± 0.6 | 5.5 ± 0.7 | 7.2 ± 0.8 | 4.1 ± 0.8 | 3.9 ± 0.6 | 4.0 ± 0.7 | 4.2 ± 0.8 | 4.7 ± 0.8 |
| MAP (mmHg) | 91 ± 3 | 88 ± 2 | 88 ± 1 | 87 ± 2 | 90 ± 2 | 91 ± 4 | 93 ± 4 | 92 ± 3 | 92 ± 3 | 94 ± 3 |
| Heart rate (beats min−1) | 59 ± 1 | 54 ± 2 | 59 ± 1 | 60 ± 1 | 58 ± 2 | 63 ± 2 | 63 ± 2 | 64 ± 2 | 63 ± 2 | 63 ± 1 |
FBF = absolute blood flow in the infused forearm; MAP = mean arterial pressure. Values are means ± s.e. mean, n = 8.
Figure 2.
Comparison of the effect of intra-arterial adrenomedullin on forearm blood flow when co-infused with either thiorphan (□) or noradrenaline (▪). n = 8; ⋆P = 0.03 (AUC).
Discussion
The aims of the present study were to compare the response to ADM and PAMP in forearm resistance vessels in vivo, and to examine the effect of the NEP inhibitor thiorphan and the control vasoconstrictor noradrenaline on the response to ADM. The main findings were that, although ADM and PAMP both produced vasodilatation in the forearm vascular bed in healthy male subjects, PAMP was ∼60 times less potent than ADM, and that the response to ADM was substantially potentiated by thiorphan.
Adrenomedullin is a potent vasodilator in a number of vascular beds in several species including the rat [8] and cat [10, 26], and we have previously demonstrated vasodilatation to ADM in the human forearm vascular bed [12]. Proadrenomedullin N-terminal 20 peptide causes hypotension when injected intravenously into anaesthetized rats [2], and is a vasodilator in cat mesenteric and hind limb resistance vessels [10, 11], although in these models it is between 30 and 100 times less potent than ADM. Only one previous study has investigated the relative potencies of the two peptides in the human forearm vascular bed, concluding that PAMP is ∼100 times less potent than ADM, in Japanese subjects [15]. The results of the present study are in broad agreement with these observations as we found PAMP to be ∼60 times less potent a vasodilator than ADM, in Caucasian subjects. The lack of change in the control arm provides evidence that the dose-dependent vasodilatation occurs in response to a local effect of ADM or PAMP in the forearm, rather than via a centrally mediated mechanism. However, given that the circulating concentrations of ADM and PAMP are similar (in the range 5–10 pmol l−1), and that the local concentration of PAMP in the forearm during the highest infusion rate would have been ∼60 nmol l−1 (assuming a FBF of 50 ml min−1), it seems unlikely that PAMP is an important circulating physiological regulator of arterial tone. Although PAMP may be more important in regulating blood flow in other vascular beds, it should be recognized that the forearm has proved a reliable predictor of the systemic response to number of peptides; including angiotensin II [27, 28], endothelin-1 [29, 30], bradykinin [31, 32], and ADM [12, 13]. However, the issue of the physiological relevance of PAMP can only really be resolved with the use of receptor antagonists, which are not currently available. It has also been suggested that PAMP may produce vasodilatation through inhibition of peripheral sympathetic neurotransmission [9], although others have been unable to confirm this [10]. If, indeed, PAMP does inhibit sympathetic activity, then it may produce a more profound vasodilatation in situations of increased sympathetic nervous system activity, such as congestive heart failure.
Plasma ADM levels are increased in subjects with heart failure [4, 16, 33, 34], possibly as a compensatory response protecting against further elevation of peripheral vascular resistance [16, 33]. This view is supported by the observation that, as with other neurohormones, plasma concentrations of ADM decrease following effective treatment for heart failure [34]. In vivo studies indicate that NEP may be involved in the breakdown of ADM [18]. Therefore, in the present study we investigated the effect of the selective NEP inhibitor, thiorphan, on haemodynamic responses to ADM.
We have previously demonstrated that thiorphan reduces FBF by ∼15% in healthy subjects [20]. Therefore, we elected to use noradrenaline as a control vasoconstrictor, to reduce FBF to a similar degree, to avoid any potential confounding influence that preconstriction might have on the response to ADM [35, 36]. As expected, thiorphan and noradrenaline produced similar reductions in resting FBF and there was little difference in the FBF ratio immediately before infusion of ADM. Despite this, the increase in FBF produced by the highest dose of ADM (30 pmol min−1) was substantially greater when it was co-infused with thiorphan rather than noradrenaline, as was the area under the dose–response curve, indicating a potentiation of the response to ADM by NEP inhibition. Although, in vitro evidence supporting a role for NEP in ADM degradation is, at present, conflicting [37, 38], our observations are consistent with the work of Lisy et al. [18], who have previously reported a rise in plasma ADM, and potentiation of the natriuretic effect of ADM, in dogs following administration of the selective NEP inhibitor candoxatrilat [18]. However, since the response to ADM was reduced during co-infusion of noradrenaline, compared with infusion of ADM alone, we cannot exclude the possibility that there was physiological antagonism of the dilator effect of ADM by noradrenaline. Nevertheless, the degree of vasoconstriction induced by noradrenaline and thiorphan was similar.
In summary, PAMP is a less potent vasodilator in human resistance vessels than ADM, and the haemodynamic response to ADM is potentiated by NEP inhibition. This may be clinically relevant since infusion of ADM has a beneficial haemodynamic effect in patients with congestive cardiac failure [17]. Indeed, potentiation of haemodynamic and natriuretic properties of endogenous ADM may be a useful strategy for patients with heart failure and may, in part, explain the favourable haemodynamic response to NEP inhibitors [39] and combined NEP/angiotensin converting enzyme inhibitors in patients with heart failure [40].
Acknowledgments
Professor D. J. Webb is currently in receipt of a Research Leave Fellowship from the Wellcome Trust (052633). We would like to thank Mrs Fiona Strachan for technical assistance.
References
- 1.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:159–164. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura K, Kangawa K, Ishiyama Y, et al. Identification and hypotensive activity of proadrenomedullin N-terminal 20 peptide (PAMP) FEBS Lett. 1994;351:35–37. doi: 10.1016/0014-5793(94)00810-8. [DOI] [PubMed] [Google Scholar]
- 3.Richards AM, Nicholls MG, Lewis L, Lainchbury JG. Adrenomedullin. Clin Sci. 1996;91:3–16. doi: 10.1042/cs0910003. [DOI] [PubMed] [Google Scholar]
- 4.Cheung B, Leung R. Elevated plasma levels of human adrenomedullin in cardiovascular, respiratory, hepatic and renal disorders. Clin Sci. 1997;92:59–62. doi: 10.1042/cs0920059. [DOI] [PubMed] [Google Scholar]
- 5.Taniyama M, Kitamura K, Ban Y, Sugita E, Ito K, Katagiri T. Elevation of circulating proadrenomedullin N, terminal 20-peptide in thyrotoxicosis. Clin Endocrinol. 1997;46:271–274. doi: 10.1046/j.1365-2265.1997.1220934.x. [DOI] [PubMed] [Google Scholar]
- 6.Sugo S, Minamino N, Kangawa K, et al. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. doi: 10.1006/bbrc.1994.1827. 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- 7.Kato J, Kitamura K, Kangawa K, Eto T. Receptors for adrenomedullin in human vascular endothelial cells. Eur J Pharmacol. 1995;289:383–385. doi: 10.1016/0922-4106(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic effects of human and rat adrenomedullin in conscious rats. Br J Pharmacol. 1995;114:584–591. doi: 10.1111/j.1476-5381.1995.tb17179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimosawa T, Fujita T. Hypotensive effect of a newly identified peptide, proadrenomedullin N-terminal 20 peptide. Hypertension. 1996;28:325–329. doi: 10.1161/01.hyp.28.3.325. [DOI] [PubMed] [Google Scholar]
- 10.Champion HC, Erickson CC, III, Simoneaux ML, et al. Proadrenomedullin NH2-terminal 20 peptide has cAMP-mediated vasodilator activity in the mesenteric vascular bed of the cat. Peptides. 1996;17:1379–1387. doi: 10.1016/s0196-9781(96)00240-9. 10.1016/s0196-9781(96)00240-9. [DOI] [PubMed] [Google Scholar]
- 11.Champion HC, Murphy WA, Coy DH, Kadowitz PJ. Proadrenomedullin NH2-terminal 20 peptide has direct vasodilator activity in the cat. Am J Physiol. 1997;272:R1047–R1054. doi: 10.1152/ajpregu.1997.272.4.R1047. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft JR, Noon JP, Gardner-Medwin J, Bennett T. Haemodynamic effects of adrenomedullin in human resistance and capacitance vessels. Br J Clin Pharmacol. 1997;44:57–60. doi: 10.1046/j.1365-2125.1997.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lainchbury JG, Cooper GJS, Coy DH, et al. Adrenomedullin: a hypotensive hormone in man. Clin Sci. 1997;92:467–472. doi: 10.1042/cs0920467. [DOI] [PubMed] [Google Scholar]
- 14.McEniery CM, Wilkinson IB, Webb DJ, Cockcroft JR. The effect of adrenomedullin (ADM) and pro-adrenomedullin N-terminal 20 peptide (PAMP) in the human forearm vascular bed. Br J Clin Pharmacol. 1999;48:873P. doi: 10.1046/j.0306-5251.2001.1420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Yoshida H, Hiramori K. Comparison of vasodilator potency of adrenomedullin and proadrenomedullin N-terminal 20 peptide in humans. Life Sci. 1999;65:2151–2156. doi: 10.1016/s0024-3205(99)00480-4. 10.1016/s0024-3205(99)00480-4. [DOI] [PubMed] [Google Scholar]
- 16.Jougasaki M, Wei C-M, McKinley LJ, Burnett JC., Jr Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92:286–289. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]
- 18.Lisy O, Jougasaki M, Schirger JA, Chen HH, Barclay PT, Burnett JC., Jr Neutral endopeptidase inhibition potentiates the natriuretic actions of adrenomedullin. Am J Physiol. 1998;275:F410–F414. doi: 10.1152/ajprenal.1998.275.3.F410. [DOI] [PubMed] [Google Scholar]
- 19.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 20.Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- 21.Calver A, Collier J, Moncada S, Vallance P. Effect of local intra-arterial N (G)-monomethyl-l-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens. 1992;10:1025–1031. [PubMed] [Google Scholar]
- 22.Haynes WG, Strachan FE, Webb DJ. Endothelin ET (A) and ET (B) receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- 23.Chang PC, Verlinde R, van Brummelen P. A microcomputer-based R-wave triggered system for hemodynamic measurements in the forearm. Computer Biol Med. 1998;18:157–163. doi: 10.1016/0010-4825(88)90042-x. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self measurement of blood pressure according to the revised British Hypertension Society protocol: the Omron HEM-705CP, Philips HP5332 and Nissei DS-175. Blood Press Monitoring. 1996;1:55–61. [PubMed] [Google Scholar]
- 25.Greenfield ADM, Patterson GC. Reactions of the blood vessels of the human forearm to increases in transmural pressure. J Physiol. 1954;125:508–524. doi: 10.1113/jphysiol.1954.sp005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santiago JA, Garrison EA, Ventura VL, et al. Synthetic human adrenomedullin and adrenomedullin 15–52 have potent short-lived vasodilator activity in the hindlimb vascular bed of the cat. Life Sci. 1994;55:85–90. doi: 10.1016/0024-3205(94)00652-0. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin N, Cockcroft JR, Collier JG, et al. Local inhibition of converting enzyme and vascular responses to angiotensin and bradykinin in the human forearm. J Physiol. 1989;412:543–555. doi: 10.1113/jphysiol.1989.sp017630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collier JG, Robinson BF, Vane JR. Reduction of pressor effects of angiotensin I in man by synthetic nonapeptide (B.P.P.9a or SQ 20, 881) which inhibits converting enzyme. Lancet. 1973;i:72–74. doi: 10.1016/s0140-6736(73)90468-6. [DOI] [PubMed] [Google Scholar]
- 29.Clarke JG, Benjamin N, Larkin SW, Webb DJ, Davies GJ, Maseri A. Endothelin is a potent long-lasting vasoconstrictor in men. Am J Physiol. 1989;257:H2033–H2035. doi: 10.1152/ajpheart.1989.257.6.H2033. [DOI] [PubMed] [Google Scholar]
- 30.Vierhapper H, Wagner O, Nowotny P, Waldhausl W. Effect of endothelin-1 in man. Circulation. 1990;81:1415–1418. doi: 10.1161/01.cir.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 31.Cockcroft JR, Chowienczyk PJ, Brett SE, Ritter JM. Effect of N (G)-monomethyl-l-arginine on kinin-induced vasodilation in the human forearm. Br J Clin Pharmacol. 1994;38:307–310. doi: 10.1111/j.1365-2125.1994.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonner G, Preis S, Schunk U, Toussaint C, Kaufmann W. Hemodynamic effects of bradykinin on systemic and pulmonary circulation in healthy and hypertensive humans. J Cardiovasc Pharmacol. 1990;15:S46–S56. [PubMed] [Google Scholar]
- 33.Kato J, Kobayashi K, Etoh T, et al. Plasma adrenomedullin concentration in patients with heart failure. J Clin Endocrinol Metab. 1996;81:180–183. doi: 10.1210/jcem.81.1.8550749. [DOI] [PubMed] [Google Scholar]
- 34.Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26:1424–1431. doi: 10.1016/0735-1097(95)00338-X. 10.1016/0735-1097(95)00338-x. [DOI] [PubMed] [Google Scholar]
- 35.O'Kane KPJ, Webb DJ, Collier JG, Vallance PJT. Local L-N (G)-monomethyl-arginine attenuates the vasodilator action of bradykinin in the human forearm. Br J Clin Pharmacol. 1994;38:311–315. doi: 10.1111/j.1365-2125.1994.tb04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newby DE, Boon NA, Webb DJ. Comparison of forearm vasodilatation to substance P and acetylcholine: contribution of nitric oxide. Clin Sci. 1997;92:133–138. doi: 10.1042/cs0920133. [DOI] [PubMed] [Google Scholar]
- 37.Nagatomo Y, Kitamura K, Kangawa K, Fujimoto Y, Eto T. Proadrenomedullin N-terminal 20 peptide is rapidly cleaved by neutral endopeptidase. Biochem Biophys Res Commun. 1996;223:539–543. doi: 10.1006/bbrc.1996.0930. 10.1006/bbrc.1996.0930. [DOI] [PubMed] [Google Scholar]
- 38.Lewis LK, Smith MW, Brennan SO, Yandle TG, Richards AM, Nicholls MG. Degradation of human adrenomedullin (1–52) by plasma membrane enzymes and identification of metabolites. Peptides. 1997;18:733–739. doi: 10.1016/s0196-9781(97)00005-3. 10.1016/s0196-9781(97)00005-3. [DOI] [PubMed] [Google Scholar]
- 39.Chen HH, Schirger JA, Chau WL, et al. Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation. 1999;100:2443–2448. doi: 10.1161/01.cir.100.24.2443. [DOI] [PubMed] [Google Scholar]
- 40.Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]