Abstract
Aims
NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione (2-(4-methylsulphonyl-2-nitrobenzoyl)-1,3-cyclohexanedione) are inhibitors of 4-hydroxyphenyl pyruvate dioxygenase (HPPD). NTBC has been successfully used as a treatment for hereditary tyrosinaemia type 1 (HT-1), while mesotrione has been developed as an herbicide. The pharmacokinetics of the two compounds were investigated in healthy male volunteers following single oral administration. The aim of the NTBC study was to assess the bioequivalence of two different formulations and to determine the extent of the induced tyrosinaemia. The mesotrione study was performed to determine the magnitude and duration of the effect on tyrosine catabolism. Additionally, the urinary excretion of unchanged mesotrione was measured to assess the importance of this route of clearance and to help develop a strategy for monitoring occupational exposure.
Methods
A total of 28 volunteers participated in two separate studies with the compounds. In the first study, the relative bioavailability of NTBC from liquid and capsule formulations was compared and the effect on plasma tyrosine concentrations measured. In the second study the pharmacokinetics of mesotrione were determined at three doses. Plasma tyrosine concentrations were monitored and the urinary excretion of mesotrione and tyrosine metabolites was measured.
Results
Both compounds were well tolerated at the dose levels studied. Peak plasma concentrations of NTBC were rapidly attained following a single oral dose of 1 mg kg−1 body weight of either formulation and the half-life in plasma was approximately 54 h. There were no statistical differences in mean (± s.d.) AUC(0,∞) (capsule 602 ± 154 vs solution 602 ± 146 µg ml−1 h) or t½ (capsule 55 ± 13 vs solution 54 ± 8 h) and these parameters supported the bioequivalence of the two formulations. Mesotrione was also rapidly absorbed, with a significant proportion of the dose eliminated unchanged in urine. The plasma half-life was approximately 1 h and was independent of dose and AUC(0,∞) and Cmax increased linearly with dose. Following administration of 1 mg NTBC kg−1 in either formulation, the concentrations of tyrosine in plasma increased to approximately 1100 nmol ml−1. Concentrations were still approximately 8 times those of background at 14 days after dosing, but had returned to background levels within 2 months of the second dose. Administration of mesotrione resulted in an increase in tyrosine concentrations which reached a maximum of approximately 300 nmol ml−1 following a dose of 4 mg kg−1 body weight. Concentrations returned to those of background within 2 days of dosing. Urinary excretion of tyrosine metabolites was increased during the 24 h immediately following a dose of 4 mg mesotrione kg−1, but returned to background levels during the following 24 h period.
Conclusions
NTBC and mesotrione are both inhibitors of HPPD, although the magnitude and duration of their effect on tyrosine concentrations are very different. When normalized for dose, the extent of the induced tyrosinaemia after administration of NTBC and over the duration of these studies, was approximately 400 fold greater than that following administration of mesotrione. The persistent and significant effect on HPPD following administration of NTBC make it suitable for the treatment of patients with hereditary tyrosinaemia type 1 (HT-1), whilst the minimal and transient effects of mesotrione minimize the likelihood of a clinical effect in the event of systemic exposure occurring during occupational use.
Keywords: HPPD inhibitors, HT-1, mesotrione, NTBC, pharmacodynamics, pharmacokinetics, triketones, tyrosine
Introduction
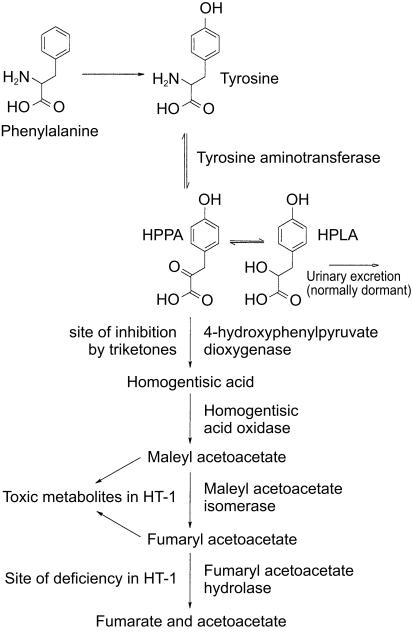
The major route of tyrosine catabolism in mammals (Figure 1) proceeds via transamination by tyrosine aminotransferase to yield 4-hydroxyphenyl pyruvic acid (HPPA). This product is then oxidized by 4-hydroxyphenyl pyruvate dioxygenase (HPPD) to produce homogentisic acid. Further catabolism occurs via malonyl acetoacetate and fumaryl acetoacetate to produce fumarate and acetoacetate, the normal products of intermediary metabolism.
Figure 1.
The tyrosine catabolism pathway.
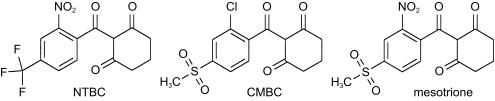
The triketones are a series of compounds discovered by Lee which exhibit herbicidal activity [1]. NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione (2-(4-(methylsulphonyl)-2-nitrobenzoyl)-1, 3-cyclohexanedione) are members of the series which have been evaluated as herbicides (Figure 2).
Figure 2.
Structures of NTBC, CMBC and mesotrione.
The herbicidal mode of action of triketones is known to be due to inhibition of HPPD in the target plants resulting in effects on the biosynthesis of plastoquinones and tocopherols. These effects result in a decrease in phytoene desaturase activity and lead to bleaching of the plants [2]. During evaluation of the triketones in laboratory animals, it was discovered that this class of compounds also inhibit mammalian HPPD [3]. This inhibition resulted in a reversible increase in plasma tyrosine concentrations, the magnitude of which is species dependent, and an associated increase in the excretion of HPPA and its metabolite 4-hydroxyphenyl lactic acid (HPLA) [4]. In vitro experiments have also been used to characterize the interaction of the triketones with mammalian HPPD [5].
Hereditary tyrosinaemia type 1 (HT-1) is a severe inherited disease of humans caused by a deficiency of fumaryl acetoacetate hydrolase (FAH). This deficiency leads to accumulation of fumaryl and maleyl acetoacetate and their saturated derivatives resulting in progressive liver and kidney damage [6]. Inhibition of HPPD prevents production of homogentisic acid and the resulting accumulation of toxic products in patients with a FAH deficiency. Consequently, the triketone NTBC has been proposed for the treatment of HT-1 [7].
This report describes and compares the results of two studies in healthy male volunteers with the triketones, NTBC and mesotrione. The aim of the NTBC study was to compare the relative bioavailability of NTBC from two different formulations and to determine the extent and duration of the induced tyrosinaemia from measurements of tyrosine in plasma. The mesotrione study was performed to investigate the pharmacokinetics of the chemical over a range of doses and to determine the effect on tyrosine catabolism via inhibition of HPPD by monitoring plasma tyrosine concentrations and also the urinary excretion of HPPA and HPLA. Urinary excretion of unchanged mesotrione was measured to determine whether this was a significant route of clearance. Although occupational exposure to mesotrione would not be expected to involve primarily oral ingestion, this route of administration was selected to allow a direct comparison of the pharmacokinetics and pharmacodynamics with those of NTBC.
Methods
The NTBC study was performed at Cardiff Clinical Trials Ltd, Cardiff, Wales and the mesotrione study at Inveresk Clinical Research, Edinburgh, Scotland, following review and approval of the study protocols by the appropriate Ethics Committees. The studies were performed in accordance with the Declaration of Helsinki (1964) including all amendments up to and including the South African revision (1996). A total of 28 male volunteers aged between 19 and 53 years and considered to be healthy on the basis of medical history, a normal physical examination, clinical chemistry, haematology, urinalysis, drugs of abuse screen, ophthalmoscopy and electrocardiographic tests were recruited. Volunteers gave written, witnessed and informed consent prior to participating and were free to withdraw from the studies at any time. The well being of the volunteers was monitored throughout both studies and any adverse events occurring were recorded. Oral temperature, pulse rate and blood pressure were monitored at intervals throughout the postdosing period and any subjective symptomology was determined by questioning. A post study medical examination including vital signs, ECG, ophthalmoscopy, haematology, clinical chemistry and urinalysis was performed.
NTBC with a purity of 99.8% w/w was supplied by Swedish Orphan AB, Stockholm, Sweden as either a powder mixed with lactose and dispensed into hard gelatine capsules containing 5, 6, 8 or 10 mg NTBC or as a solution containing 2 mg NTBC ml−1 in pH 7 phosphate buffer. Mesotrione with a purity of 99.7% w/w was supplied by Zeneca Agrochemicals, Fernhurst, Haslemere, Surrey, UK in powdered form. The required quantities were weighed into hard gelatine capsules prior to dosing.
Study 1
In the NTBC study, 10 volunteers in two groups of five were each given a single oral dose of 1 mg NTBC kg−1 as either a solution or in a capsule (period 1) followed at least 14 days later by a second dose of the alternative formulation (period 2). The drug was administered with 150 ml water. Volunteers were fasted for approximately 10 h prior to dosing and until 4 h after dosing. Volunteers remained in the clinic until 48 h after dosing during which time they each received the same meals. No other concomitant medications were permitted to be taken during the study period unless judged necessary for the well being of the volunteer. Alcohol intake was prohibited from 48 h prior to the initial dose until the end of the study. Caffeine intake was prohibited from admission to the clinic until 120 h after each dose.
Venous blood samples were taken from each volunteer predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 24, 48, 72, 96 and 120 h after administration of each dose. Blood samples were collected into tubes treated with lithium heparin and centrifuged to separate the plasma. The collected plasma was stored refrigerated prior to analysis. The concentrations of tyrosine and NTBC were determined in all plasma samples. Total urinary output was collected from 0 to 12, 12–24, 24–48, 48–72 and 72–96 h after dosing.
Study 2
In the mesotrione study, three groups, each consisting of six volunteers, were given a single oral dose of either 0.1, 0.5 or 4 mg mesotrione kg−1. Each volunteer received a single dose which was administered with 100 ml of water. Volunteers were fasted from approximately 9 h prior to dosing until 4 h after dosing. Doses were administered in ascending order and the resulting plasma tyrosine concentrations at each dose level were reviewed prior to administration of the next dose. The volunteers were admitted to the clinic prior to dosing and fed identical standard meals throughout both the 96 h predose and postdose periods. The meals were provided each day at comparable times in order to minimize variability in background plasma tyrosine concentrations. The menu selected was designed to provide a consistent rather than reduced intake of tyrosine. No other drugs, with the exception of paracetamol, were permitted to be taken during the 5 day periods prior to and following dosing. Alcohol, tobacco and caffeine intake were prohibited from 1 day prior to dosing and until 5 days after dosing.
Venous blood samples were taken from each volunteer at 72, 71, 70, 69, 68, 67, 66, 64, 60, 48, 42, 36, 24, 18, 12 and 0 h prior to dosing in order to define the background variability in tyrosine concentrations. Further samples were taken at 1, 2, 3, 4, 5, 6, 8, 12, 24, 30, 36, 48, 60, 72, 84 and 96 h after dosing. Blood samples were collected into tubes treated with lithium heparin and centrifuged and the resultant plasma was stored refrigerated prior to analysis. All plasma samples were analysed for tyrosine concentration and post dose samples were analysed for mesotrione. Total urinary output was collected at 12 h intervals from 72 h prior to dosing until immediately before administration of the dose. Further collections were made following dosing from 0 to 4, 4–8, 8–12 and then over 12 h intervals until 96 h after dosing. Aliquots of each urine sample collected following dosing were stored frozen prior to analysis for mesotrione. Pre- and postdose urine samples from the volunteers that received an oral dose of 4 mg mesotrione kg−1 were analysed for tyrosine metabolites.
Analytical procedures
Samples were transferred to either The Biomedicin Central Laboratory, Apoteksbolaget AB, Sweden for determination of NTBC concentrations or Zeneca Central Toxicology Laboratory for determination of mesotrione, tyrosine and tyrosine metabolite concentrations. The concentrations of free NTBC and mesotrione were measured in these studies since animal studies have demonstrated that there is no significant urinary excretion of conjugates of this class of compound [8].
The concentrations of NTBC in plasma were determined by reverse phase liquid chromatography with u.v. detection (LC-u.v.). Aliquots of each sample were diluted with pH 2 phosphate buffer and injected onto a 100 × 3 mm BioTrap acid C18 precolumn. NTBC was transferred onto a 150 × 3 mm YMC-basic 3 µm column using a mobile phase containing sulphuric acid and then eluted with acetonitrile/pH 2 phosphate buffer (55 : 45 v/v). The eluate from the chromatographic column was monitored using a u.v. detector at 278 nm. The assay was validated prior to use and the relationship between detector response and concentration was shown to be linear. Although no internal standard was used, assay performance was acceptable. The intra-assay precision ranged from 2% at 800 ng ml−1 to 18% at 40 ng ml−1, and the lower limit of quantification was 20 ng ml−1.
Plasma and urine samples were analysed for mesotrione using reverse phase liquid chromatography-mass spectrometry (LC-MS) with selected ion monitoring (SIM). A structural analogue of mesotrione was added to each sample as an internal standard. Calibration series were prepared in each sample matrix and analysed concurrently with each batch of samples. Plasma proteins were precipitated with acetonitrile; following centrifugation the supernatant was retained, reduced to dryness and re-dissolved in methanol/acetate buffer prior to analysis using LC-MS. Urine samples were acidified with hydrochloric acid, centrifuged and the supernatant analysed by LC-MS. Chromatographic separation was achieved using a 150 × 4.6 mm Kromasil KR100 C18 5 µm column eluted with 1 ml min−1 of methanol/acetate buffer (45 : 55 v/v for plasma and 50 : 50 v/v for urine). The chromatographic system was connected to either a Finnigan MAT TSQ 7000 mass spectrometer via an atmospheric pressure chemical ionization (APCI) interface or a UV detector operated at 260 nm. The assay response for mesotrione in both plasma and urine was linear with a lower limit of quantification of 5 ng ml−1. The interassay precision ranged from 2.1% at 6 µg ml−1 to 6.7% at 5 ng ml−1 in plasma and from 3.6% at 6 µg ml−1 to 8.8% at 5 ng ml−1 in urine.
Concentrations of tyrosine in plasma were determined using reverse phase liquid chromatography (LC) with detection by u.v. absorbance at 274 nm. Aliquots of each plasma sample were either centrifuged or transferred to a Centrifree micropartition device and then centrifuged to prepare a filtrate containing the analyte. Calibration series were prepared concurrently with each batch of samples. Aliquots of each standard and sample were analysed by LC using a 250 × 4.6 mm Hichrom S5ODS2 5 µm column eluted with a mobile phase of water/acetonitrile/trifluoroacetic acid (950 : 50 : 2 v/v/v) at a flow rate of 1 ml min−1. During validation, the intra-assay precision ranged from 0.54% at 400 µg ml−1 (2210 nmol ml−1) to 2.4% at 2 µg/ml (11 nmol ml−1). The assay response was linear with a lower limit of quantification of 1 µg ml−1.
Urinary excretion of the tyrosine metabolites HPPA and HPLA was estimated using a semiquantitative, proton nuclear magnetic resonance (NMR) method. Peak integrals of the regions of interest in the NMR spectra were quantified by comparison with those obtained by adding a known amount of 3-(trimethylsilyl) propionic 2, 2, 3, 3 -D4 acid to each urine sample.
Data analysis
Pharmacokinetic parameters for NTBC and mesotrione were determined by either inspection of the data (Cmax and tmax) or calculated (AUC(0,∞) and t½) using either PC-Nonlin version 2 (NTBC) or WinNonlin version 1.5 (mesotrione). Statistical analysis of the parameters calculated using either compartmental or noncompartmental methods was performed. The paired differences in the pharmacokinetic parameters for NTBC after administration as the capsule and liquid formulations were evaluated by the Pitman randomization test based on the Wilcoxon matched paired sign rank test [9]. Bioequivalence of the formulations was established using the Westlake 95% interval for un-transformed values [10, 11] and the Hauschke-Steinijans nonparametric test for bioequivalence [12].
Differences between the pre and post dose AUC values for tyrosine from each group in the mesotrione study were compared using a paired Student's t-test and post dose values were compared across dose groups using an analysis of covariance allowing for predose values.
Results
Both NTBC and mesotrione were well tolerated with no compound related adverse events or clinically significant, compound related changes in the safety parameters either during the studies or between the pre and post study medical screens.
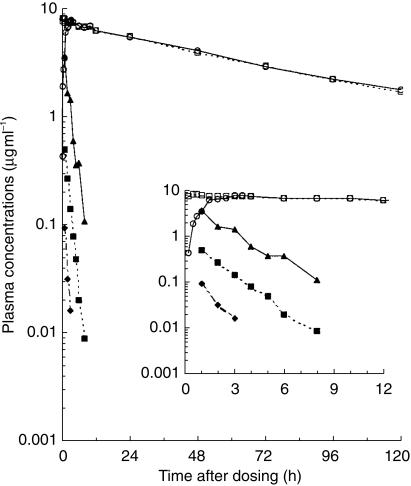
Peak plasma concentrations of NTBC were rapidly attained following administration of either formulation (Table 1a) and were still above the limit of quantification (0.02 µg ml−1) of the assay at the final time point 120 h after administration (Figure 3). There were no statistical differences in the values for AUC(0,∞) and t½ obtained for the two groups that received NTBC and these parameters supported the bioequivalence of the two formulations (Table 1a). The values for Cmax and the ratio Cmax/AUC(0,∞) were higher following administration of the liquid formulation than following the capsule formulation and did not meet the criteria of the nonparametric test for bioequivalence. Whilst comparison of the Cmax values using Westlake's test did not support bioequivalence, this was not the case for the ratio Cmax/AUC(0,∞).
Table 1.
Mean pharmacokinetic parameters for NTBC, mesotrione and tyrosine.
a) NTBC (mean (n = 10) ± s.d.)
| Dose (mg kg–1) | Cmax (µg ml−1) | tmax (h) | AUC (µg ml−1 h) Compartmental | AUC (µg ml−1 h) Non-compartmental | t½ (h) |
|---|---|---|---|---|---|
| 1 (capsule) | 7.81 ± 1.17 | 1.5–4 | 599 ± 153 | 602 ± 154 | 54.5 ± 13.0 |
| 1 (solution) | 8.19 ± 1.04 | 0.25–3.5 | 598 ± 142 | 602 ± 146 | 53.6 ± 8.2 |
| Parameter | Differences in formulations | Westlake's 95% interval | Non-parametric bioequivalence evaluation | ||
|---|---|---|---|---|---|
| AUC1 | P = 0.99 | 92.4 to 107.6% | 94.5 to 105.6% | ||
| AUC2 | P = 0.99 | 93.1 to 106.9% | 95.4 to 105.9% | ||
| t1/2 | P = 0.78 | 87.5 to 112.5% | 90.8 to 109.1% | ||
| Cmax | P = 0.023 | 77.4 to 122.6% | 102.6 to 124.3% | ||
| Cmax/AUC | P = 0.002 | 81.3 to 118.7% | 105.2 to 121.5% | ||
| b) mesotrione (mean (n = 6) ± s.d.) | ||||
|---|---|---|---|---|
| Dose (mg kg−1) | Cmax (µg ml−1) | tmax (h) | AUC (µg ml−1 h) | t½ (h) |
| 0.1 | 0.09 ± 0.05 | 1 | 0.15 ± 0.06 | 0.9 ± 0.3 |
| 0.5 | 0.58 ± 0.23 | 1–3 | 1.08 ± 0.26 | 1.3 ± 0.5 |
| 4 | 3.93 ± 1.85 | 1–6 | 8.50 ± 1.11 | 0.9 ± 0.2 |
| c) tyrosine AUC (nmol ml−1 h) values measured from 72 h prior to dosing to 72 h post dosing | |||||
|---|---|---|---|---|---|
| Dose of mesotrione (mg kg−1) 0.1 | 0.5 | 4 | |||
| Post dose | Mean | 5871 | 6246 | 10026 | |
| s.d. | 927 | 145 | 1227 | ||
| Adjusted for pre-dose | 6095 | 6751 | 9298 | ||
| P value vs 0.1 mg kg−1 | 0.21 (NS) | < 0.01** | |||
| P value vs 0.5 mg kg−1 | <0.01** | ||||
Non-compartmental analysis
Compartmental analysis.
NS not significant
statistically significant difference at the 1% level.
Figure 3.
Mean concentrations of NTBC and mesotrione in plasma following oral administration of either NTBC (○ 1 mg kg−1 bodyweight in solution; □ 1 mg kg−1 body weight in capsule) or mesotrione (♦ 0.1 mg kg−1 body weight; ▪ 0.5 mg kg−1 body weight; ▴ 4 mg kg−1 body weight). Inset graph shows the concentration data at early time points. For clarity, error bars have not been included.
At each dose level in the mesotrione study, peak plasma concentrations of mesotrione were observed within 6 h after dosing (Table 1b) and had declined to below the limit of quantification of the assay (0.009 µg ml−1) within 12 h after dosing (Figure 3). The estimated half-life of mesotrione in plasma was approximately 1 h and was independent of dose over the range studied. Mean values for both Cmax and AUC(0,∞) increased in proportion to the administered dose of mesotrione (Table 1b).
Unchanged mesotrione was rapidly eliminated in urine at each dose level with the majority of the recovered dose being excreted within 12 h of dosing (Table 2). The values for the mean total percentages of the doses excreted at the three dose levels were similar.
Table 2.
Total urinary excretion of mesotrione following a single oral dose (mean (n = 6) ± s.d.)
| Dose (mg kg−1) | Total urinary excretion of mesotrione (% of dose) |
|---|---|
| 0.1 | 53.3 ± 22.2 |
| 0.5 | 48.5 ± 12.5 |
| 4 | 72.0 ± 18.7 |
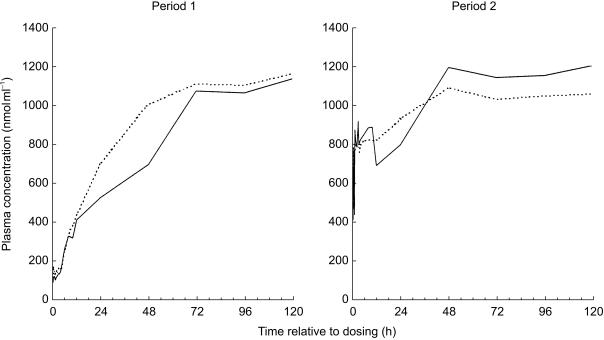
Prior to the initial dose of NTBC in Period 1 of the study, the mean concentrations of tyrosine in plasma were approximately 100 nmol ml−1. Following dosing, concentrations of tyrosine increased reaching maximum concentrations of approximately 1100 nmol ml−1 which persisted until after the final time point at 120 h after dosing (Figure 4). In Period 2 of the study, plasma samples taken immediately prior to administration of the dose showed that the concentrations of tyrosine had fallen to approximately 800 nmol ml−1. Administration of the second dose of NTBC again resulted in an increase in tyrosine concentrations to approximately 1100 nmol ml−1, these values persisted until after the final plasma sample was obtained at 120 h after the second dose. In each period of the study, the two formulations resulted in a similar magnitude and duration of effect on the concentrations of tyrosine in plasma.
Figure 4.
Mean concentrations of tyrosine in plasma following single oral doses of 1 mg NTBC kg−1 (____ solution; - - - - capsule). For clarity, error bars have not been included.
An additional plasma sample was obtained from each volunteer approximately 2 months after the end of the study and was analysed for tyrosine concentration. Fasted samples were provided by 7 of the 10 volunteers whilst the remainder provided nonfasted samples. Tyrosine concentrations varied between 57 and 109 nmol ml−1 in the fasted samples and between 108 and 140 nmol ml−1 in the nonfasted samples.
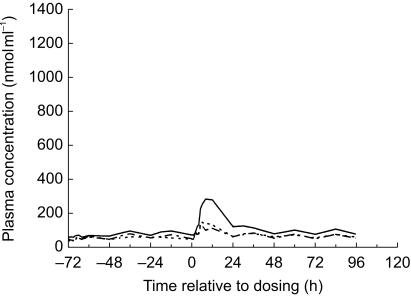
In the mesotrione study, the mean concentrations of tyrosine in plasma from each group of volunteers prior to dosing showed considerable interindividual variation and also varied throughout each 24 h period (Figure 5). Although these temporal variations were more pronounced in some volunteers, the overall trend was the same with higher concentrations throughout the day than during the night. At each dose level, the mean peak concentrations of tyrosine in plasma during the 24 h period following dosing were greater than the corresponding values during the predose period. This increase in mean peak tyrosine concentrations was transient and with the exception of the volunteers who received a dose of 4 mg mesotrione kg−1 where the effect persisted beyond 24 h, concentrations had returned to the predose range within 24 h of dosing. Mean concentrations at the top dose group had returned to predose levels by 48 h after dosing.
Figure 5.
Mean concentrations of tyrosine in plasma following single oral doses of mesotrione (_ _ _ _ 0.1 mg kg−1 body weight; - - - - - 0.5 mg kg−1 body weight; ______ 4 mg kg−1 body weight). For clarity, error bars have not been included.
Comparison of the mean AUC(0,∞) (Table 1) and Cmax values for tyrosine during the 72 h periods prior to and post dosing showed that for both parameters, at each dose level, there was a statistically significant (P < 0.01) increase in the values. The values following a dose of 4 mg mesotrione kg−1 were statistically significantly greater than those following doses of either 0.1 or 0.5 mg mesotrione kg−1 (P ≤0.01) but there was no statistical differences between the effects of the 0.1 and 0.5 mg mesotrione kg−1 doses.
The mean total quantities of the tyrosine metabolites HPPA and HPLA excreted in the urine of volunteers prior to and following dosing with 4 mg mesotrione kg−1 were calculated (Table 3). During the predose period the concentrations of the metabolites in the urine of each volunteer were below the lower limit of quantification of the assay. Excretion increased to 267 mg in the 24 h period immediately following dosing, but was again below the LLOQ during the period from 24 to 48 h after dosing.
Table 3.
Urinary excretion of the tyrosine metabolites HPPA and HPLA (mean (n = 6) ± s.d.) prior to and following a single oral dose of 4 mg mesotrione kg−1.
| Collection interval (h) | Urinary excretion of tyrosine metabolites (mg 24 h−1) |
|---|---|
| Predose | N/D |
| 0–24 | 267 ± 102 |
| 24–48 | N/D |
N/D – Not detected.
Discussion
To date, clinical administration of NTBC for the treatment of HT-1 has been limited to the use of the capsule formulation [13]. The use of a liquid formulation could allow easier and more accurate individualization of the dose particularly in children and adolescents. In this study, a comparison of the plasma AUC(0,∞) values for NTBC supports the bioequivalence of the two formulations and indicates that the liquid formulation could be used for future treatments. The observed differences in the values for Cmax are considered not to be important, particularly following repeated administration.
Although NTBC and mesotrione have similar chemical structures and both compounds are rapidly absorbed, resulting in significant plasma concentrations within 2 h of dosing, their plasma half-lives are very different. The fate of mesotrione has been investigated in other mammalian species and the absence of metabolism prior to urinary excretion has been established [8]. In man, mesotrione is rapidly cleared with a plasma half-life of approximately 1 h. Since a significant proportion of the administered dose was recovered unchanged in the urine, the rapid clearance is likely to be due to renal elimination. NTBC has a long plasma half-life of approximately 54 h which may reflect a requirement for metabolism prior to excretion.
Plasma samples taken from the volunteers in both studies contained background concentrations of tyrosine. During the NTBC study where there was no control of diet, these predose concentrations were between 76 and 136 nmol ml−1. When the volunteers returned to the clinic for their poststudy medical examination, the concentrations in samples from volunteers that had fasted were in the range 57–109 nmol ml−1, whilst in volunteers that had not fasted the corresponding concentrations ranged from 108 to 140 nmol ml−1. In the mesotrione study, where diet was controlled with each volunteer receiving the same meals throughout the study, the concentrations of tyrosine in plasma samples prior to administration of mesotrione were in the range 36–105 nmol ml−1. These ranges are likely to be representative of the normal fasted and nonfasted ranges in healthy males. The predose sampling schedule in the mesotrione study identified a diurnal variation in the concentrations of tyrosine in plasma with higher values during the day than during the night. This is thought to be due to a combination of dietary intake and higher levels of protein catabolism during the day. The overall pattern of normal tyrosine concentrations in plasma within the population studied is within a range from 36 to 140 nmol ml−1 with the higher values occurring during the day and following meals.
Exposure to both NTBC and mesotrione resulted in an increase in the concentrations of tyrosine in plasma although the magnitude and duration of the effect differed. Comparison of the increase above background in the AUC values for tyrosine calculated over the 14 day period after administration showed that whilst a dose of 4 mg mesotrione kg−1 body weight resulted in an increase of approximately 3000 nmol ml−1 h, the corresponding increase following a dose of 1 mg NTBC kg−1 was almost 300000 nmol ml−1 h. When the difference in dose is taken into account, this corresponds to an approximately 400 fold difference in the magnitude of the induced tyrosinaemia.
The observed differences can be explained on the basis of differences in the pharmacokinetics and pharmacodynamics of the compounds. Mesotrione, with a short plasma half-life, resulted in a moderate and short lived effect whilst NTBC, with a far longer half-life, resulted in an effect greater in both magnitude and persistence. The peak concentrations of tyrosine observed following each administration of NTBC are likely to represent the maximum achievable and were independent of the very different initial concentrations at the time of dosing in the two phases. An additional factor which may contribute to the differences in effect on tyrosine between the two compounds is the nature of the binding of the administered compound to HPPD. An in vitro study [5] with the triketones NTBC and CMBC (Figure 2) showed that, while both compounds bound rapidly to rat HPPD resulting in inhibition, the rate of recovery of the enzyme activity differed. Following inhibition with NTBC, enzyme activity recovered with a half-life of approximately 63 h whereas following inhibition with CMBC the corresponding value was approximately 10 h. The chemical structure of mesotrione is similar to that of CMBC, both compounds having a methylsulphonyl group on the 4 position of the aromatic ring. This structural similarity may be related to the short duration of elevation of tyrosine by mesotrione observed in this study.
The magnitude of the increase in tyrosine exposure resulting from administration of mesotrione is within the range expected to result from dietary factors and even the significant increase seen following repeated administration of NTBC over long periods of treatment has only infrequently been associated with adverse clinical effects [14].
The observed transient increase in the urinary excretion of the tyrosine metabolites HPPA and HPLA is consistent with inhibition of the enzyme HPPD by mesotrione. This enzyme is responsible for the conversion of tyrosine to HPPA and, since this process is reversible, inhibition results in an increase in the amounts of both tyrosine and HPPA in the body. While both compounds undergo renal clearance, tyrosine is reabsorbed resulting in elevated concentrations in plasma while HPPA and its metabolite HPLA are excreted in the urine (Figure 1).
NTBC and mesotrione are both inhibitors of HPPD, although the magnitude and duration of their effect on tyrosine concentrations are very different. The persistent and significant effect following administration of NTBC make it suitable for the treatment of patients with HT-1. Systemic exposure to mesotrione could occur during manufacture or field application. The development and use of mesotrione, which has been shown to result in a minimal and transient effect on tyrosine catabolism rather than an analogue such as NTBC which can lead to a substantial effect, should minimize the likelihood of a clinical effect in occupationally exposed workers.
Following oral administration of mesotrione, a significant proportion of the dose was excreted unchanged in urine. Provided that it could be shown that this was also the case following exposure by routes relevant to occupational handling, measurement of urinary excretion of unchanged mesotrione could provide a simple noninvasive marker of worker exposure to the herbicide during field application. Additionally, plasma tyrosine measurements could provide a marker of biological effect.
References
- 1.Lee DL, Prisbylla MP, Cromartie TH, et al. The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci. 1997;45:601–609. [Google Scholar]
- 2.Mayonado DJ, Hatzios KK, Orcutt DM, Wilson HP. Evaluation of the mechanism of action of the bleaching herbicide SC-0051 by HPLC analysis. Pestic Biochem Physiol. 1989;35:138–145. [Google Scholar]
- 3.Lock EA, Ellis MK, Provan WM, Smith LL. The effect of NTBC on enzymes involved in tyrosine catabolism in the rat. Toxicologist. 1994;14:826. [Google Scholar]
- 4.Wadman SK, Duran M, Ketting D, et al. Urinary excretion of deuterated metabolites in patients with tyrosinaemia type 1 after oral loading with deuterated l-tyrosine. Clin Chem Acta. 1983;130:231–238. doi: 10.1016/0009-8981(83)90120-1. [DOI] [PubMed] [Google Scholar]
- 5.Ellis MK, Whitfield AC, Gowans LA, et al. Inhibition of 4-hydroxyphenylpyruvate dioxygenase by 2-(2-nitro-4-trifluoromethylbenzoyl) -cyclohexane-1, 3-dione and 2-(2-chloro-4-methanesulphonylbenzoyl)- cyclohexane-1,3-dione) Toxicol Appl Pharmacol. 1995;133:12–19. doi: 10.1006/taap.1995.1121. 10.1006/taap.1995.1121. [DOI] [PubMed] [Google Scholar]
- 6.Lindblad B, Lindstedt S, Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci USA. 1977;74:4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 8.Gledhill A, Jones BK, Laird WJD. Metabolism of 2-(4-methylsulphonyl-2-nitrobenzoyl) -1,3-cyclohexanedione (mesotrione) in the rat and mouse. Xenobiotica. 2001 doi: 10.1080/00498250110052760. in press. [DOI] [PubMed] [Google Scholar]
- 9.Dallal GE. PITMAN: a FORTRAN program for exact randomization tests. Comput Biomed Res. 1988;21:9–15. doi: 10.1016/0010-4809(88)90037-7. [DOI] [PubMed] [Google Scholar]
- 10.Westlake WJ. Use of confidence intervals in analysis of comparative bioavailability. J Pharm Sci. 1972;61:1340–1341. doi: 10.1002/jps.2600610845. [DOI] [PubMed] [Google Scholar]
- 11.Westlake WJ. Symmetrical confidence intervals for bioequivalence trials. Biometrics. 1976;32:741–744. [PubMed] [Google Scholar]
- 12.Hauschke D, Steinijans VW, Diletti E. A distribution-free procedure for the statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol. 1990;28:72–78. [PubMed] [Google Scholar]
- 13.Swedish Orphan AB. 1996. NTBC Physician's Brochure.
- 14.Holme E, Lindstedt S. Diagnosis and management of tyrosinaemia type I. Curr Opin Pediatrics. 1995;7:726–732. doi: 10.1097/00008480-199512000-00017. [DOI] [PubMed] [Google Scholar]