Abstract
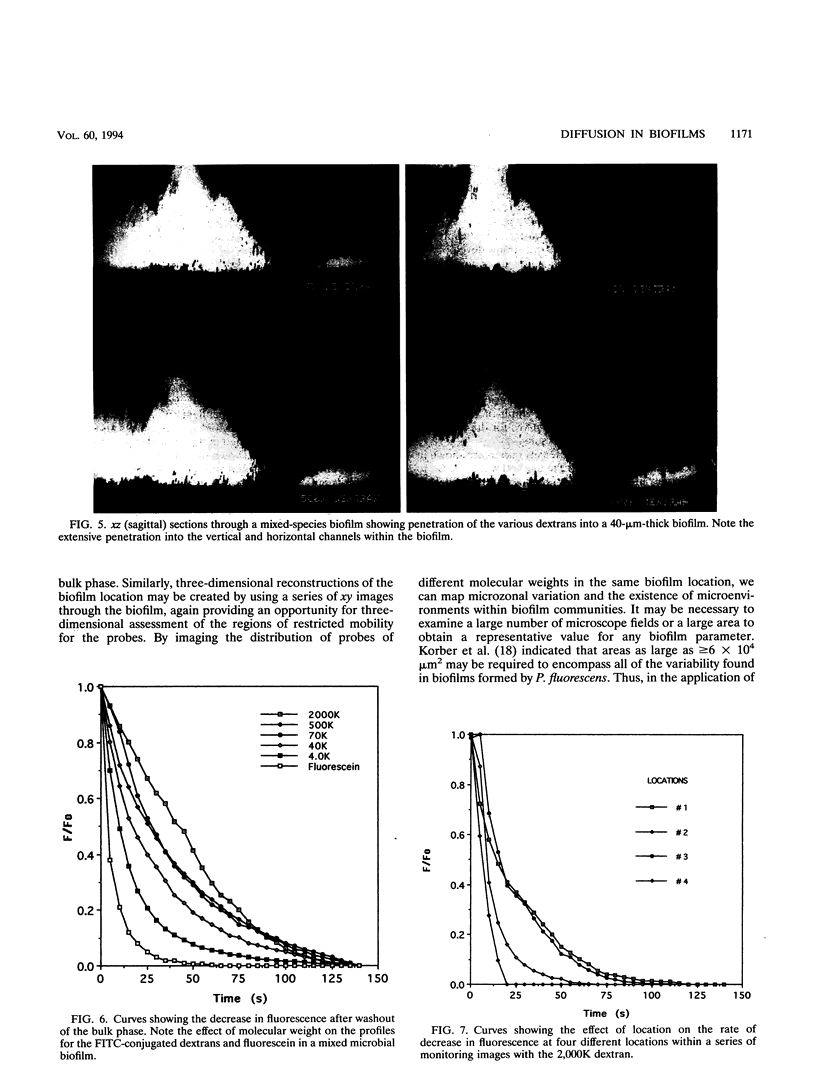
Microbial exopolymer may hinder the diffusion of nutrients, antibiotics, and other materials to the cell surface. Studies of diffusion in biofilms have been limited to indirect measurements. This study demonstrated the use of fluorescein and size-fractionated fluor-conjugated dextrans in conjunction with scanning confocal laser microscopy to directly monitor and determine diffusion coefficients within biofilms. The monitoring approaches were simple and, when combined with computerized image collection, allowed assembly of a data set suitable for calculation of one-dimensional diffusion coefficients for biofilm regions. With these techniques, it was shown that regional variability in the mobility of the dextrans occurred within mixed-species biofilms. Some regions exhibited rapid diffusion of all test molecules, while adjacent regions were only penetrated by the lower-molecular-weight compounds. The effective diffusion coefficients (De) determined in a mixed-species biofilm were a function of the molecular radius of the probe (i.e., fluorescein, De = 7.7 × 10-8 cm2 s-1; 4,000 molecular weight, De = 3.1 × 10-8 cm2 s-1; and 2,000,000 molecular weight, De = 0.7 × 10-8 cm2 s-1). These results demonstrated that diffusion in the biofilm was hindered relative to diffusion in the bulk solution. The study indicated that in situ monitoring by scanning laser microscopy is a useful approach for determining the mobility of fluorescently labeled molecules in biofilms, allowing image acquisition, appropriate scales of study, both xy and xz monitoring, and calculation of De values.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., STEERE R. L. Restricted diffusion of macromolecules through agar-gel membranes. Biochim Biophys Acta. 1962 May 7;59:137–149. doi: 10.1016/0006-3002(62)90704-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Cronenberg C. C., van den Heuvel J. C. Determination of glucose diffusion coefficients in biofilms with micro-electrodes. Biosens Bioelectron. 1991;6(3):255–262. doi: 10.1016/0956-5663(91)80011-l. [DOI] [PubMed] [Google Scholar]
- Hoyle B. D., Wong C. K., Costerton J. W. Disparate efficacy of tobramycin on Ca(2+)-, Mg(2+)-, and HEPES-treated Pseudomonas aeruginosa biofilms. Can J Microbiol. 1992 Nov;38(11):1214–1218. doi: 10.1139/m92-201. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wojcieszyn J. The translational mobility of substances within the cytoplasmic matrix. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6747–6751. doi: 10.1073/pnas.81.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Zhang F., Tsay T. T. Fluorescence recovery after photobleaching techniques to measure translational mobility in microscopic samples. Scanning Microsc. 1991 Jun;5(2):357–362. [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Hoyle B. D., Costerton J. W., Caldwell D. E. Optical sectioning of microbial biofilms. J Bacteriol. 1991 Oct;173(20):6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson T. G., Snaddon D. M. Biological oxidation of sewage by films of microorganisms. Air Water Pollut. 1966 Nov-Dec;10(11):865–881. [PubMed] [Google Scholar]
- Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988 Apr;34(4):415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]
- Wolfaardt G. M., Lawrence J. R., Hendry M. J., Robarts R. D., Caldwell D. E. Development of steady-state diffusion gradients for the cultivation of degradative microbial consortia. Appl Environ Microbiol. 1993 Aug;59(8):2388–2396. doi: 10.1128/aem.59.8.2388-2396.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfaardt G. M., Lawrence J. R., Robarts R. D., Caldwell S. J., Caldwell D. E. Multicellular organization in a degradative biofilm community. Appl Environ Microbiol. 1994 Feb;60(2):434–446. doi: 10.1128/aem.60.2.434-446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]