Abstract
Aims
To investigate the influence of combined ritonavir (RTV) and saquinavir (soft-gelatin capsule formulation; SQV) on systemic exposure to SQV with a view to optimizing the dosing regimen of combined RTV and SQV antiretroviral therapy.
Methods
In this open labelled, randomized, parallel group study, SQV and RTV were administered twice daily for 14 days to groups of eight healthy subjects. The two antiretrovirals were either administered alone (800 mg SQV, regimen A, and 400 mg RTV, B) or in combination at various dose levels (RTV:SQV: 400:400 mg, C; 300:600 mg, D; 200:800 mg, E; 300:800 mg, F; 400:800 mg, G; and 400:600 mg, H). Pharmacokinetic parameters of saquinavir and ritonavir were determined and adverse events, vital signs, and clinical laboratory variables recorded.
Results
RTV substantially increased the plasma concentration of saquinavir for all dose combinations, compared with SQV alone. Based on the primary statistical analysis there was an overall 17-, 22-, and 23-fold increase in saquinavir AUC(0,24 h) on day 14 with regimens E, F, and G, respectively (with confidence intervals of 10–30, 13–37, and 13–39). The lowest combination dose of RTV (200:800 mg; E) significantly increased the saquinavir AUC(0,24 h) from below 5 to 57 µg ml−1 h, which was higher than the exposure obtained with the 400:400 mg twice daily regimen (i.e. 36 µg ml−1 h). RTV also reduced intersubject variability in AUC(0,24 h) for saquinavir from 105% to 32–68%, and Cmax(0,24 h) from 124% to 30–49%. In contrast, SQV showed no clinically significant effect on the pharmacokinetics of ritonavir. The combination regimens were well tolerated, with the least number of adverse events recorded for the 200:800 mg (RTV:SQV) combination regimen.
Conclusions
RTV significantly increases saquinavir exposure as a consequence of inhibiting SQV metabolism and possibly P-glycoprotein efflux. Pharmacokinetic and safety profiles obtained in the current study indicate that the use of a combination with a lower dose of RTV and a higher dose of SQV than the 400:400 mg combination frequently used in clinical practice should be further explored.
Keywords: pharmacokinetics, ritonavir, saquinavir, soft gelatin capsule
Introduction
Saquinavir is a highly potent and specific HIV protease inhibitor that is clinically effective in combination antiretroviral regimens for the treatment of Human Immunodeficiency Virus (HIV) [1–4]. The HIV protease is essential for the maturation of infectious virions [5, 6]. Although originally developed as a hard-gelatin capsule formulation (SQV-HGC; Invirase®), a soft-gelatin formulation of saquinavir (SQV-SGC; Fortovase®) has subsequently been introduced which substantially increases plasma exposure to this drug [7, 8]. Doses of 1200 mg three times daily of SQV-SGC produced plasma concentrations approximately eight-fold greater than obtained with SQV-HGC at the recommended dose (600 mg three times daily) when administered to HIV-infected adults [7]. As the antiviral activity of SQV is related to its plasma drug concentration [8, 9], the soft-gelatin capsule formulation is significantly superior at reducing plasma HIV-1 RNA [4, 7], and also substantially increases CD4+ T-lymphocyte cell count [3, 4, 7, 10]. Furthermore, studies have shown that SQV-SGC is well tolerated [10].
When administered in the original hard-gelatin formulation, the bioavailability of saquinavir is low and variable due to its incomplete absorption and high first-pass metabolism by P450 enzymes, particularly CYP3A [11]. The development of the soft-gelatin formulation has helped to improve saquinavir absorption.
It has become generally accepted in the treatment of HIV to coadminister SQV-HGC with ritonavir (RTV, 400:400 mg twice daily) – a potent CYP3A inhibitor, as well as a potent protease inhibitor with demonstrated clinical benefit [11–17]. The combination of SQV-HGC and RTV, both at doses of 400 mg twice daily, has indicated that this regimen produces a sustained reduction in viral load. For example, in a cohort of predominantly reverse transcriptase-experienced patients after 1 year of therapy, 89% of those remaining on therapy achieved a viral load of < 200 copies ml−1 (74% by an intent-to-treat analysis) [14]. Furthermore, after 3 years of therapy viral suppression was maintained in the majority of patients receiving this combination regimen [18].
With this combined regimen of drugs, both at 400 mg twice daily doses, apparent oral clearance of saquinavir is reduced to approximately 25 l h−1 (steady-state), from > 1000 l h−1 with SQV-HGC alone [19]. Furthermore, the area under the curve (AUC) and the peak concentration (Cmax) of saquinavir was increased by over 50- and 22-fold, respectively, when the two antiretroviral agents were used in combination, compared with SQV-HGC alone in healthy volunteers or people with HIV [12, 20, 21].
Although inhibition of CYP3A by RTV is one of the most likely causes of the increased saquinavir exposure, another possibility is the influence of protease inhibitors on P-glycoprotein. There is evidence to suggest that saquinavir is transported by P-glycoprotein in vitro [22] and that this transmembrane protein limits the oral bioavailability of protease inhibitors [23]. It is therefore possible that RTV inhibits the transport of saquinavir via this transmembrane protein and thereby reduces the overall first-pass elimination of saquinavir [23, 24].
Irrespective of the mechanism involved, the combined use of RTV and SQV has been shown to reduce HIV RNA levels to below the level of quantification and increase the CD4 + cell count in both protease inhibitor naive and experienced HIV-positive patients [14, 17]. The main limitation with RTV therapy is its associated side-effects, which include nausea, vomiting, oral paraesthesia, and taste perversion [25, 26]. However, recognizing the ability of RTV to inhibit CYP3A, novel strategies have been designed which incorporate low doses of RTV in both once-daily and twice-daily SQV regimens [27–30].
Recognizing the benefit that is obtained by combining SQV-HGC with RTV, it is essential to explore this combination regimen using SQV-SGC. Therefore, the objective of the current study in healthy adult volunteers was to investigate the influence of different doses of combined RTV and SQV-SGC on systemic exposure to saquinavir, and propose an optimum dosing regimen for combining RTV with SQV-SGC in HIV therapy.
Methods
Study design
This was an open-label, randomized, parallel group, multiple-dose, single-centre study of SQV-SGC and RTV, alone and in combination. Healthy subjects were randomized to receive one of eight treatment regimens given in Table 1. The study was performed in accordance with the Declaration of Helsinki and its amendments, and the protocol approved by the Victory Memorial Hospital Institutional Review Board. Written informed consent was obtained from all subjects.
Table 1.
Treatment regimens and the assignment of subjects to each.
| Regimen | Drug treatment | n Female/Male |
|---|---|---|
| A | 800 mg twice daily SQV-SGC | 1/7 |
| B | 400 mg twice daily RTV | 2/6 |
| C | 400 mg twice daily SQV-SGC plus 400 mg twice daily RTV | 2/6 |
| D | 600 mg twice daily SQV-SGC plus 300 mg twice daily RTV | 0/8 |
| E | 800 mg twice daily SQV-SGC plus 200 mg twice daily RTV | 0/8 |
| F | 800 mg twice daily SQV-SGC plus 300 mg twice daily RTV | 1/7 |
| G | 800 mg twice daily SQV-SGC plus 400 mg twice daily RTV | 1/7 |
| H | 600 mg twice daily SQV-SGC plus 400 mg twice daily RTV | 1/7 |
All subjects received a single dose of treatment on day 1, followed by twice daily dosing (i.e. a dose every 12 h) at the doses described in Table 1 for a further 13 days. The RTV dosing in groups G and H was escalated such that subjects received 300 mg on day 1, 300 mg twice daily on day 2, and 400 mg twice daily on days 3–14. Subjects in regimens E, F, and G received SQV-SGC 600 mg on day 1, 600 mg twice daily on day 2, and 800 mg twice daily on days 3–14. For other regimens, the maximum required dose was administered from day 1. All treatment doses were taken within approximately 15 min of completing a meal (i.e. breakfast on day 1, and breakfast and the evening meal on days 2–14).
Subjects
Subjects in all treatment groups were given a thorough health assessment 14 days prior to the first day of treatment. Subjects were only included in the study if they had no known pre-existing medical conditions, were aged between 18 and 50 years old, and were nonsmokers.
After subjects were enrolled on the trial, they were confined to the Clinical Pharmacology Research Unit from 16 h before the first drug dose until study completion. Following the treatment period, subjects were allowed to return home but returned to the clinic for the follow-up assessment (at day 18–24), which included a physical examination, serum pregnancy test, and measurement of vital signs.
Assessments
Pharmacokinetic assessments
Venous blood samples (5 ml) were collected before drug administration on days 1, 11 and 14, and at regular intervals up to 12 h after drug administration. In addition, on days 1 and 14 only, blood samples were taken at 14, 16, 20, and 24 h after drug administration and additionally, for day 14 only, at 13, 15, 17, 18, 22, 26, 30, 34, 38, 42, and 48 h after drug administration. The blood samples were collected into heparinized tubes, before being centrifuged (1500 g, 4 °C for 10 min), and stored at ≤ −20 °C until analysis.
Saquinavir and ritonavir were analysed using the assay methodology routinely used by the sponsor companies (r.i.a. by Roche for saquinavir and h.p.l.c.-u.v. by Abbott for ritonavir). Plasma samples for saquinavir assay were analysed by a sensitive radioimmunoassay (r.i.a.) with a lower limit of quantification of 0.5 ng ml−1 for the assay of undiluted samples and 25 ng ml−1 for samples diluted 1 in 50 with blank plasma. The mean precision (%CV) of the undiluted sample assay, as determined from the analysis of the quality control samples, was 10%. The mean precision (%CV) of the diluted sample assay, as determined from the quality control samples, was 9.9%. Plasma samples for ritonavir assay were analysed by reverse phase high-performance liquid chromatography (h.p.l.c.) with ultraviolet (u.v.) detection with a lower limit of quantification of 10 ng ml−1 [19]. The overall precision for the ritonavir calibration curve standards and quality control samples, as measured by (%RSD), was less than or equal to 12%, and the overall accuracy (% REC) for these calibration standards and quality control samples ranged from 87% to 104%.
Physiological assessments
Blood (20 ml) and urine (10 ml) samples were taken for laboratory safety assessments at screening, 1 day predose, and on days 3, 7, 11 and 14 of the study, and at follow-up. Fasting glucose, triglycerides, and cholesterol were among the determinants measured. In addition to the readings taken at screening and follow-up, vital signs were measured at regular intervals throughout the study. A 12-lead ECG was performed at screening, and on days 3 and 7 (predose and 3–5 h postdose), and also at follow-up.
Evaluation
Pharmacokinetic evaluation
The pharmacokinetics of saquinavir and ritonavir were derived by noncompartmental methods [31]. The principal parameters were those obtained on day 14, although parameters were also obtained for days 1 and 11. On day 14 subjects received two doses of study medication. Consequently two dose intervals were evaluated. The nomenclature adopted in this study is therefore described below:
AUC(0,24 h) is the AUC calculated over an observed 24 h period which covers two dose intervals.
Cmax(0,24 h) is the highest observed plasma concentration from either the first or the second dose interval.
Cmin(0,24 h) is the observed concentration in the blood sample nominally collected at 24 h post the first dose of study medication on day 14 (alternatively this could be described as 12 h after the second dose of study medication on day 14).
Safety and tolerability
Safety and tolerability were assessed by evaluation of vital signs, laboratory tests, ECG, and the occurrence of adverse events. The latter were graded according to their intensity (i.e. mild, moderate or severe) and the extent to which they were related to treatment (i.e. unrelated, remote, possible or probable). Results from the laboratory safety tests were evaluated according to whether each parameter measured fell significantly below or above the laboratory normal reference range.
Statistical evaluation
An analysis of covariance (ancova) with the factor dose regimen and the covariable body weight (BW) was applied to the logarithmically transformed and dose-normalized day 14 AUC(0,24 h) and Cmax(0,24 h) for saquinavir to test the primary null hypothesis that the kinetic parameters for SQV-SGC administered alone (800 mg twice daily; regimen A) do not differ from the geometric mean of the kinetic parameters obtained when coadministered with RTV (200, 300, 400 mg twice daily; regimens E, F, and G, respectively). Intersubject variability for AUC(0,24 h) and Cmax(0,24 h) was calculated from the ancova. An exploratory analysis of covariance with the factor dose regimen and the covariable body weight (BW) was applied to the logarithmically transformed day 14 AUC(0,48 h) and Cmax(0,48 h) for RTV to test the secondary null hypothesis that the kinetic parameters of RTV administered alone (400 mg twice daily; regimen B) do not differ from geometric mean of the kinetic parameters obtained when coadministered with SQV-SGC (400, 600, 800 mg twice daily; regimens C, H and G, respectively). No statistical analyses were conducted on the safety or tolerability data.
Results
It was planned that 64 healthy subjects would participate in the study. Eight subjects were randomised to each treatment group, but two subjects withdrew (two females, one each from regimens C and G) and were replaced by two other subjects (one male and one female). The 66 subjects who participated in total (57 males and nine females) were aged between 18 and 50 years (mean age 30.8 years), and weighed between 49.4 and 103.0 kg (mean weight 74.1 kg). There were no apparent differences in terms of age or weight between treatment groups. All 66 subjects were included in the safety analysis and the 64 subjects who completed the study were evaluable for pharmacokinetic analysis.
Effect of ritonavir on pharmacokinetics of saquinavir
Based on the primary statistical analysis (ancova), the addition of RTV to SQV-SGC (800 mg twice daily) treatment resulted in an approximate overall 20-fold (95% confidence limits 13–32) and 9.6-fold (95% confidence limits 6.4–14.5) increase in saquinavir AUC(0,24 h) and Cmax(0,24 h). There was an overall 17-, 22-, and 23-fold increase in saquinavir AUC(0,24 h) with regimens E, F, and G, respectively (with confidence intervals of 10–30, 13–37, and 13–39, respectively, P = 0.0001 for all comparisons). Similar increases in drug exposure were also obtained on day 11 (data not shown). Body weight was found to have no statistically significant effect on the pharmacokinetic parameters for saquinavir (P = 0.78 and 0.84 for in AUC and ln Cmax, respectively).
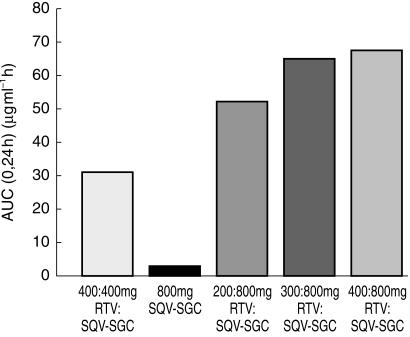
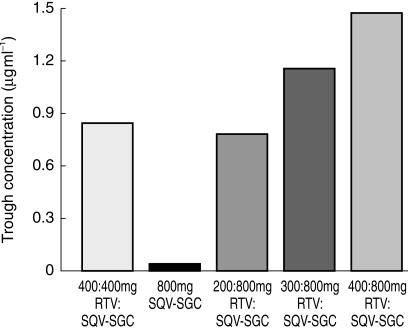
Indeed in all combination treatments the presence of RTV greatly increased the plasma concentration of saquinavir (Table 2, Figure 1, Figure 2). With 400:400 RTV:SQV-SGC (regimen C), the mean Cmax(0,24 h) and AUC(0,24 h) were 3.10 µg ml−1 and 35.51 µg ml−1 h, respectively. Even the lowest combination dose of RTV (i.e. 200:800 RTV:SQV-SGC, regimen E) substantially increased the saquinavir plasma concentration level, such that the mean Cmax(0,24 h) and AUC(0,24 h were 4.71 µg ml−1 and 56.82 µg ml−1 h, respectively, on day 14 with this regimen compared with 0.90 µg ml−1 and 4.60 µg ml−1 h, respectively, when SQV-SGC 800 mg twice daily was taken alone. Further increases in saquinavir plasma concentration levels were obtained with the higher RTV doses (i.e. regimens F and G), although these were only marginally higher. Geometric mean trough concentrations (i.e. Cmin, predose plasma concentration on day 14) were also substantially higher for the combination regimens, compared with SQV-SGC alone (Figure 2).
Table 2.
Summary of the pharmacokinetic parameters for saquinavir obtained on day 14 with the different SQV-SGC regimens.
| Parameter | A 800 mg SQV-SGC | C 400:400 mg ritonavir:SQV-SGC | D 300:600 mg ritonavir:SQV-SGC | E 200:800 mg ritonavir:SQV-SGC | F 300:800 mg ritonavir:SQV-SGC | G 400:800 mg ritonavir:SQV-SGC | H 400:600 mg ritonavir:SQV-SGC | |
|---|---|---|---|---|---|---|---|---|
| Cmax(0,24 h) (µg ml−1) | Geometric mean | 0.53 | 2.92 | 3.94 | 4.52 | 5.78 | 5.06 | 4.78 |
| Range | 0.20–3.99 | 1.78–4.72 | 2.36–9.63 | 2.80–7.25 | 3.33–8.44 | 2.62–6.75 | 3.16–7.65 | |
| AUC(0,24 h) (µg ml−1 h) | Geometric mean | 3.03 | 30.92 | 41.93 | 52.17 | 65.21 | 67.72 | 58.93 |
| Range | 1.31–18.74 | 14.68–70.07 | 25.01–154.43 | 29.56–105.22 | 40.07–89.64 | 32.81–93.44 | 42.36–115.56 | |
| Cmin(0,24 h) (µg ml−1) | Geometric mean | 0.04 | 0.84 | 0.74 | 0.79 | 1.16 | 1.48 | 1.41 |
| Range | 0.02–0.12 | 0.32–1.71 | 0.22–2.70 | 0.43–2.68 | 0.50–1.66 | 0.65–2.88 | 0.86–4.32 | |
| t1/2 (h) | Harmonic mean | 9.6 | 6.0 | 6.4 | 5.4 | 4.8 | 5.4 | 5.5 |
| Geometric mean | 9.7 | 6.0 | 6.6 | 5.5 | 4.9 | 5.5 | 5.5 | |
| Range | 7.6–12.6 | 5.1–7.8 | 4.8–10.2 | 4.9–8.7 | 4.2–6.3 | 4.1–7.5 | 4.6–7.3 |
Figure 1.
Geometric mean AUC(0,24 h) values for saquinavir on day 14 when given at a dose of 800 mg twice daily, alone (regimen A), in combination with RTV at various doses (regimens E, F and G), and when given in an equivalent dose to RTV (i.e. 400:400 mg; regimen C).
Figure 2.
Geometric mean trough values (i.e. Cmin) on day 14 when given at a dose of 800 mg twice daily, alone (regimen A), in combination with RTV at various doses (regimens E, F and G), and when given in an equivalent dose to RTV (i.e. 400:400 mg; regimen C).
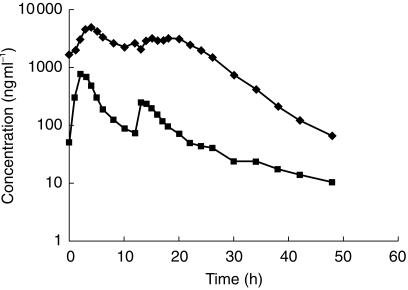
Apparent t½ was derived from the individual plasma concentration-time profiles. Surprisingly, the geometric mean t½ for saquinavir decreased from 9.7 h with SQV-SGC alone to 4.9 h with the 300 mg RTV twice daily dose and to 5.5 h with the 200 and 400 mg RTV twice daily doses when used in combination with SQV-SGC 800 mg twice daily. The t½ was generally estimated from the time points beyond 20–24 h post the first dose of day 14 (i.e. 8–12 h post the final dose). Visual inspection of the mean plasma concentration-time profiles (Figure 3) suggests that immediately postpeak, the plasma concentration of saquinavir declines less rapidly when combined with RTV. Furthermore, it cannot be excluded that if the blood collection phase had been extended beyond 48 h the regimens including RTV may have exhibited a later and longer t½ comparable with the SQV-SGC alone regimen.
Figure 3.
Plasma concentration-time profiles for subjects taking SQV-SGC monotherapy (800 mg, regimen A, ▪), or 400:800 mg (regimen G, ♦) combination RTV:SQV-SGC therapy twice daily.
Effect of saquinavir on pharmacokinetics of ritonavir
The exploratory statistical analysis results showed that additional administration of SQV-SGC did not lead to a statistically significant interaction effect for either AUC(0,48 h) or mean Cmax(0,48 h) (both P value 0.91). The ratio of the geometric means of dosage regimens C, H and G (individually) to the geometric mean of regimen B was shown to be 0.97 (95% confidence limits 0.71–1.34) for AUC(0,48 h) and 0.97 (95% confidence limits 0.75–1.27) for Cmax(0,48 h) (regimen C to regimen B); 0.90 (95% confidence limits 0.65–1.25) for AUC(0,48 h) and 0.82 (95% confidence limits 0.63–1.08) for Cmax(0,48 h) (regimen G to regimen B); and 0.91 (95% confidence limits 0.66–1.26) for AUC(0,48 h) and 0.91 (95% confidence limits 0.70–1.19) for Cmax(0,48 h) (regimen H to regimen B).
Thus coadministration of SQV-SGC and RTV had no relevant effect on the pharmacokinetics of ritonavir. Furthermore as described in Table 3 there was no apparent difference between the AUC(0,24 h) or Cmax(0,24 h) values obtained with RTV administered alone (400 mg twice daily; regimen B) compared with the values obtained when combined with SQV-SGC (400, 600 and 800 mg twice daily; regimens C, H, and G).
Table 3.
Summary of the pharmacokinetic parameters for ritonavir obtained on day 14 with the different ritonavir regimens.
| Parameter | B 400 mg ritonavir | C 400:400 mg ritonavir:SQV-SGC | D 300:600 mg ritonavir:SQV-SGC | E 200:800 mg ritonavir:SQV-SGC | F 300:800 mg ritonavir:SQV-SGC | G 400:800 mg ritonavir:SQV-SGC | H 400:600 mg ritonavir:SQV-SGC | |
|---|---|---|---|---|---|---|---|---|
| Cmax (0−24) (µg ml−1) | Geometric mean | 7.95 | 7.73 | 5.75 | 3.32 | 5.42 | 6.51 | 7.16 |
| Range | 4.83–18.74 | 6.72–9.26 | 4.58–6.55 | 2.52–4.15 | 4.34–7.66 | 3.91–9.88 | 3.40–10.19 | |
| AUC(0,24 h) (µg ml−1 h) | Geometric mean | 79.36 | 76.65 | 53.30 | 35.11 | 58.30 | 73.63 | 73.31 |
| Range | 39.54–171.36 | 48.93–100.68 | 32.45–90.11 | 21.96–51.47 | 50.68–65.06 | 50.27–126.20 | 33.29–117.33 | |
| Cmin(0,24 h) (µg ml−1) | Geometric mean | 2.35 | 2.83 | 1.63 | 0.87 | 1.49 | 2.08 | 2.39 |
| Range | 1.13–3.32 | 2.02–4.98 | 0.98–2.90 | 0.38–1.57 | 1.12–1.87 | 1.47–3.69 | 1.25–3.49 | |
| t½ (h) | Harmonic mean | 5.3 | 5.0 | 4.3 | 4.1 | 3.5 | 3.3 | 4.1 |
| Geometric mean | 5.4 | 5.1 | 4.5 | 4.3 | 3.6 | 3.4 | 4.2 | |
| Range | 3.3–7.1 | 4.0–6.4 | 2.5–7.1 | 2.8–9.8 | 2.8–5.7 | 2.2–5.3 | 2.9–7.1 |
Intersubject variability
The greatest intersubject variability (% coefficient of variation) in terms of the saquinavir pharmacokinetics was recorded for regimen A (SQV-SGC administered alone); approximately 105% was calculated for AUC(0,24 h) and 124% for Cmax(0,24 h). For the six combination regimens the variability was estimated to be between 32 and 68% for AUC(0,24 h) and 30–49% for Cmax(0,24 h) (Table 4).
Table 4.
Intersubject variability in terms of the saquinavir pharmacokinetics for all dosing regimens tested. Data are presented as percentage coefficient of variation.
| Coefficient of variation % | |||
|---|---|---|---|
| Regimen | Drug treatment (mg; RTV:SQV-SGC) | Saquinavir AUC(0,24 h) | Saquinavir Cmax(0,24 h) |
| A | 800 (SQV-SGC alone) | 105 | 124 |
| C | 400:400 | 63 | 39 |
| D | 300:600 | 68 | 49 |
| E | 200:800 | 46 | 32 |
| F | 300:800 | 32 | 30 |
| G | 400 800 | 36 | 32 |
| H | 400 600 | 33 | 33 |
Table 5.
Summary of the number of adverse events for each treatment regimen and a description of those occurring in more than one person. Eight subjects were randomised to receive each treatment regimen, except those marked with * which contained nine subjects. † The number of subjects reporting at least one adverse event.
| Adverse events | ||||
|---|---|---|---|---|
| Treatment regimen | Dose | Number of subjects† | Total number of events | Most common events (number of subjects reporting each events) |
| A | 800 mg twice daily SQV-SGC | 5 | 19 | Headache (2); sore throats (2); increased sweating (2) |
| B | 400 mg twice daily ritonavir | 7 | 35 | Non-specific oral hypoaesthesia (5); headache (5); nausea (3); tongue hypoaesthesia (3); taste disturbance (2); rhinitis (2) |
| C* | 400:400 mg ritonavir:SQV-SGC | 9 | 83 | Non-specific oral hypoaesthesia (7); headache (4); nausea (2); tongue hypoaesthesia (8); taste disturbance (2); paraesthesia (2); loose stools (3); dry mouth (4); dry throat (3); abdominal upper pain (2); sore throat (3); cough (2); pruritus (3); back pain (2); dysuria (2) |
| D | 300:600 mg ritonavir:SQV-SGC | 7 | 57 | Non-specific oral hypoaesthesia (4); headache (3); nausea (4); taste disturbance (4); paraesthesia (2); dizziness excluding vertigo (3); sore throat (3); myalgia (2); muscle cramps (2) |
| E | 200:800 mg ritonavir:SQV-SGC | 5 | 17 | Headache (2); constipation (2) |
| F | 300:800 mg ritonavir:SQV-SGC | 7 | 37 | Non-specific oral hypoaesthesia (5); headache (2); nausea (4); tongue hypoaesthesia (2); weakness (2); paraesthesia circumoral (2); sore throat (2) |
| G* | 400:800 mg ritonavir:SQV-SGC | 7 | 57 | Non-specific oral hypoaesthesia (5); headache (4); nausea (6); taste disturbance (4); dizziness excluding vertigo (2); weakness (2); vomiting (2); feeling hot (2); pyrexia (2); sore throat (2); cough (2) |
| H | 400:600 mg ritonavir:SQV-SGC | 8 | 50 | Non-specific oral hypoaesthesia (6); headache (2); nausea (3); tongue hypoaesthesia (3); taste disturbance (3); oral paraesthesia (3); dizziness excluding vertigo (3); feeling hot (2); sore throat (2) |
Safety and tolerability
Adverse events
All 66 subjects were eligible for the safety evaluation. The majority of reported adverse events were mild (98%) in intensity with only seven adverse events reported as being of moderate intensity. All resolved without sequelae. Two subjects withdrew from the study due to adverse events classified as probably related to treatment (one in regimen C who developed a rash and one in regimen G who suffered from nausea and vomiting). There were no reports of severe or serious adverse events during the study.
The most frequently reported adverse events were oral hypoaesthesia, headache, nausea, taste disturbance and dizziness (Table 1). Oral hypoaesthesia, nausea, and taste disturbance are common side-effects associated with RTV therapy and were probably related to treatment. Subjects who were administered regimens A (no RTV) or E (200 mg RTV: 800 mg SQV) reported relatively few adverse effects.
Laboratory safety tests
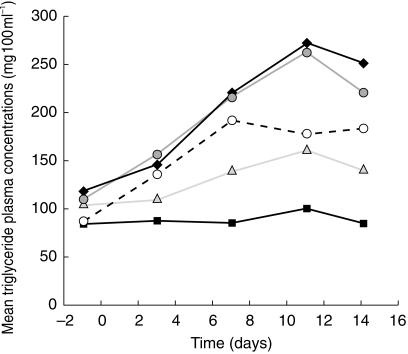
There were no changes of clinical relevance in vital signs or electrocardiograms during the study. Increases in some laboratory parameters, such as alanine and aspartate amino transferases or gamma glutamyl transpeptidase, were seen that were outside the laboratory reference ranges (one subject in each of the regimens involving the highest doses of SQV-SGC). However, these levels returned to within the normal range, or approaching normal, after discontinuation of drug treatment. Increases in triglyceride levels were obtained in the majority of subjects on RTV-containing regimens (Figure 4). The highest incidence of laboratory abnormalities was reported in regimen G, which involved the highest combined doses of RTV and SQV-SGC.
Figure 4.
Geometric mean triglyceride concentrations with time recorded in subjects taking either SQV-SGC monotherapy (800 mg, regimen A, ▪), or 200:800 mg (regimen E,  300:800 mg (regimen F,
300:800 mg (regimen F,  ), or 400:800 mg (regimen G, ♦) combination RTV: SQV-SGC therapy twice daily.
), or 400:800 mg (regimen G, ♦) combination RTV: SQV-SGC therapy twice daily.  RTV 400 mg (regimen B).
RTV 400 mg (regimen B).
Discussion
This is the first study to investigate the interaction between RTV and the soft-gelatin formulation of saquinavir – SQV-SGC. The results support other findings showing that, presumably, RTV substantially inhibits the metabolism of SQV and leads to a several-fold increase in saquinavir exposure [20, 21, 28, 29, 32]. From the results of this study, it also appears that the AUC(0,24 h) of saquinavir with the 400:400 mg twice daily combination RTV:SQV-SGC dosing regimen is similar to that obtained in a previous study using 400:400 mg twice daily RTV:SQV-HGC combination dosing (i.e. AUC(0,24 h) was also 36 µg ml−1 h) [32]. However, it should be noted that saquinavir plasma concentrations obtained in the current study and in the previous SQV-HGC study were assayed by two different methods.
As mentioned earlier, it has become generally accepted in the treatment of HIV to coadminister SQV-HGC with ritonavir, particularly at doses of 400:400 mg twice daily [12–14, 17]. Although care should be taken when conducting cross-study comparisons, the mean AUC(0,24 h) for saquinavir was 57 µg ml−1 h with the 200:800 mg twice daily RTV:SQV-SGC in the current study, which was higher than the exposure (AUC(0,24 h)) obtained with the 400:400 twice daily regimen and substantially higher than the median AUC(0,24 h) obtained with 1200 mg three times daily SQV-SGC monotherapy in HIV infected adults (i.e. approximately 20 µg ml−1 h) [7, 8]. Therefore, from the perspective of saquinavir, it may be possible to reduce the RTV dose used in combination RTV:SQV-SGC therapy still further (e.g. to 100 mg twice daily) in clinical practice. A previous therapeutic study in HIV patients showed that a regimen including SQV-HGC (1000 mg twice daily) with a lower dose of RTV (100 mg twice daily) decreased median plasma viral load by 1.20 log10 copies ml−1 and increased median CD4 cell count by 60 cells/mm3 (from 4.31 log10 copies ml−1 and 258 cells/mm3 at baseline, respectively) after 24 weeks [27]. Seventy-one percent of the patients (all of whom had failed previously on highly active antiretroviral therapy) achieved a plasma viral load of less than 500 copies ml−1. Preliminary studies have also shown that adding only 100 mg once daily of RTV to high dose SQV-SGC (1600 mg, also once daily) dramatically increases saquinavir exposure. Indeed the 24 h trough for the 1600:100 mg once daily regimen was around 0.5 µg ml−1 compared with around 0.09 µg ml−1 for the 8 h trough in the control group receiving SQV-SGC 1200 mg three times daily alone (3600 mg day−1) [28]. However, it should also be recognized that administration of a pharmacologically enhancing dose of ritonavir with the once daily dose of saquinavir is critical to maintain adequate blood levels of saquinavir. Patients should be advised that failing to take the booster dose of ritonavir could lead to suboptimal saquinavir exposure for prolonged periods of time.
The use of a low RTV dose in combination therapy is supported by the safety profile of this protease inhibitor. In the present study, lower doses of RTV were associated with fewer adverse events, rarely occurring in those subjects who received the lowest RTV combination-dosing regimen (i.e. 200:800 mg twice daily RTV: SQV-SGC). RTV administration was also associated with elevations in plasma triglyceride concentrations. These findings confirm those from previous studies showing that RTV therapy is associated with neurological and gastrointestinal side-effects [14, 25] and elevations in triglycerides levels [14, 26]. The use of low dose RTV to boost saquinavir exposure to very high levels, even with low total daily SQV-SGC doses, may also lead to a reduction in typical saquinavir-related gastrointestinal effects [4, 10].
As a potent inhibitor of CYP3A and P-glycoprotein, RTV alters the pharmacokinetics of saquinavir. The presence of RTV jointly diminishes first-pass extraction and efflux (and therefore increases bioavailability) and reduces clearance of systemically available saquinavir, thereby producing the large increase in exposure to saquinavir observed in vivo after oral dosing. As a further consequence, RTV also reduces the intersubject variability in saquinavir plasma concentrations observed when SQV-SGC is administered alone. Drugs, such as saquinavir, which undergo extensive gut wall and hepatic metabolism through CYP3A and efflux by P-glycoprotein are associated with extensive intersubject variability in the respective drug plasma levels due to large intersubject variability in CYP3A and P-glycoprotein expression in intestinal and hepatic cells [21, 33, 34]. In the current study, administration of SQV-SGC with RTV reduced the intersubject variability from 105% and 124% for AUC(0,24 h) and Cmax(0,24 h), respectively, with SQV-SGC alone, to less than 68% and 49%, respectively, when RTV was added to the treatment.
It is also well established that ritonavir as well as being an inhibitor is also an inducer of CYP3A and P-glycoprotein. One possible explanation for the unexpected effects of RTV on the terminal half-life of saquinavir is that as plasma ritonavir exposure falls that induction of liver CYP3A overrides inhibition leading to an increased clearance and decreased half life.
In agreement with previous studies, no effect of saquinavir on the overall pharmacokinetics of ritonavir was observed [20, 32]. This is not surprising, given the strength of RTV as a CYP3A inhibitor and the influence it has on its own metabolism.
In conclusion, based on the known relationship between saquinavir plasma concentration and HIV RNA and other surrogate markers [8, 9], the favourable effect of RTV and SQV-SGC combination therapy on saquinavir plasma concentration levels can be considered to be clinically relevant. The pharmacokinetic and safety profiles obtained in the current study indicate that the use of a low dose RTV–high dose SQV-SGC combination therapy should be further explored for use in clinical practice. Additional investigation will be necessary as only the pharmacokinetic properties of RTV are being considered in these circumstances and such a low dose of RTV may be suboptimal with regard to its antiviral contribution in such a combination regimen.
Acknowledgments
This study was supported by Roche Products Ltd and Abbott Laboratories and carried out by the Abbott Clinical Pharmacology Research Unit, Waukegen, IL, USA.
References
- 1.Stellbrink H-J. Abstracts from the Sixth European Conference on Clinical Aspects and Treatment of HIV-Infection. Hamburg, Germany: 1997. Clinical and survival benefit of saquinavir (SQV) in combination with zalcitabine (DDC) and zidovudine (ZDV) in untreated minimally treated HIV-infected patients; p. 21. abstract 212. [Google Scholar]
- 2.Haubrich R, Lalezari J, Follansbee SE, et al. Improved survival and reduced clinical progression in HIV-infected patients with advanced disease treated with saquinavir plus zalcitabine. Antiviral Ther. 1998;3:33–42. [Google Scholar]
- 3.Cohen Stuart JWT, Schuurman R, Burger DM, et al. Randomized trial comparing saquinavir soft gelatin capsules versus indinavir as part of triple therapy (CHEESE study) AIDS. 1999;13:F53–F58. doi: 10.1097/00002030-199905070-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuyasu RT, Skolnik PR, Cohen SR, et al. Activity of the soft gelatin formulation of saquinavir in combination therapy in antiretroviral-naive patients. AIDS. 1998;12:F103–F109. doi: 10.1097/00002030-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kohl NE, Emini EA, Schleif WA, et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng C, Bao K, Chang TW, Chang NT. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989;63:2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalezari J. Selecting the optimum dose for a new soft gelatin capsule formulation of saquinavir. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:195–196. doi: 10.1097/00042560-199810010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gieschke R, Fotteler B, Buss N, Steimer J-L. Relationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin Pharmacokin. 1999;37:75–86. doi: 10.2165/00003088-199937010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Schapiro JM, Winters MA, Stewart F, et al. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gill MJ. Safety profile of soft gelatin formulation of saquinavir in combination with nucleosides in a broad patient population. AIDS. 1998;12:1400–1402. [PubMed] [Google Scholar]
- 11.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempf DJ, Marsh KC, Kumar G, et al. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter CCJ, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997: updated recommendations of the International AIDS Society – USA Panel. JAMA. 1997;277:1962–1969. [PubMed] [Google Scholar]
- 14.Cameron DW, Japour AJ, Xu Y, et al. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13:213–224. doi: 10.1097/00002030-199902040-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lal R, Hsu A, Chen P, et al. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Toronto, Canada: 1997. Single dose pharmacokinetics of ABT-378 in combination with ritonavir; p. 279. (abstract I-194) [Google Scholar]
- 16.Lal R, Hsu A, Granneman GR, et al. Abstracts 5th Conference on Retroviruses and Opportunistic Infections. Chicago, USA: 1998. Multiple dose safety, tolerability and pharmacokinetics of ABT-378 in combination with ritonavir; p. 201. Abstract 647. [Google Scholar]
- 17.Tebas P, Patick AK, Kane EM, et al. Virologic responses to ritonavir-saquinavir-containing regimen in patients who had previously failed nelfinavir. AIDS. 1999;13:F23–F28. doi: 10.1097/00002030-199902040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cameron DW, Xu Y, Roce R, et al. 7th Conference on Retroviruses and Opportunistic Infections. San Francisco, USA: 2000. Three-year follow-up and conditional outcomes survival analysis of ritonavir (RTV) plus saquinavir (SQV) therapy in HIV infection. abstract 533. [Google Scholar]
- 19.Hsu A, Granneman GR, Bertz RJ. Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokin. 1998;35:275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hsu A, Granneman GR, Cao G, et al. Pharmacokinetic interactions between two human immunodeficiency virus protease inhibitors, ritonavir and saquinavir. Clin Pharmacol Ther. 1998;63(4):453–464. doi: 10.1016/S0009-9236(98)90041-8. [DOI] [PubMed] [Google Scholar]
- 21.Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11:F29–F33. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Profit L, Eagling VA, Back DJ. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 23.Washington CB, Duran GE, Sikic BI, Blaschke TF. Saquinavir is a high affinity substrate for the multidrug transporter, P-glycoprotein. Clin Pharmacol Ther. 1997;61:193. [Google Scholar]
- 24.Wacher VJ, Wu C-Y, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P4503A and P–glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 25.Gatti G, Di Biagio AD, Casazza R, et al. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- 26.Abbott Laboratories. Norvir (ritonavir capsules) soft gelatin (ritonavir oral solution) Prescribing Information. 1999 June. [Google Scholar]
- 27.Pikety C, Race E, Castiel P, et al. Efficacy of a five-drug combination including ritonavir, saquinavir and efavirenz in patients who failed on a conventional triple-drug regimen: phenotypic resistance to protease inhibitors predicts outcome of therapy. AIDS. 1999;13:F71–F77. doi: 10.1097/00002030-199907300-00001. [DOI] [PubMed] [Google Scholar]
- 28.Saag MS, Kilby M, Ehrensing E, Buss N, Oo CY. 7th European Conference on Clinical Aspects and Treatment of HIV-infection. Lisbon, Portugal: 1999. Modulation of saquinavir steady-state pharmacokinetics with ‘baby’ doses of ritonavir in healthy volunteers; p. 210. Abstract 829. [Google Scholar]
- 29.Van Heeswijk RPG, Veldkamp AI, Mulder JW, et al. 7th European Conference on Clinical Aspects and Treatment of HIV-infection. Lisbon, Portugal: The steady-state pharmacokinetics of saquinavir in a once daily dosing regimen with a low dose of ritonavir; p. 211. Abstract 830. [Google Scholar]
- 30.Kurowski M, Müller M, Donath F, Mrozikiewicz M, Möcklinghoff C. Single daily doses of saquinavir achieve HIV-inhibitory concentrations when combined with ‘baby-dose’ ritonavir. Eur J Med Res. 1999;4:101–104. [PubMed] [Google Scholar]
- 31.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 32.Hsu A, Granneman GR, Sun E, et al. Abstracts from XI International Conference on AIDS. Vancouver, Canada: 1996. Assessment of single- and multiple-dose interactions between ritonavir and saquinavir. [Google Scholar]
- 33.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolars JC, Lown KS, Schmiedlin-Ren P, et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics. 1994;4:247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]