Abstract
Aims
To assess the effect of local hyperthermia on the systemic absorption of mitomycin C (MMC) during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma of the bladder, and to establish the likely safety of this procedure.
Methods
Group 1 (n = 12) received 20 mg intravesical MMC plus local hyperthermia, group 2 (n = 13) 20 mg MMC alone, group 3 (n = 16) 40 mg MMC plus local hyperthermia and group 4 (n = 10) 40 mg MMC alone. Patients in groups 1, 2, and 4 underwent post-tumour resection adjuvant treatment, whereas those in group 3 still had tumour present and were treated to eradicate it. Intravesical instillation lasted 60 min, with the solution (50 ml) being replaced after the first 30 min. Blood samples were taken before, and every 15 min during instillation. MMC concentrations in plasma and in urine were determined by h.p.l.c.
Results
The highest MMC plasma concentration (67.9 ng ml−1) occurred in a patient in group 3. This value was well below the threshold concentration (400 ng ml−1) for myelosuppression. Local hyperthermia associated with the intravesical chemotherapy enhanced plasma MMC concentrations at 30, 45 and 60 min compared with chemotherapy alone (Group 1 vs 2, P ≤ 0.008). Systemic exposure to MMC was not significantly increased by doubling the intravesical dose when intravesical chemotherapy alone was administered. Patients in group 3 displayed the highest degree of MMC absorption and the greatest variability in pharmacokinetics between patients.
Conclusions
Local hyperthermia enhances the systemic absorption of MMC during intravesical chemotherapy for bladder cancer. In the doses used, plasma MMC concentrations were always more than six times lower than those shown to cause toxicity.
Keywords: bladder hyperthermia, h.p.l.c., intravesical chemotherapy, superficial transitional cell carcinoma, systemic absorption
Introduction
Epidemiological data show that between 50 and 70% of patients treated with transurethral resection (TUR) for superficial transitional cell carcinoma of the bladder (STCCB) will develop recurrences, with progression of tumour grade and stage in 10–15% of cases [1, 2]. A combination of TUR and adjuvant intravesical chemotherapy (ICT) or immunotherapy has contributed to lowering the recurrence rate to 30–40%. However, the progression rate seems to have remained unchanged in spite of local adjuvant treatment [3].
When local hyperthermia between 40 and 45 °C is administered in combination with selected cytostatic drugs [4, 5], a supra-additive or at least an additive antitumoural effect has been reported. This may be a therapeutic option for STCCB, due to its endocavitary superficial location, and the ease of accessibility via the urethra [6]. However, the lack of suitable technology has so far prevented the implementation of this anticancer regimen.
At our Institute, a new advanced system (SB-TS 101–1, Synergo®), specifically designed for the combined administration of ICT and local hyperthermia by means of an intravesical microwave applicator, has been developed and clinically tested in STCCB patients. Since 1987 many clinical trials have shown that administration of mitomycin C (MMC) combined with LHT is safe and more effective in tumour ablation and in preventing recurrences after TUR than using ICT alone. Furthermore, the procedure can prevent or at least delay the need for cystectomy in a large number of patients affected by multifocal and unresectable bladder tumours [7–13].
MMC is absorbed systemically to some extent after intravesical administration. Many studies in humans [14–21] have indicated that plasma concentrations after normal doses do not reach a critical toxic value of 400 ng ml−1 reported by Crooke et al. [22]. The aim of the present study was to examine the systemic absorption of MMC during microwave-induced hyperthermia and to determine whether concentrations remain below toxic levels. Moreover, attempts have been made to correlate MMC pharmacokinetics with the type of therapy given, drug administration schedules and clinical parameters.
Methods
Chemicals
MMC, Mitomycin-C® (injectable powder at 4% purity in NaCl) and porfiromycin (> 99% pure), used as the internal standard for the h.p.l.c. analysis of MMC, were from Kyowa Hakko Kogyo Co., Ltd. (Tokyo, Japan). Organic solvents (HPLC grade) were obtained from J. T. Backer (Deventer, The Netherlands). The MMC solution used for intravesical instillation was freshly prepared in bi-distilled water prior to each treatment.
The SB-TS 101–1 Synergo® system
A description of this technique as well as its advantages over earlier methods of inducing hyperthermia, have been extensively reported by us [7–12].
Patients
This investigation was performed after approval by our Institute's local Human Investigation Committee and in accordance with a good clinical practice protocol. Patients were enrolled for the pharmacokinetic study after a full explanation of what was involved and they provided verbal agreement to blood collection during treatment. Thirty-nine patients undergoing a complete TUR of STCCB 21–40 days before this study were considered eligible. They were randomly assigned to group 1 (MMC at 20 mg dose in combination with hyperthermia), to group 2 (MMC alone at 20 mg) or to group 4 (MMC alone at 40 mg) in an adjuvant setting to prevent recurrence. For technical reasons the enrolment was stopped at 35 patients (n = 13, n = 12, n = 10, in group 1, 2, 4, respectively). An additional 16 patients suffering from STCCB but whose tumours could not be eradicated by TUR, were assigned to group 3 and received MMC at 40 mg dose combined with hyperthermia as a debulking approach with bladder sparing intent [11]. For staging purposes all patients underwent pelvic ultrasounds, video-cystoscopy and pyelogram. Abdominal and pelvic CT was performed for patients showing high rate of recurrence. Tumour size was estimated to be less than 5 cm in 2 patients, between 5 and 10 cm in 10 patients, and more than 10 cm in 4 patients. Five patients had never undergone resection, 9 patients had undergone 6 or less TUR, and 1 had undergone 22 TUR before this study. One patient presented with ulcerated lesions. For ethical reasons a clinically matched normothermic control group was not used, as previous work has failed to confirm the ability of ICT alone to ablate tumours [11]. Clinical details of the patients are shown in Table 1.
Table 1.
Patient information.
| Gender | Tumour stage | Tumour grade | Tumours number | Recurrences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | Patients age (years) | M | F | Ta | T1 | G1 | G2-G3 | < 5 | ≥ 5 | < 3 | ≥ 3 |
| 1 | 12 | 37–76 | 10 | 2 | 6 | 6 | 3 | 9 | 1 | 11 | 3 | 9 |
| 2 | 13 | 36–83 | 10 | 3 | 7 | 6 | 3 | 10 | 10 | 3 | 12 | 1 |
| 3 | 16 | 39–72 | 3 | 13 | 5 | 11 | 0 | 16 | 7 | 9 | 7 | 9 |
| 4 | 10 | 40–82 | 0 | 10 | 7 | 2 | 2 | 8 | 3 | 7 | 10 | 0 |
Group 1 patients were given MMC (20 mg) combined with hyperthermia. Group 2 patients were given MMC alone at the same dose as group 1. Group 3 patients were given ICT (40 mg) combined with hyperthermia. Group 4 patients were given ICT alone at the same dosage as Group 3. Groups 1, 2 and 4 patients underwent the first treatment session within 21–40 days after a complete TUR of tumours, while group 3 patients were treated by leaving their tumour intact as a debulking approach for a bladder sparing purposes.
Treatment
The operative catheter of the Synergo® System was inserted into the empty bladder of patients in groups 1 and 3, and the balloon inflated with 15 ml of bi-distilled water. The three thermocouples were spread out to contact the bladder wall and 50 ml of the MMC solution (preheated to 42.5 °C) was instilled. The intravesical location of the balloon, heat applicator and thermocouples were assessed by suprapubic ultrasounds. The power of the microwave source was regulated to a range of 20–30 Watts to obtain an intravesical temperature of 42–45 °C. The temperature of the bladder wall (mean of three thermocouples) was recorded every 15 min. The bladder was emptied after 30 min and infused with fresh MMC solution at the same dosage. The bladder was again emptied at the end of the second 30 min period. Patients in the groups undergoing normothermic treatment were subjected to exactly the same procedure except that the microwave source was disconnected and the cytostatic solution was instilled at ambient temperature. The bladder wall temperatures ranged from 36.5 to 37 °C. Possible physical damage to the bladder wall due to the microwave probes was excluded by examination with a flexible cystoscope after the treatment. Subjective symptoms before, during and after treatment were assessed by a questionnaire.
In all cases the treatment protocol included 8 once-weekly sessions, each lasting 60 min. Pre- and post-treatment evaluation included clinical history, haematology, blood biochemistry and urine analysis. The pharmacokinetic study was performed during the first session to exclude any effects of temperature or residual drug in the bladder wall. All patients received sodium bicarbonate (500 mg) to be taken orally both the evening before and 2 h before treatment.
Sampling
Blood samples were collected in heparinized tubes immediately after filling the bladder with MMC solution (time zero) and at 15, 30, 45, and 60 min after instillation. Plasma was separated as soon as possible and stored at −80 °C until h.p.l.c. analysis. The volume and pH of the solution recovered from the bladder were measured after both the first 30 min (when the bladder was emptied and the drug solution replaced) and the second 30 min instillation period. Aliquots were stored immediately at −80 °C.
MMC analysis by h.p.l.c
MMC concentrations in plasma and urine were determined by h.p.l.c. using a method previously developed and validated by us [23]. Briefly the recovery of MMC was equal to or greater than 89%, its limit of quantification was 0.5 µg l−1, the intra-day coefficient of variation was ≤6.0% (n = 6) and the reproducibility of the assay over 1 year was ≤10.7% (n = 10).
In vitro degradation of MMC
MMC at concentrations of 0.8 mg ml−1 was prepared in bi-distilled water, in urine at pH 5.5 and in urine at pH 7.5. Triplicate samples were incubated for up to 30 min at 37 °C or 45 °C. Samples were diluted 250-fold in water and injected onto the h.p.l.c.
Data analysis
Statistical analysis was performed using the Sigma Stat statistical package (Jandel Scientific GmbH, Erkrath, Germany). The fraction of the dose recovered after 30 min was calculated as the amount in urine drained at the end of the instillation period through the catheter, divided by the dose instilled. Data were expressed as the mean±standard deviation (± s.d.). To identify significant differences between groups, the parametric Student's t-test was used. When data were not normally distributed and showed dishomogeneous variance, they were expressed as medians (interquartile range) and the nonparametric Mann-Whitney test on ranks was applied. Correlation between variables was assessed by using the nonparametric Spearman's Rank Order Correlation test. Statistical significance was assumed at P ≤ 0.05.
Results
MMC degradation
After incubation in water at 45 °C for 30 min, less than 2% of MMC was lost. Incubation of MMC for 30 min in urine at pH 5.5 and 7.5 resulted in a mean loss of 6.3 ± 0.9 and 6.5 ± 0.8% at 37 °C and 15 ± 1.2 and 7.9 ± 1.1% at 45 °C, respectively.
Application of local hyperthermia
A similar degree of bladder hyperthermia was achieved in groups 1 and 3 over the entire period (Table 2). However, more power to the heat applicator was required to achieve these temperatures in group 3 than in group 1 (P ≤ 0.004), suggesting that in the former patients blood flow in the bladder wall may be greater due to the continued presence of the papillary tumour.
Table 2.
Bladder temperatures and heating power required for local hyperthermia
| Temperature (°C) | Power (watts) | |||
|---|---|---|---|---|
| Time (min) | Group 1 (n = 12) | Group 3 (n = 16) | Group 1 (n = 12) | Group 3 (n = 16) |
| 15 | 42.8 ± 0.8 | 43.8 ± 1.14 | 22.8 ± 1.6* | 25.9 ± 2.9 |
| 30 | 43.4 ± 0.8 | 43.5 ± 1.0 | 24.5 ± 2.7** | 28.5 ± 2.7 |
| 45 | 42.9 ± 0.63 | 43.7 ± 2.0 | 23.9 ± 3.2# | 29.0 ± 3.3 |
| 60 | 43.6 ± 0.9 | 44.0 ± 1.4 | 25.1 ± 3.6## | 29.0 ± 3.3 |
Values are expressed as mean (± s.d.).
P ≤ 0.005 vs group 3 (95% confidence interval for the means 1.02, 5.18 watts).
P ≤ 0.002 vs group 3 (95% confidence interval for the mean 1.69, 6.28 watts).
= P ≤ 0.001 vs group 3 (95% confidence interval for the mean 2.3, 7.8 watts).
= P ≤ 0.004 vs group 3 (95% confidence interval for the mean 1.5, 6.9 watts).
Safety of local hyperthermia
The systemic absorption of MMC reached a plateau between 45 and 60 min, with a median value of 5.56 (3.46–11) and 19.4 (5.2–42.9) ng ml−1 in groups 1 and 3, respectively. The highest MMC plasma concentration (67.9 ng ml−1) was recorded in a patient from group 3 (40 mg dose plus hyperthermia). This concentration is six times lower than the reported threshold concentration for toxicity (400 ng ml−1). None of the patients enrolled in the study complained of nausea or vomiting during the treatment. No evidence of myelosuppression was detected in any patients. The haematologic data for each group are reported in Table 3.
Table 3.
Haematological data before and after 8 weeks of intravesical chemotherapy
| Group | Time | WBC (109/l) | Hb (g l−1) | PLT (109/l) |
|---|---|---|---|---|
| Pre | 5.1 | 12.9 | 233 | |
| 1 | (4.5–6.0) | (11.0–13.9) | (217–250) | |
| Post | 6.7 | 13.7 | 228 | |
| (5.7–9.9) | (13.4–14.2) | (201–270) | ||
| Pre | 7.5 | 15.0 | 234 | |
| 2 | (6.6–11.2) | (14.4–15.9) | (221–239) | |
| Post | 6.8 | 14 | 247 | |
| (6.4–7.0) | (13.5–15.4) | (144–258) | ||
| Pre | 7.9 | 13.6 | 226 | |
| 3 | (5.7–8.8) | (11.5–15.7) | (188–261) | |
| Post | 8.0 | 14.1 | 271 | |
| (6.9–11.1) | (11.3–15) | (231–328) | ||
| Pre | 6.5 | 14 | 251 | |
| 4 | (5.6–9.1) | (13–15) | (152–260) | |
| Post | 6.7 | 13.9 | 248 | |
| (6.4–7.1) | (11–15) | (220–260) |
Data are expressed as the median (interquartile range). Group 1 patients were given MMC (20 mg) combined with hyperthermia. Group 2 patients were given MMC alone at the same dose as group 1. Group 3 patients were given MMC (40 mg) combined with hyperthermia. Group 4 patients were given ICT alone at the same dosage as Group 3. Groups 1, 2 and 4 underwent the first treatment session within 21–40 days after a complete TUR of tumours, while group 3 patients were treated by leaving their tumour intact as a debulking approach for a bladder sparing purposes.
Plasma MMC concentration-time profiles
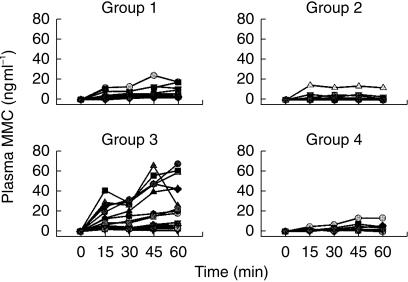
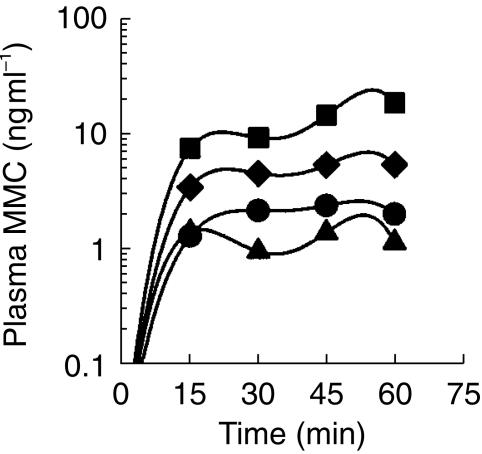
Figure 1 shows the plasma MMC concentration-time profiles for each patient. ‘Outliers’ from all groups were evident, with a particularly high variability among patients in group 3. Figure 2 shows the median plasma concentration-time curves for each group. After the first 30 min of instillation, hyperthermia with ICT at 20 mg dose significantly enhanced the passage of MMC through the bladder into the systemic circulation compared with ICT alone at the same dose (4.6 (2.5–6.8) vs 0.9 (0.2–2.7) ng ml−1, P ≤ 0.008). This effect persisted after 45 min (5.5 (3.3–10.9) vs 1.5 (0.8–2.3) ng ml−1, P ≤ 0.003) and 60 min (5.6 (3.4–11.0) vs 1.1 (0.4–1.5) ng ml−1, P ≤ 0.001). Comparing the two normothermic groups (20 and 40 mg), a dose-dependent effect was noted, although this did not reach statistical significance. Group 3 patients, whose tumours were still present in the bladder, showed the highest MMC plasma concentrations of all the four groups (Figure 2).
Figure 1.
Individual concentration-time profiles of MMC in plasma during a 60-min intravesical instillation. Group 1, ICT (20 mg) combined with hyperthermia; group 2, ICT alone at the same dose as group 1; group 3, ICT (40 mg) combined with hyperthermia; group 4, ICT alone at the same dose as group 3.
Figure 2.
Median concentration-time profiles of MMC in plasma during a 60-min intravesical instillation. The bladder contents were flushed out after 30 min and replaced with fresh MMC solution (♦), ICT (20 mg) combined with hyperthermia (▴), ICT (20 mg) alone (▪), ICT (40 mg) combined with hyperthermia (•), ICT (40 mg) alone. Median MMC concentrations after ICT (20 mg) plus hyperthermia (♦) were significantly different from those ater ICT alone at the same dose (▴), (P ≤ 0.008, P ≤ 0.003 and P ≤ 0.001 at 30, 45 and 60 min, respectively).
Residual urine and MMC recovery at the end of instillation
The volume and pH of the residual bladder contents were similar for the 0–30 and 30–60 min instillation periods and these data were averaged. Plasma creatinine assessed prior to treatment was in the physiological range for all patients. The pH of the bladder content drained after the 30 min instillation period was 7.6 ± 0.8 (mean±s.d., n = 51), with no differences between treatments groups. At this pH value, the half-life of MMC disappearance from the bladder has been reported to be 38 h [19, 21]. Dilution of the drug proved to be greater when the bladder was hyperthermic. MMC concentration in the bladder declined from 0.4 mg ml−1 to 0.13 (0.09–0.15) in group 1 and 0.17 (0.12–0.15) mg ml−1 in group 2, and from 0.8 mg ml−1 to 0.2 (0.11–0.28) in group 3 and 0.37 (0.19–0.4) mg ml−1 in group 4. Following the instillation of 50 ml of MMC solution into the bladder at time 0, a median volume of 82 (70–92) and 95 (95–100) ml was recovered from the normothermic groups, whereas 132 (99–193) and 130 (98–207) ml were recovered in the hyperthermic groups. Hyperthermia did not appear to significantly enhance intravesical nonenzymatic degradation of MMC, since the recovery of drug (% of the initial value) from the bladder at the end of treatment did not differ significantly from normothermic patients (80 (55–98)%, n = 28 vs 79 (62–97)%, n = 23, respectively).
Discussion
In most patients with STCCB, drug exposure at the target site is an important determinant of treatment efficacy, whereas systemic exposure is an important determinant of host toxicity. Wientjes et al. [20] found an approximately 35-fold concentration ratio between the urine and urothelium/lamina propria interface when MMC was instilled endovesically. Dalton et al. [17], using a mean value of 10 ml min−1 kg−1 for MMC plasma clearance, have estimated that under standard prophylactic conditions, less than 6% of an intravesical dose was absorbed into the systemic circulation.
The present study showed that plasma uptake of MMC from the bladder increases when one tumour is present, suggesting that patients with several tumours, or one tumour with a large surface area, could be at risk of systemic toxicity. However, in all patients, plasma MMC concentrations remained between 10 and 100 times below the value of 400 ng ml−1 associated with myelosuppression [22]. Subjective assessment of patients together with the haematological data collected after multiple treatments confirm the safety of the thermo-chemotherapy tested in the first treatment session.
Wientjes et al. [20] analysed MMC concentration-depth profiles in the bladder wall using two kinetics models: the diffusion and the distributed model. The former model does not account for drug removal by tissue blood flow and predicts a linear concentration decline from the urothelium to the serosal surface. The latter model describes a log-linear decline through the bladder wall, combining drug diffusion and removal from the bladder, the latter process being influenced by parietal blood flow. Thus, the observed effects of thermo-chemprophylaxis on bladder MMC pharmacokinetics may be caused by modified blood perfusion, increased cellular membrane permeability and/or altered intracellular metabolism [24].
In the present study hyperthermia may have induced modifications in tissue microcirculatory functions, causing a greater production of urine plus exudate concomitant to a dilution of the drug into the bladder. However, heat also tended to increase the passage of the drug from the bladder to the plasma. A further finding was that when ICT was administered alone, systemic exposure to the drug was not significantly affected by the endovesical dose. However, when the bladder was hyperthermic, MMC plasma concentrations almost doubled after the higher dose. This observation suggests that modifications in urothelium permeability induced by thermotherapy are more crucial for MMC systemic absorption than the trans-wall bladder drug concentration gradient. However, a comparison of group 3 patients with the other groups is not totally valid since their tumours were still present. Group 3 showed the greatest intersubject variability, which was not related to disease history (number of previous TUR), neoplastic staging or grading and tumour size. The different vascularity of tumours, which is not necessarily related to their size, may contribute to inter-subject variability. The more abundant vascularization of the tumoural mass may also account for the higher heat energy needed in these patients to reach the same target temperatures of group 1, in whom the tumour was not present.
Urothelium cicatrizations after a number of previous TUR could also modify bladder vascularization, thus influencing MMC penetration through the bladder wall. However, cicatrization status is difficult to define a priori and depends on both the number and extent of previous resections and on the effectiveness of urothelium repair after surgery.
In conclusion, this study, supports the safety of adjuvant microwave-induced hyperthermia in combination with ICT at the doses used and provides a possible rationalization for the clinical effectiveness of the SB-TS 101–1 System (Synergo®).
Acknowledgments
We thank Dr Giliola Calori for her invaluable help in the statistical analysis and Sig. Giuseppe Collura for his skilful technical assistance in the treatment of patients. This work was supported by grants from C.N.R. and the ‘Associatione Italo-Brasiliana per la Diagnosi e la terapia delle Malattie dell’Apparato Urinario'.
References
- 1.Torti FM, Lum DL, Aston D. Superficial bladder cancer: the primacy of grade in the development of invasive disease. J Clin Oncol. 1987;5:125–130. doi: 10.1200/JCO.1987.5.1.125. [DOI] [PubMed] [Google Scholar]
- 2.Heney NM, Ahmed S, Flanagan MJ, et al. Superficial bladder cancer: progression and recurrence. J Urol. 1983;136:1083–1086. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MA, Ratliff TL, Gillen DP, Haaff EO, Dresner SM, Catalona WS. Single course versus maintenance bacillus Calmete-Guerin therapy for superficial bladder tumours: a prospective randomized trial. J Urol. 1987;138:295–299. doi: 10.1016/s0022-5347(17)43125-9. [DOI] [PubMed] [Google Scholar]
- 4.Hahn GM. Potential for therapy of drugs and hyperthermia. Cancer Res. 1979;39:2264–2268. [PubMed] [Google Scholar]
- 5.Dahl O. Hyperthermia and drugs. In: Wathmong DJ, Ross WM, editors. Hyperthermia. Glasgow: Blackie; 1986. pp. 121–153. [Google Scholar]
- 6.Herr HW. Intravesical therapy. A critical review. Urol Clin North Am. 1987;14:399–404. [PubMed] [Google Scholar]
- 7.Rigatti P, Lev A, Colombo R. Combined intravesical chemotherapy with Mytomicin C and local bladder microwave-induced hyperthermia as a preoperative therapy for superficial bladder tumours. A preliminary clinical study. Eur Urol. 1991;20:204–210. doi: 10.1159/000471701. [DOI] [PubMed] [Google Scholar]
- 8.Colombo R, Lev A, Da Pozzo LF, Freschi M, Gallus G, Rigatti P. A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J Urol. 1995;153:959–963. [PubMed] [Google Scholar]
- 9.Colombo R, Da Pozzo LF, Lev A, et al. Local microwave hyperthermia and intravesical chemotherapy with Mitomycin C as neoadjuvant treatment for selected multifocal and urresectable superficial bladder tumours. Acta Urol Ital. 1995;4:167–171. [Google Scholar]
- 10.Colombo R, Da Pozzo LF, Lev A, Freschi M, Gallus G, Rigatti P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol. 1996;155(4):1227–1232. [PubMed] [Google Scholar]
- 11.Colombo R, Da Pozzo LF, Lev A, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumours. J Urol. 1998;159:783–787. [PubMed] [Google Scholar]
- 12.Leib Z, Baniel J, Servadio C, et al. Mytomicin C (MMC) vs MMC combined with local microwave hyperthermia (LMWH) as prophylaxis of recurrence of superficial transitional bladder cancer: a preliminary report. Eur Urol. 1996;30:198. [Google Scholar]
- 13.Colombo R, Brausi M, Da Pozzo LF, et al. Thermo-chemotherapy and electromotive drug administration of Mitomycin C in superficial bladder cancer eradication. A pilot study on marker lesion. Eur Urol. 2001;39:95–100. doi: 10.1159/000052419. [DOI] [PubMed] [Google Scholar]
- 14.Aeikens B, Niermann R, Schindler E. Investigation about the penetration depth in the normal bladder wall and tumour by local instillation of Mitomycin into the urinary bladder. Urol Int. 1982;37:389–393. doi: 10.1159/000280844. [DOI] [PubMed] [Google Scholar]
- 15.den Hartigh J, McVie JG, van Oort WJ, Pinedo HM. Pharmacokinetics of Mitomycin C in humans. Cancer Res. 1983;43:5017–5021. [PubMed] [Google Scholar]
- 16.Wientjes MG, Dalton JT, Badalament RA, Dasani BM, Drago JR, Au JL-S. A method to study drug concentration-depth profiles in tissues: Mitomycin C in dog bladder wall. Pharm Res. 1991;8:168–173. doi: 10.1023/a:1015827700904. [DOI] [PubMed] [Google Scholar]
- 17.Dalton JT, Wientjes MG, Badalament RA, Drago JR, Au JL-S. Pharmacokinetics of intravesical Mitomycin C in superficial bladder cancer patients. Cancer Res. 1991;51:5144–5152. [PubMed] [Google Scholar]
- 18.Schmittgen TD, Au JL-S, Wientjes MG, Badalament RA. Pharmacodynamics of Mitomycin C in cultured human bladder tumours. Cancer Res. 1991;51:3849–3856. [PubMed] [Google Scholar]
- 19.Wientjes MG, Dalton JT, Badalament RA, Drago JR, Au JL-S. Bladder wall penetration of intravesical Mitomycin C in dogs. Cancer Res. 1991;51:4347–3454. [PubMed] [Google Scholar]
- 20.Wientjes MG, Badalament RA, Wang RC, Hassan F, Au JL-S. Penetration of Mytomicin C in human bladder. Cancer Res. 1993;53:3314–3320. [PubMed] [Google Scholar]
- 21.Gao X, Au JL-S, Badalament RA, Wientjes MG. Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin Cancer Res. 1998;3(1):139–143. [PubMed] [Google Scholar]
- 22.Crooke ST, Henderson M, Samson M, Baker LH. Phase I study of oral Mitomycin C. Cancer Treat Rep. 1976;60:1633–1636. [PubMed] [Google Scholar]
- 23.Paroni R, Arcelloni C, De Vecchi E, Fermo I, Mauri D, Colombo R. Plasma Mitomycin C concentrations determined by HPLC coupled to solid-phase extraction. Clin Chem. 1997;43:615–618. [PubMed] [Google Scholar]
- 24.Dewhirst HW, Sim DA, Gross J, Kundrat MA. Effect of heating rate in tumour and normal tissue microcirculatory function. In: Overgaard J, editor. Hyperthermic Oncology. Vol. 1. London, Philadelphia: Taylor & Francis; 1984. pp. 77–180. [Google Scholar]