Abstract
Aims
Smoking is a major risk factor for developing atherosclerosis. In order to understand the vascular abnormalities observed in smokers, we investigated vascular responsiveness in cigarette smokers.
Methods
We performed two consecutive matched group comparative studies to investigate vascular responsiveness using venous occlusion plethysmography. The mean effects of three incremental doses of each vasoactive agent are presented. Both studies compared smokers with nonsmokers.
Results
The first investigated 68 subjects (smokers = 29; mean ± s.d. ages; 24 ± 6 vs 25 ± 5 years; P = NS) and found smoking was associated with a significant blunting of the flow ratio between treated and untreated arms to endothelium-dependent vasodilatation to acetylcholine (mean ± s.d., nonsmokers vs smokers) 4.07 ± 2.18 vs 3.42 ± 1.79 (P = 0.04, 95% CI 0.02, 1.12). By contrast, there was no significant difference in the responses to the endothelium-independent vasodilators sodium nitroprusside and verapamil. Smoking was also associated with a significant impairment in endothelium-dependent vasoconstriction induced by monomethyl-l-arginine (L-NMMA) 0.78 ± 0.22 vs 0.87 ± 0.21 (P = 0.006, 95% CI −0.14, −0.02) and a trend to blunted endothelium-independent vasoconstrictor responses to noradrenaline. In the second study we investigated the response to angiotensin I and II in 23 subjects (smokers = 12; mean ± s.d. ages; 34 ± 10 vs 32 ± 11 years). There was significant impairment in smokers of the mean vasoconstrictor response to angiotensin I 0.51 ± 0.15 vs 0.59 ± 0.16 (nonsmokers vs smokers; P = 0.003, 95% CI −0.13, −0.03) and a nonsignificant trend towards impairment of the response to angiotensin II.
Conclusions
Cigarette smoking in male volunteers is associated with blunted basal and stimulated nitric oxide bioactivity. Endothelial independent vasodilator responses (to nitroprusside and verapamil) were unaltered in smokers. A defect in the vasoconstrictor response to angiotensin I was also seen.
Keywords: angiotensin I, endothelium, nitric oxide, smoking
Introduction
Smokers have a two and a half fold increase in coronary artery disease compared with nonsmokers [1] and increasing exposure to cigarette smoke increases the severity of atherosclerotic disease in animals [2] and man [3]. Cigarette smoke is directly toxic to the vascular endothelium [4, 5] and endothelial cells appear to be the principal target for cardiovascular risk factors in early atherogenesis [6]. Changes in nitric oxide (NO) bioactivity are thought be a contributor to smoking damage although previous studies have not produced a consistent picture.
Another effect of smoking could be that smoking has a significant impact on the vascular renin angiotensin system (RAS). Smoking increases the conversion of angiotensin 1 to angiotensin II [7] in isolated rat hearts, and angiotensin II increases free radical production [8], which can cause endothelial cell damage. Therefore there is logical reason to investigate the role of smoking on the RAS, with the expectation from previous in vitro work that we might observe accentuated angiotensin I responses because of increased conversion of angiotensin I to II. If so, an activated vascular RAS might be a contributor to smoking induced vascular damage.
In order to clarify the effect of smoking on vascular responses we performed two consecutive studies looking at smokers and nonsmokers, the first of which investigated the vascular responses to acetylcholine, nitroprusside, verapamil, L-NMMA and noradrenaline. The second study looked at the effects of angiotensin I and angiotensin II.
Methods
General clinical protocol
All subjects gave written informed consent to participate in both studies which were approved by the Tayside Medical Ethics Committee. For those subjects who rolled their own cigarettes, 25 g tobacco/week was deemed equivalent to 10 manufactured cigarettes per day. None had evidence of cardiovascular disease as determined by history or clinical examination.
Subjects attended a temperature-controlled room (23 °C) in our research unit at 08.45 h, following a 12 h fast where water was permitted. Volunteers refrained from cigarette consumption for a least 1 h prior to the study. After 20 min supine rest, baseline BP measurements were recorded. The brachial artery of the nondominant forearm was cannulated with a 26 gauge cannula mounted on a 16 gauge epidural catheter.
Vascular function was assessed using forearm venous occlusion plethysmography [9] (Medasonics, Mountain View, CA, USA) using bilateral strain gauges. Pneumatic cuffs were placed around both wrists and upper arms and those at the wrist inflated to 200 mmHg to isolate arterial circulation at the wrist and intermittently both upper arm cuffs were inflated to 30 mmHg to occlude venous return. The change in forearm volume was measured by mercury filled strain gauges (stretched to forearm circumference + 20%). Each data point is the mean of five repeated measures of forearm blood flow taken in the last minute of a 5 min drug infusion and were always taken with both sets of cuffs inflated. All drugs and doses were infused at 1 ml min−1, a rate found not to alter basal blood flow appreciably. The order of each drug infusion was the same for all subjects.
Blood was collected at the screening visit and on each study day for plasma urea, creatinine, cholesterol and high-density lipoprotein (HDL) cholesterol analysis.
Specific clinical protocol
Study 1: Forearm blood flow measurements were performed at baseline and then following each of three, 5 min incrementally increasing doses of acetylcholine (25, 50 and 100 nmol ml−1 of infusate) [10], sodium nitroprusside (4.2, 12.6 and 37.8 nmol ml−1 of infusate) [11] and verapamil (10, 20 and 40 nmol ml−1 of infusate). A period of 15–20 min was allowed for blood flow to return to baseline between each drug infusion. One week later in the same volunteers, we investigated the effect of endothelial-dependent vasoconstriction utilizing intra-arterial L-NMMA (1, 2 and 4 µmol ml−1 of infusate) and endothelial-independent vasoconstriction using noradrenaline (1, 2 and 4 pmol ml−1 of infusate). L-NMMA is a net vasoconstrictor due to a reduction in tonic NO production by endothelial cells. Noradrenaline is a direct vasoconstrictor (via its effects on α-adrenoceptors), but it has also been shown to release substantial amounts of NO, which makes this agent pharmacologically less clean than is desirable. Unfortunately, there are few better choices.
Study 2: Endothelial responses to intra-arterial infusions of angiotensin I and II were assessed. Forearm blood flow measurements were performed at baseline and then following each of two, 7 min incrementally increasing doses of angiotensin I (16 and 64 pmol min−1). This was followed by an infusion of saline to allow blood flow to return to baseline and then two doses of angiotensin II (4 and 16 pmol min−1) were infused.
Statistical analysis (studies 1 and 2)
Flow values were measured as ml 100 ml−1 forearm volume min−1; they are presented as the ratio between the values of the treated and the untreated arm [12, 13]. Blood flow ratios for individual subjects were compared by a general linear model using blood flow ratio at all doses except baseline as a response and smoking habit and dose of infusate as factors for the model. 95% confidence intervals for the differences between smokers and nonsmokers were calculated using Bonferroni's test for pairwise comparisons for the main effect in the model, for both between- and within-subjects factors.
The data are presented as the mean (± s.e. mean) flow ratio in response to the three incremental doses of each vasodilator. Differences were considered statistically significant at P < 0.05.
The baseline variability of our data was less than 10%, when blood flow was analysed repeatedly in steady state, in a quiet environment. The variability of repeated analysis of the same raw plethysmographic data was less than 5%.
Results
Study 1
Sixty-eight male subjects (age 24 ± 6 vs 25 ± 5 years; P = NS: nonsmokers vs smokers) completed the study of whom 29 smoked, the median duration of smoking was 8 years (range 2–30 years) and median consumption was 10 cigarettes per day (range 2–20). There was no difference in BP between nonsmokers and smokers; baseline BP was 117/74 ± 9/8 and 118/73 ± 8/7 mmHg. There were no significant differences in cholesterol (3.9 ± 0.8 vs 4.2 ± 0.9 mmol l−1, P = 0.21); HDL-cholesterol (1.2 ± 0.2 vs 1.2 ± 0.3 mmol l−1, P = 0.90); serum ACE (35.9 ± 15.9 vs 34.5 ± 16.4 IU l−1, P = 0.55) or body mass index (23.1 ± 2.2 vs 23.3 ± 2.8 kg m−2, P = NS).
Forearm blood flow
Baseline blood flow: There was no significant difference in baseline absolute blood flow between nonsmokers and smokers on any study day (Table 1).
Table 1.
Blood pressure plasma indices and basal blood flow (mean ± s.d.).
| Nonsmokers | Smokers | P value | ||
|---|---|---|---|---|
| Study 1 | ||||
| Absolute baseline blood flow | Day 1 | 3.1 ± 2.3 | 2.6 ± 2.2 | 0.24 |
| (ml 100 ml−1 min−1) | Day 2 | 2.8 ± 2.0 | 2.4 ± 1.9 | 0.62 |
| Study 2 | ||||
| Absolute baseline blood flow | 2.8 ± 1.0 | 2.6 ± 0.4 | 0.54 | |
| (ml 100 ml−1 min−1) | ||||
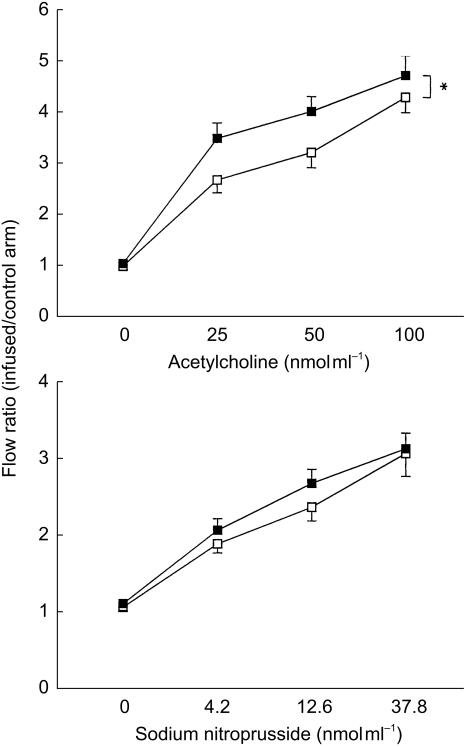
Vasodilators: We found smoking was associated with a significant impairment in endothelial-dependent vasodilatation to acetylcholine with values of 4.07 ± 2.18 and 3.42 ± 1.79 in the nonsmoking and smoking groups, respectively (P = 0.04, 95% CI 0.02, 1.12). There was no significant difference between endothelial-independent vasodilators; sodium nitroprusside (2.61 ± 1.19 vs 2.43 ± 1.32; P = 0.75, 95% CI −0.71, 0.99) and verapamil (4.87 ± 3.44 vs 4.74 ± 3.56; P = 0.76, 95% CI −0.77, 1.05) (Figure 1).
Figure 1.
Dose-response curves for acetylcholine and sodium nitroprusside. Non-smokers ▪, smokers □. ⋆P < 0.05. Mean ± s.e. mean.
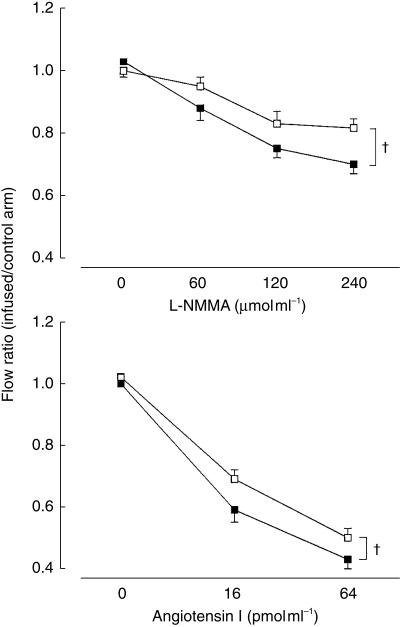
Vasoconstrictors: Smokers had a significant impairment in endothelial-dependent vasoconstriction; monomethyl-l-arginine (0.78 ± 0.22 vs 0.87 ± 0.21; P = 0.006; 95% CI −0.14, −0.02) (Figure 2). The corresponding figure for noradrenaline failed to reach statistical significance (0.61 ± 0.20 vs 0.68 ± 0.17; P = 0.20, 95% CI −1.97, 0.41).
Figure 2.
Dose-response curves for L-NMMA and angiotensin 1. Non-smokers ▪, smokers □. †P < 0.005. Mean ± s.e. mean.
Correlation analysis
The maximal vasodilator response to acetylcholine was negatively correlated with plasma cholesterol (P = 0.05) and BMI (P = 0.03). There was no relationship between maximal acetylcholine response and duration of smoking (P = NS).
Study 2
Twenty-three male subjects completed the study of whom 11 smoked, the mean duration of smoking was 18 years (range 8–35 years) and the median consumption was 22 cigarettes per day (range 10–40). There was no difference in blood pressure (131/72 ± 8/11 vs 133/77 ± 12/11 mmHg, P = 0.64/0.29); age (32 ± 11 vs 34 ± 10 years, P = NS) and BMI (23.42.4 vs 24.74.1 kg m−2; P = 0.36) between nonsmokers and smokers.
Forearm blood flow
Baseline blood flow
There was no significant difference in baseline blood flow between nonsmoking and smoking groups on either study day (Table 1).
Angiotensin 1
Smokers had a significant impairment in response to angiotensin I 0.51 ± 0.15 vs 0.59 ± 0.16 (nonsmokers vs smokers; P = 0.03, 95% CI −0.13, −0.03) (Figure 2).
Angiotensin II
Smokers had a nonsignificant reduction in response to angiotensin II 0.58 ± 0.15 vs 0.65 ± 0.20 (nonsmokers vs smokers; P = 0.13, 95% CI −0.13, 0.02).
Plasma variables
There was no significant difference between smokers and nonsmokers in the plasma levels of angiotensin II; 17.7 ± 7.1 vs 22.3 ± 15.1 pg ml−1 (nonsmokers vs smokers; P = 0.37, 95% CI −5.8, 15.0).
Discussion
Cigarette smoking is a major risk factor for the pathogenesis of atheromatous disease and endothelial dysfunction may occur early in this process [5]. The association between endothelial dysfunction and smoking has been demonstrated in some studies but not in all [14–16]. We demonstrated a blunted vasodilatory response to acetylcholine, usually regarded as a marker of blunted NO release, although acetylcholine also releases EDHF and PGI2. In addition we also demonstrated significantly blunted vasoconstrictor responses to L-NMMA and angiotensin I. The noradrenaline and angiotensin II responses follow the same trend, in that smokers had a blunted vasoconstrictor response but were not statistically significant. We saw no correlation between numbers of cigarettes smoked, duration of smoking and endothelial function.
Previous studies of endothelium-dependent vasodilatation in smokers have not produced a consistent picture. Of the six previous papers, two found no difference in endothelial function between smokers and nonsmokers [17, 18], three found a deficit in endothelial function [14–16], while one even found an augmented response after smoking acutely [19]. The three that found blunting of endothelial function used flow mediated dilatation which assesses conduit artery function, while the two that found no deficit used plethysmography, which assesses resistance vessel function.
Contrary to previously presented data, plethysmographic responses do differ between smokers and nonsmokers. Thus endothelial function in this highly metabolically active muscle bed is also abnormal. Previous studies of endothelium-independent vasodilatory responses are also inconsistent. Some previous authors have demonstrated associations with cigarette smoking [14, 15] while others have not [16]. We found no abnormality with nitroprusside or verapamil, suggesting that non-NO responses are not blunted in smokers.
Basal tonic NO production has been investigated in only two previous papers [18, 20] but here the previous data are consistent and we confirm this finding.
Previous studies are also inconsistent with regard to vasoconstrictor responses and have found both blunted [21] and normal [20] responses to noradrenaline. We observed a nonsignificant trend towards blunted vasoconstrictor responses, which may suggest a degree of blunting of the response to noradrenaline. The explanation may be down regulation of α-adrenoreceptors by endogenous catecholamines [20] because long-term smokers may have increased plasma levels of noradrenaline [22]. Cigarette smoking may specifically interfere with noradrenaline induced vasoconstriction or smoking could be associated with a more generalized defect in vasoconstrictor responses.
Our first study did not clarify whether all vasoconstrictors produce a blunted response in smokers or whether this only occurs with selected vasoconstrictors. Thus we performed the second study. Another rationale for this second study was that in vitro work had suggested that smoking activates the renin-angiotensin system observed a blunting of the vasoconstrictor response to angiotensin I in vivo in cigarette smokers. This is the first description of this phenomenon although the associated nonsignificant blunting with the vasoconstrictor angiotensin II makes interpretation of these results difficult.
Our findings are in contrast with the previous in vitro study [7]. The difference is probably because ours was an in vivo study in man where alternative data came from an animal in vitro study, i.e. methodology differences may explain the different result. Further investigation of the impact of smoking the vascular RAS is required to clarify this issue.
Taken together, there are two potential explanations for our findings. Firstly there may be specific defects only in angiotensin I and L-NMMA responses which are associated with smoking. These could be because smoking blunts both NO production and vascular ACE activity. Secondly, there may be a generalized defect to all vasoconstrictors associated with smoking. We cannot differentiate between these possibilities on the basis of the data available in this study and therefore further work is necessary.
These data strongly suggest that cigarette smoke causes endothelial dysfunction by virtue of reduced vascular responsiveness in smokers and specifically impairs both basal and stimulated NO bioactivity.
Acknowledgments
RB is supported by a project grant from the British Heart Foundation. This study was also supported by equipment grants from Tenovus Tayside, a local Anonymous Trust and the Nuffield Foundation.
References
- 1.Fielding JE. Smoking: health effects and control (1) N Engl J Med. 1985;313:491–498. doi: 10.1056/NEJM198508223130807. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman MJ. Comparison of the free radical-scavenging ability of captopril and ascorbic acid in an in-vitro model of lipid oxidation. Implications for reperfusion injury and ACE inhibitor therapy. J Pharm Pharmacol. 1994;46:217–220. doi: 10.1111/j.2042-7158.1994.tb03782.x. [DOI] [PubMed] [Google Scholar]
- 3.Herderschee D, Hijdra A, Algra A, Koudstaal PJ, Kappelle LJ, van Gijn J. Silent stroke in patients with transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. Stroke. 1992;23:1220–1224. doi: 10.1161/01.str.23.9.1220. [DOI] [PubMed] [Google Scholar]
- 4.Asmussen I, Kjeldsen K. Intimal ultrastructure of human umbilical arteries. Observations on arteries from newborn children of smoking and nonsmoking mothers. Circ Res. 1975;36:579–589. doi: 10.1161/01.res.36.5.579. [DOI] [PubMed] [Google Scholar]
- 5.Davis JW, Shelton L, Eigenberg DA, Hignite CE, Watanabe IS. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin Pharmacol Ther. 1985;37:529–533. doi: 10.1038/clpt.1985.83. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Yu XL, Jin XR, Wang DX. Effects of cigarette smoking on the function of metabolized arachadonic acid and angiotensin I in the isolated perused rat lung. J Tongji Med University. 1992;12:201–204. doi: 10.1007/BF02887849. [DOI] [PubMed] [Google Scholar]
- 8.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 10.Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 11.Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 12.Whitney RJ. The measurement of volume changes in human limbs. J Physiol. 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenfield ADM, Patterson GC. Reactions of the blood vessels of the human forearm to increases in transmural pressure. J Physiol. 1954;125:508–524. doi: 10.1113/jphysiol.1954.sp005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs MC, Lenders JW, Kapma JA, Smits P, Thien T. Effect of chronic smoking on endothelium-dependent vascular relaxation in humans. Clin Sci. 1993;85:51–55. doi: 10.1042/cs0850051. [DOI] [PubMed] [Google Scholar]
- 18.McVeigh GE, Lemay L, Morgan D, Cohn JN. Effects of long-term cigarette smoking on endothelium-dependent responses in humans. Am J Cardiol. 1996;78:668–672. doi: 10.1016/s0002-9149(96)00391-8. 10.1016/s0002-9149(96)00391-8. [DOI] [PubMed] [Google Scholar]
- 19.Kool MJ, Hoeks AP, Struijker BH, Reneman RS, Van Bortel LM. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol. 1993;22:1881–1886. doi: 10.1016/0735-1097(93)90773-t. [DOI] [PubMed] [Google Scholar]
- 20.Kiowski W, Linder L, Stoschitzky K, et al. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Jensen EW, Espersen K, Kanstrup IL, Christensen NJ. Plasma noradrenaline and ageing: effects of smoking habits. Eur J Clin Invest. 1996;26:839–846. doi: 10.1111/j.1365-2362.1996.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 22.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]