Abstract
Aims
Noradrenaline increases thermal hyperalgesia in skin sensitized to heat by the topical application of capsaicin. The aim of this study was to determine whether desensitization to the hyperalgesic effects of noradrenaline would develop after repeated local administrations of noradrenaline in the skin of the forearm.
Methods
Noradrenaline and saline were administered to the forearm by iontophoresis (200 µA, 2 min, over a surface area of 3.1 cm2) two times per day for 4–10 days in 19 healthy subjects. The adequacy of the desensitization procedure was evaluated by measuring noradrenaline-induced vasoconstriction with laser Doppler fluxmetry. Thresholds and pain ratings to heat were then investigated at treated and control sites before and after the topical application of capsaicin, and after the iontophoresis of noradrenaline.
Results
At previously untreated sites blood flow was 49 ± 14% (± 95% confidence intervals) lower than flow at reference sites after the iontophoresis of noradrenaline. Vascular signs of adrenergic desensitization developed after 4–5 days of repeated local administration of noradrenaline in the majority of subjects. In those whose vessels constricted after the acute administration of noradrenaline, the adrenergic response averaged 23 ± 15% at the desensitized site compared with 61 ± 9% at previously untreated sites (P < 0.001). However, similar signs developed after repeated iontophoreses of saline (adrenergic response 7 ± 16% compared with 58 ± 15% at previously untreated sites, P < 0.001). Both the noradrenaline and saline treatments inhibited thermal hyperalgesia after the topical application of capsaicin. Heat-pain thresholds averaged 43.2 ± 2.5 °C and 43.0 ± 2.3 °C at the noradrenaline and saline pretreated sites compared with 41.4 ± 2.7 °C at the control site (P < 0.05 and P < 0.06, respectively). On a 0–10 scale, heat-pain ratings to a 7 s, 45 °C stimulus averaged 3.8 ± 1.6 and 3.5 ± 1.7 at the noradrenaline and saline pretreated sites compared with 5.3 ± 1.6 at the control site (P < 0.05). After the iontophoresis of noradrenaline heat-pain ratings increased 1.6 ± 1.4 at the site pretreated with saline (P < 0.05) compared with only 0.4 ± 1.0 at the site pretreated with noradrenaline (not significant), consistent with local adrenergic desensitization.
Conclusions
We conclude that repeated iontophoreses of noradrenaline or saline inhibit vasoconstriction to noradrenaline, and also inhibit increases in thermal hyperalgesia evoked by capsaicin. The release of endogenous stores of noradrenaline by iontophoretic currents might contribute to these effects.
Keywords: adrenergic desensitization, iontophoresis, noradrenaline, pain, thermal hyperalgesia
Introduction
In some circumstances sympathetic nerve activity increases nociceptor discharge in the presence of inflammation. In one of the first experiments to demonstrate this effect, inflammation was induced in rat paws by a cocktail of painful substances [1]. In the presence of inflammation, electrical stimulation of sympathetic nerves or local injection of noradrenaline increased activity in C polymodal nociceptors. Similar effects were subsequently reported after adjuvant inflammation in rats [2, 3]. In humans, local administration of noradrenaline increases sensitivity to heat-pain in skin inflamed by capsaicin, which itself selectively sensitizes C polymodal nociceptors to heat [4]. The nociceptive effect of endogenously released noradrenaline during inflammation [5] appears to be masked by analgesic influences during normal sympathetic activation [6, 7]. Nevertheless, blocking α-adrenergic receptors at the site of intradermal capsaicin injection with phentolamine inhibits spontaneous pain, hyperalgesia to punctate stimulation, and flaring around the site of the capsaicin injection [8]. When administered after the onset of inflammation α-adrenergic antagonists reverse the painful effect of noradrenaline [5, 9, 10], suggesting that noradrenaline directly stimulates excitatory α-adrenergic receptors on nociceptors.
After prolonged exposure to catecholamines, vascular smooth muscle loses some of its contractile responsiveness [11–13]. Adrenergic desensitization begins within 2 h of exposure to high concentrations of adrenaline [14], is specific to adrenergic agonists [11], and is well-established after 48 h [12]. Various mechanisms appear to contribute to α-adrenergic desensitization of vascular smooth muscle, including alterations in receptor function [13], a decrease in receptor concentration [12] and an enhanced release of endothelium-derived relaxing factors such as nitric oxide [15].
The aim of the present study was to investigate the effect of repeated local administrations of noradrenaline on vasoconstriction and sensitivity to heat-pain in human skin. We expected that vascular signs of adrenergic desensitization would develop at treated sites. Since noradrenaline increases thermal hyperalgesia in inflamed skin [4, 5, 9, 16, 17], it was hypothesized that thermal hyperalgesia would be lower than normal in skin desensitized to noradrenaline.
Methods
Subjects
The sample consisted of 12 females and seven males aged between 18 and 44 years (mean age 23.2 years). Each subject gave their informed consent for the procedures, which were approved by the Murdoch University Human Research Ethics Committee.
Procedures
The experiments were conducted in a temperature controlled laboratory maintained at 21 ± 2 °C (± s.d.). The experimental sequence is summarized in Table 1.
Table 1.
Summary of procedures.
| Stage | Procedure | Sites |
|---|---|---|
| 1. Pretreatment (n = 19) | Iontophoresis twice/day for 4–10 days | A: noradrenaline |
| B: saline | ||
| C: saline (n = 10) | ||
| 2. Assessment of adrenergic vasoconstriction (n = 19) | (i) iontophoresis of noradrenaline | (i) A, C, D, E |
| (ii) iontophoresis of saline | (ii) B, F | |
| (iii) skin heated in 42 °C water bath | (iii) entire forearm | |
| (iv) blood flow measurement | (iv) A–F and two reference sites | |
| 3. Assessment of thermal hyperalgesia (n = 14) | (i) at baseline | (i) A, B, G |
| (ii) after topical capsaicin | (ii) A, B, G | |
| (iii) after iontophoresis of noradrenaline | (iii) A, B, G |
Note that Sites D–G received no pretreatment.
Stage 1: Pretreatment
Drug and skin preparation
Noradrenaline ((–)-arterenol bitartrate, Sigma, St. Louis, MI, USA) was prepared daily at 0.5 mm with distilled water from a 10 mm stock solution and introduced into the skin of the forearm by iontophoresis. To ensure that oxidization of noradrenaline was minimal, the stock solution was stored in an airtight container at 4 °C and replaced fortnightly. In preparation for iontophoresis the skin was shaved, if necessary, and cleaned with an isopropyl alcohol swab.
Iontophoresis
Perspex capsules with an internal diameter of 20 mm were attached with adhesive washers and filled with the noradrenaline solution. A 3 cm by 5 cm silver electrode was coated with electrode gel and attached to the dorsal aspect of the hand or to the forearm near the wrist to complete the electrical circuit. Direct current (200 µA) was passed through the solution for 2 min to introduce the noradrenaline into the skin. To investigate the nonspecific effects of iontophoresis, the current was also passed through another capsule containing 0.9% saline.
The position of the iontophoresis capsules on the forearm was marked with a permanent ink marker. In pilot studies noradrenaline was administered 2–3 times/day and adrenergic vasoconstriction was measured daily. Vascular signs of adrenergic desensitization usually developed within 3–5 days. In the present study the iontophoreses were repeated twice daily for 4–5 days. Daily administrations were separated by a minimum of 4 h. In six cases, the iontophoreses were continued for another 2–6 days because assessment was delayed by timetabling difficulties or illness (two subjects) or because vascular signs of adrenergic desensitization had not developed after 4–5 days (four subjects; the criterion used for adrenergic desensitization is described below). In 10 subjects an additional site was iontophoresed with saline to investigate the effect of repeated saline iontophoreses on adrenergic vasoconstriction.
Stage 2: assessment of adrenergic vasoconstriction
Drug administration
Noradrenaline was iontophoresed at the site of repeated noradrenaline pretreatment and at two previously untreated sites (200 µA for 2 min). Saline was iontophoresed at the site of repeated saline pretreatment and at an additional site (200 µA for 2 min). In addition, noradrenaline was iontophoresed at the second site of repeated saline pretreatment in 10 subjects. The order of the iontophoreses was randomised across subjects.
Measuring skin blood flow
In general, the skin of the forearm is poorly perfused [18]; however, local heating to 42 °C reliably induces maximal skin blood flow [19, 20]. Thus, to avoid floor effects the forearm was heated to 42 °C for 5 min in a water bath starting 5 min after the final iontophoresis. Blood flow was then measured from near the centre of each iontophoresis site and from two additional reference sites in the forearm with a Moor MBF3D dual-channel laser Doppler flowmeter (Moor Instruments, Axminster, U.K.). The flowmeter emitted near infra-red laser beams at 810 nm, and changes in wavelength (reflecting changes in blood flow) were detected with wide surface area probes over a volume approximately 7 mm2 in surface area and 1 mm deep. During the recordings, the forearm was positioned just below heart level and the water bath's heating element and circulating jet were switched off to prevent data artefacts caused by probe agitation. To ensure that the probe distance from the skin was uniform, tape was applied to the probes so that they were positioned 1 mm above the skin surface when inserted into plastic probe holders. Probe positions were changed after 20 s of stable flow at each site. Recordings were taken twice from each site by each probe, with the order randomised across probes and subjects. Measurements took an average of 11.5 ± 1.2 min during which time the water temperature fell from 42.1 ± 0.3 °C to 41.1 ± 0.3 °C.
Stage 3: assessment of thermal hyperalgesia
Sensory testing began 243 min (range 130–301 min) after the assessment of adrenergic vasoconstriction (except for two subjects where sensory testing began 17–19 h after the assessment of adrenergic vasoconstriction because of timetabling constraints). Sensitivity to thermal stimulation was investigated at the site of repeated noradrenaline pretreatment, a site of repeated saline pretreatment, and at another previously untreated control site.
Measuring thermal hyperalgesia
To identify the heat-pain threshold, the radiant heat from a halogen globe was focused through a 6 mm diameter aperture placed just above the skin. Skin temperature was monitored and servo-controlled via a spring-mounted thermocouple bead (0.8 mm diameter) that indented the skin slightly in the centre of the aperture. Skin temperature was brought to 32 °C with the servo-controlled heat lamp and maintained at that temperature for 10–15 s. Skin temperature was then increased at 0.5 °C s−1 until the subject signalled pain or to a maximum of 49 °C, and returned passively to around 32 °C over the next 10–15 s. The heat-pain threshold at each site was calculated as the average threshold from two or three temperature ramps. The skin was also heated to 45 °C for 7 s, and subjects rated pain intensity verbally on a scale ranging from 0 (no pain) to 10 (extreme pain).
Topical application of capsaicin
Next, 2.5 cm by 2.5 cm gauze pads containing 250 µl of 0.02 m (0.6%) capsaicin solution were applied to the noradrenaline pretreated site, one of the saline pretreated sites and to the previously untreated control site. The capsaicin solution was prepared by dissolving capsaicin powder (Sigma Chemical Company, Sydney, Australia) in 50% ethanol in distilled water. The gauze dressings were covered with plastic tape to retard evaporation of the capsaicin solution. After 30 min the dressings were removed and the skin was washed with soap. Thermal hyperalgesia was measured at all three sites after spontaneous pain had subsided (on average, 30 min after the capsaicin had been washed from the skin except for three cases where testing was delayed for 75, 91 and 127 min, respectively, because of the persistence of spontaneous pain).
Iontophoresis of noradrenaline
Noradrenaline was iontophoresed at each site (200 µA for 60 s). Thermal hyperalgesia was measured again, starting 7 min after the final iontophoresis.
Data reduction and statistical approach
Adrenergic vasoconstriction
The mean blood flow at each site was calculated by averaging across probes and repeated measurements. In addition, blood flow was averaged across the two reference sites and across the two sites of acute noradrenaline administration (i.e. sites that had received no prior treatment with saline or noradrenaline). Since the laser Doppler flowmeter measured blood flow in relative rather than absolute terms, flow at sites of drug administration was expressed as a percentage of flow at the reference sites. The criterion used for adrenergic vasoconstriction was that blood flow was at least 25% lower at sites of acute noradrenaline administration than at the reference sites. The criterion used for adrenergic desensitization was that blood flow was greater at the pretreated site than at the sites of acute noradrenaline administration.
Student's paired t-test was used to determine whether blood flow differed significantly between sites of acute noradrenaline administration and the site of acute saline administration (to test for adrenergic vasoconstriction), between sites of acute noradrenaline administration and the site of repeated noradrenaline pretreatment (to test for adrenergic desensitization) and between sites of acute noradrenaline administration and the site of saline pretreatment (to test for nonspecific effects of the iontophoretic pretreatment on adrenergic vasoconstriction). Student's paired t-test was also used to determine whether blood flow differed significantly between the noradrenaline pretreated site and the saline pretreated site after the iontophoresis of noradrenaline (to compare the strength of adrenergic desensitization), and between the site of saline pretreatment and the site of acute saline administration after the iontophoresis of saline (to investigate any nonspecific effect of iontophoretic pretreatment on blood flow). No correction was made for multiple comparisons because each of these tests investigated independent hypotheses in different subgroups of subjects.
Thermal hyperalgesia
Student's paired t-test was used to investigate whether thermal hyperalgesia intensified after the iontophoresis of noradrenaline at the control site. The one-tailed criterion was used to test this directional confirmatory hypothesis [4, 5, 9, 16, 17]. Next, changes in heat-pain thresholds and ratings were investigated in separate 3 × 3 [Site (noradrenaline pretreatment, saline pretreatment, control)×Time (baseline, after capsaicin, after noradrenaline)] analyses of variance (anova) for repeated measures. The source of significant main effects and interactions was explored at each time point with simple contrasts between the control site and each of the pretreated sites.
Data are reported as the mean± 95% confidence interval.
Results
Adrenergic vasoconstriction
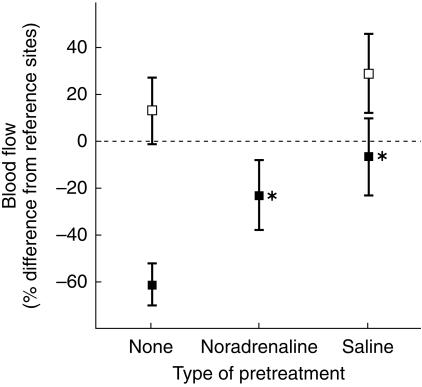
In the group as a whole, blood flow was lower at sites of acute noradrenaline administration than at the site of acute saline administration (49 ± 14% lower vs 13 ± 14% greater than flow at reference sites, Student's paired t-test P < 0.001). In 15 of the 19 subjects, blood flow was 25% to 86% lower at sites of acute noradrenaline administration than at the two reference sites (mean decrease 61 ± 9%, see Figure 1). In the other four subjects, blood flow at sites of acute noradrenaline administration was, respectively, 14% greater, 4% lower, 5% lower and 9% lower than blood flow at the two reference sites. These subjects were excluded from analyses of adrenergic desensitization because the minimal constrictive response to acutely administered noradrenaline ruled out detection of adrenergic desensitization.
Figure 1.
Cutaneous blood flow (± 95% confidence intervals) in heated skin, expressed as a percentage of flow at reference sites that did not receive drug administration. Blood flow after the acute administration of saline did not differ between the saline pretreated and control sites (open squares, n = 19). However, blood flow was greater at the site of saline pretreatment than at reference sites (95% confidence interval > 0). The response to noradrenaline is presented for subjects who met the criterion for adrenergic vasoconstriction after the acute administration of noradrenaline (filled squares, n = 15, except for the site of saline pretreatment where n = 7). Blood flow was greater at pretreated sites than at the site of acute administration of noradrenaline (*P < 0.001).
In 14 of the 15 remaining subjects, blood flow was greater at the site of repeated noradrenaline pretreatment than at sites of acute noradrenaline administration (Sign test, P < 0.001; Student's paired t-test, P < 0.001). In the other subject, blood flow at the site of noradrenaline pretreatment was 68% lower than flow at reference sites but was only 51% lower than reference flow at sites of acute noradrenaline administration. This subject was excluded from analysis of the effect of adrenergic desensitization on thermal hyperalgesia because the criterion for desensitization was not met.
The effect of saline pretreatment on adrenergic vasoconstriction was investigated in seven subjects (data from another three subjects were excluded because vasoconstriction did not develop at sites of acute noradrenaline administration). In all seven cases, blood flow was greater at the site of saline pretreatment than at sites of acute noradrenaline administration (Sign test, P < 0.05; Student's paired t-test, P < 0.001) (Figure 1). Blood flow did not differ significantly between the noradrenaline pretreated site and the saline pretreated site after the acute administration of noradrenaline.
Blood flow was greater at sites of acute saline administration than at the reference sites (Figure 1), thus ruling out a vasoconstrictive influence of saline iontophoresis. Blood flow did not differ significantly between the site of acute saline administration and the site of saline pretreatment.
Thermal hyperalgesia
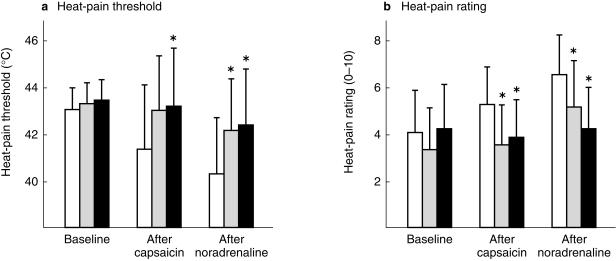
Heat-pain thresholds and ratings in the 14 subjects with vascular signs of adrenergic desensitization are presented in Figure 2. Heat-pain thresholds decreased after the application of capsaicin at the control site, and decreased further after the iontophoresis of noradrenaline (Student's paired t-test, P < 0.05). Similarly, heat-pain ratings increased after the application of capsaicin at the control site, and increased further after the iontophoresis of noradrenaline (Student's paired t-test, P < 0.05).
Figure 2.
Heat-pain thresholds and heat-pain ratings (± 95% confidence intervals) at sites of noradrenaline pretreatment (filled bars), saline pretreatment (stippled bars), and no pretreatment (open bars) in 14 subjects with vascular signs of adrenergic desensitization. Thresholds and ratings were measured at each site before and after the topical application of capsaicin and after the iontophoresis of noradrenaline. Heat pain thresholds were lower and heat pain ratings were greater at the control site than at pretreated sites after the topical application of capsaicin and iontophoresis of noradrenaline (*P < 0.05).
The heat-pain threshold differed among the three sites (anova Site main effect, P < 0.01) except at baseline (Figure 2a). In particular, the heat-pain threshold was higher at the site pretreated with noradrenaline than at the control site, both after the application of capsaicin (P < 0.05) and after the iontophoresis of noradrenaline (P < 0.05). In addition, the heat-pain threshold was higher at the site pretreated with saline than at the control site after the application of capsaicin (P < 0.06) and after the iontophoresis of noradrenaline (P < 0.01).
As shown in Figure 2b, heat-pain ratings differed among the three sites after the application of capsaicin and after the iontophoresis of noradrenaline (anova Site–Time interaction, P < 0.01). Pain ratings were similar at pretreated and untreated control sites at baseline. After the application of capsaicin, pain ratings were greater at the control site than at the sites of noradrenaline (P < 0.05) or saline pretreatment (P < 0.05). After the iontophoresis of noradrenaline, ratings remained greater at the control site than at sites of noradrenaline (P < 0.001) or saline pretreatment (P < 0.01) and did not differ significantly between the two pretreated sites. However, it is worth noting that pain ratings increased significantly after the iontophoresis of noradrenaline at the saline pretreated site (P < 0.05) but not at the site pretreated with noradrenaline.
Discussion
The main findings of the present study were that pretreating the skin with noradrenaline inhibited vasoconstriction to noradrenaline, and also inhibited thermal hyperalgesia in inflamed skin. Unexpectedly, however, similar effects developed after saline pretreatment.
In the majority of subjects, vasoconstriction to noradrenaline was attenuated in skin pretreated for 4–5 days with noradrenaline twice per day. Adrenergic vasoconstriction was quantified by comparing cutaneous blood flow at treated sites with blood flow at reference sites heated to 42 °C. In preliminary studies employing the local heating method, we identified a strong linear relationship between the strength of vasoconstriction and logarithmic increases in the dose of noradrenaline [21]. Thus, we are confident that noradrenaline provoked greater vasoconstriction at previously untreated sites than at sites of repeated noradrenaline pretreatment. Vascular signs of adrenergic desensitization developed at different rates in different subjects, presumably reflecting individual differences in, for example, adrenoceptor reserve or in the vulnerability of transmitter-receptor coupling to down-regulation.
Unexpectedly, saline pretreatment was as effective as noradrenaline pretreatment in provoking vascular signs of adrenergic desensitization. Weak direct currents increase cutaneous blood flow by stimulating the antidromic release of neuropeptides from sensory nerve terminals [22]. Pre-treating the skin repeatedly with iontophoretic currents possibly enhanced a local vasodilating mechanism (e.g. by priming release of vasoactive neuropeptides or other components of a local inflammatory response). We found recently that high levels of noradrenaline provoke axon reflex flares in human skin [23], which could augment the vasodilating effect of iontophoretic currents. Thus, an inflammatory response may have masked the constrictive effect of acutely administered noradrenaline at both of the pretreated sites. Alternatively, the iontophoretic current might have provoked the release of noradrenaline from sympathetic vasoconstrictor fibres; if so, the regular release of noradrenaline during the pretreatment may have led to adrenergic desensitization at the saline pretreated site.
Before capsaicin was applied, neither the heat-pain threshold nor ratings of pain intensity to suprathreshold heat stimuli differed between untreated sites and sites of noradrenaline or saline pretreatment. Thus, the iontophoretic pretreatment did not seem to influence the responsiveness of thermal nociceptors at baseline. However, both the noradrenaline and the saline pretreatments blocked the heat-sensitizing effect of topically applied capsaicin. Capsaicin is thought to increase thermal hyperalgesia by opening heat-sensitive channels that permit the entry of calcium into thermal nociceptors [24]. The heat-sensitizing effect of topically applied capsaicin declines after repeated applications because repeated exposure to high intracellular concentrations of calcium inhibits normal intracellular responses to calcium, thus preventing neuropeptide and neurotransmitter release [25]. However, it seems unlikely that repeated iontophoreses simulated capsaicin desensitization because this should have decreased sensitivity to heat-pain at baseline [26, 27]. Since both the saline and noradrenaline pretreatments provoked vascular signs of adrenergic desensitization, endogenously released noradrenaline might normally contribute to the hyperalgesic effect of capsaicin. In support of this possibility, Kinnman et al. [8] reported that subcutaneous injection of the α-adrenergic receptor antagonist phentolamine inhibited mechanical hyperalgesia and ongoing pain provoked by the intradermal injection of capsaicin. Furthermore, in iontophoretic studies in our laboratory, tyramine (which releases noradrenaline from adrenergic nerve terminals) increased thermal hyperalgesia in capsaicin-treated skin; this effect was blocked by pretreatment with the α-adrenergic receptor antagonist phenoxybenzamine [5].
Since adrenaline [28] and noradrenaline [4] increase thermal hyperalgesia in capsaicin-treated skin, we expected that adrenergic desensitization would block the hyperalgesic effect of noradrenaline detected in the present and previous studies [4, 5, 9]. After the iontophoresis of noradrenaline, thermal hyperalgesia was indeed lower at the site of noradrenaline pretreatment than at a control site. Furthermore, after the iontophoresis of noradrenaline heat-pain ratings did not change at the site of noradrenaline pretreatment, whereas ratings increased at the site of saline pretreatment as well as at the control site. This latter finding is consistent with a specific effect of adrenergic desensitization on thermal hyperalgesia but is not conclusive, because hypoalgesia had already developed before the iontophoresis of noradrenaline.
Noradrenaline increases thermal hyperalgesia in capsaicin-treated skin even during total arterial occlusion [9, 16], indicating that some mechanism in addition to a reduction in blood flow contributes to adrenergic hyperalgesia. Other possibilities include direct activation of adrenergic receptors on primary afferent nociceptors [29], release of inflammatory mediators such as prostaglandins or ATP from sympathetic terminals [30–32], and release of nitric oxide or nerve growth factor from the vascular endothelium [15, 33]. Whether any of these mechanisms was down-regulated by the iontophoretic current or by repeated local administration of noradrenaline awaits further investigation.
In summary, this study shows that repeated iontophoreses inhibit vasoconstriction to noradrenaline, and also inhibit the thermal hyperalgesia provoked by capsaicin. Since similar effects developed after the noradrenaline and saline pretreatments, we cannot be certain that the introduction of noradrenaline into the skin or the current-evoked release of endogenous stores of noradrenaline contributed to these effects. Nevertheless, the findings raise the possibility that iontophoretic treatments might reduce pain or hyperalgesia in conditions such as erythromelalgia and complex regional pain syndrome, where symptoms resemble those induced by capsaicin [34].
Acknowledgments
We gratefully acknowledge the support provided by the Australian Research Council, the Medical Research Fund of Western Australia and Medtronic Australasia.
References
- 1.Hu S, Zhu J. Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain. 1989;38:85–90. doi: 10.1016/0304-3959(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 2.Sato J, Suzuki S, Iseki T, Kumazawa T. Adrenergic excitation of cutaneous nociceptors in chronically inflamed rats. Neurosci Lett. 1993;164:225–228. doi: 10.1016/0304-3940(93)90897-t. [DOI] [PubMed] [Google Scholar]
- 3.Sato J, Suzuki S, Tamura R, Kumazawa T. Norepinephrine excitation of cutaneous nociceptors in adjuvant-induced inflamed rats does not depend on sympathetic neurons. Neurosci Lett. 1994;177:135–138. doi: 10.1016/0304-3940(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 4.Drummond PD. Noradrenaline increases hyperalgesia to heat in skin sensitised by capsaicin. Pain. 1995;60:311–315. doi: 10.1016/0304-3959(94)00130-7. 10.1016/0304-3959(94)00130-7. [DOI] [PubMed] [Google Scholar]
- 5.Drummond PD. Enhancement of thermal hyperalgesia by α-adrenoceptors in capsaicin-treated skin. J Auton Nerv Syst. 1998;69:96–102. doi: 10.1016/s0165-1838(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Wasner G, Borgstedt R, et al. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52:923–932. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- 7.Elam M, Olausson B, Skarphedinsson JO, Wallin BG. Does sympathetic nerve discharge affect the firing of polymodal C-fibre afferents in humans? Brain. 1999;122:2237–2244. doi: 10.1093/brain/122.12.2237. 10.1093/brain/122.12.2237. [DOI] [PubMed] [Google Scholar]
- 8.Kinnman E, Nygårds EB, Hansson P. Peripheral α-adrenoreceptors are involved in the development of capsaicin induced ongoing and stimulus evoked pain in humans. Pain. 1997;69:79–85. doi: 10.1016/s0304-3959(96)03257-5. 10.1016/s0304-3959(96)03257-5. [DOI] [PubMed] [Google Scholar]
- 9.Drummond PD. The effect of noradrenaline, angiotensin II and vasopressin on blood flow and sensitivity to heat in capsaicin-treated skin. Clin Auton Res. 1998;8:87–93. doi: 10.1007/BF02267818. [DOI] [PubMed] [Google Scholar]
- 10.Drummond PD. The effect of sympathetic activity on thermal hyperalgesia in capsaicin-treated skin during body cooling and warming. Eur J Pain. 2001 doi: 10.1053/eujp.2001.0224. in press. [DOI] [PubMed] [Google Scholar]
- 11.Carrier O, Wedell EK, Barron KW. Specific alpha-adrenergic receptor desensitisation in vascular smooth muscle. Blood Vessels. 1978;15:247–258. doi: 10.1159/000158170. [DOI] [PubMed] [Google Scholar]
- 12.Colucci WS, Grimbone MA, Alexander RW. Regulation of the postsynaptic α-adrenergic receptor in rat mesenteric artery: effects of chemical sympathectomy and epinephrine treatment. Circ Res. 1981;48:104–111. doi: 10.1161/01.res.48.1.104. [DOI] [PubMed] [Google Scholar]
- 13.Lurie KG, Tsujimoto G, Hoffman BB. Desensitisation of alpha-1 adrenergic receptor-mediated vascular smooth muscle contraction. J Pharmacol Exp Ther. 1985;234:147–152. [PubMed] [Google Scholar]
- 14.Maze M, Spiss CK, Tsujimoto G, Hoffman BB. Epinephrine infusion induces hyporesponsiveness of vascular smooth muscle. Life Sci. 1985;37:1571–1578. doi: 10.1016/0024-3205(85)90475-8. [DOI] [PubMed] [Google Scholar]
- 15.Hu ZW, Miller JW, Hoffman BB. Induction of enhanced release of endothelium-derived relaxing factor after prolonged exposure to α-adrenergic agonists: role in desensitisation of smooth muscle contraction. J Cardiovasc Pharmacol. 1994;23:337–343. [PubMed] [Google Scholar]
- 16.Drummond PD. Nitroprusside inhibits thermal hyperalgesia induced by noradrenaline in capsaicin-treated skin. Pain. 1999;80:405–412. doi: 10.1016/s0304-3959(98)00236-x. 10.1016/s0304-3959(98)00236-x. [DOI] [PubMed] [Google Scholar]
- 17.Drummond PD. Independent effects of ischaemia and noradrenaline on thermal hyperalgesia in capsaicin-treated skin. Pain. 1996;67:129–133. doi: 10.1016/0304-3959(96)03115-6. 10.1016/0304-3959(96)03115-6. [DOI] [PubMed] [Google Scholar]
- 18.Karanfilian RG, Lynch TG, Lee BC, Long JB, Hobson RW. The assessment of skin blood flow in peripheral vascular disease by laser Doppler velocimetry. Am Surg. 1984;50:641–644. [PubMed] [Google Scholar]
- 19.Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. J Appl Physiol. 1994;76:1759–1763. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- 20.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 21.Lipnicki DM, Drummond PD. Facilitating laser Doppler measurements of cutaneous adrenergic vasoconstriction: a comparison of methods. Clin Auton Res. 2001 doi: 10.1007/BF02322052. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Westerman RA, Low A, Pratt A, et al. Electrically evoked skin vasodilatation: a quantitative test of nociceptor function in man. Clin Exp Neurol. 1987;23:81–89. [PubMed] [Google Scholar]
- 23.Drummond PD, Lipnicki DM. Noradrenaline provokes axon reflex hyperaemia in the skin of the human forearm. J Auton Nerv Syst. 1999;77:39–44. doi: 10.1016/s0165-1838(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 25.Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- 26.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 27.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 28.Janssen SA, Arntz A, Bouts S. Anxiety and pain: epinephrine-induced hyperalgesia and attentional influences. Pain. 1998;76:309–316. doi: 10.1016/S0304-3959(98)00060-8. 10.1016/s0304-3959(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 29.Ouseph AK, Levine JD. α1-adrenoceptor-mediated sympathetically dependent mechanical hyperalgesia in the rat. Eur J Pharmacol. 1995;273:107–112. doi: 10.1016/0014-2999(94)00677-y. [DOI] [PubMed] [Google Scholar]
- 30.Levine JD, Taiwo YO, Collins SD, Tam JK. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postganglionic neurone terminals rather than activation of primary afferent nociceptors. Nature. 1986;323:158–160. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- 32.Khasar SG, Green PG, Chou B, Levine JD. Peripheral nociceptive effects of α2-adrenergic receptor agonists in the rat. Neurosci. 1995;66:427–432. doi: 10.1016/0306-4522(94)00562-j. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle JB, Etheridge R, Creedon DJ. Receptor-mediated stimulation and inhibition of nerve growth factor secretion by vascular smooth muscle. Exp Cell Res. 1993;208:350–361. doi: 10.1006/excr.1993.1256. 10.1006/excr.1993.1256. [DOI] [PubMed] [Google Scholar]
- 34.Ochoa J. Thermal hyperalgesia as a clinical symptom. In: Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press; 1992. pp. 151–165. [Google Scholar]