Abstract
Aims
To establish the bioavailability of tropisetron (5 mg) administered orally as capsule compared with 2 mg given intravenously.
Methods
Using a randomized crossover design, 18 healthy volunteers received a single oral dose of tropisetron (5 mg) and an intravenous bolus of tropisetron (2 mg) separated by a wash-out period of 1 week. Plasma concentrations of tropisetron were determined by h.p.l.c. and the pharmacokinetic parameters were estimated.
Results
The mean pharmacokinetic parameters for 5 mg tropisetron given orally were Cmax 3.46 ng ml−1, tmax 2.6 h, t1/2 5.7 h and AUC(0,∞) 32.9 ng ml −1 h. After intravenous administration initial plasma concentration was 15.1 ng ml−1, t1/2 5.6 h, AUC(0,∞) 20.7 ng ml −1 h, V 678 l and CL 1800 ml min−1. An inverse correlation was demonstrated between CYP2D6 activity, measured by the sparteine metabolic ratio, and the bioavailability (mean 0.60, range 0.27–0.99) of oral tropisetron.
Conclusions
Tropisetron exhibits a wide range of oral bioavailability at therapeutic doses, which is mainly determined by CYP2D6 activity.
Keywords: bioavailability, low doses, tropisetron, volunteers
Introduction
Tropisetron is a potent and selective 5-HT3 receptor antagonist, and it is used primarily in the treatment of patients with chemotherapy-induced or postoperative nausea and vomiting. Tropisetron is almost completely absorbed from the gastrointestinal tract, but undergoes dose-dependent first pass metabolism. The absolute bioavailability of oral tropisetron increases from 0.52 at a 20 mg dose to 0.66 at a 100 mg dose [1], and may even reach 1.0 at doses of 45 mg and over [2]. Tropisetron is extensively metabolized to inactive metabolites by hydroxylation of the indole moiety and further conjugation to glucuronides and sulphates. Ring hydroxylation occurs mainly via CYP2D6. Accordingly, the terminal plasma elimination half-life of tropisetron varies between 6 and 8 h in extensive metabolizers and 30–40 h in subjects with CYP2D6 deficiency [1].
Whereas the basic pharmacokinetic parameters of tropisetron have been established using doses of 20–100 mg, the typical recommended therapeutic dose of tropisetron is 5 mg orally, and 2–5 mg intravenously [1]. The aim of the present study was to determine the bioavailability of oral tropisetron 5 mg with respect to 2 mg administered intravenously, in order to provide pharmacokinetic data for comparative clinical trials investigating the potential therapeutic use of tropisetron in fibromyalgia [3]. Only subjects known to be CYP2D6 extensive metabolizers were included into the study.
Methods
Subjects
Eighteen healthy volunteers (nine males/nine females, 22–30 years, median 26 years, body mass index 19–26 kg m−2, median 22 kg m−2) were included in the study. All subjects were extensive metabolizers with respect to CYP2D6 as determined by phenotyping with sparteine. (Only 15 subjects were tested in the present study, because three subjects were known to be extensive metabolizers from previous screening). Urine was collected for 12 h after oral intake of 100 mg sparteine and urinary sparteine/2-, 5-dehydro sparteine ratios were used as estimates of CYP2D6 activity [4]. The mean metabolic ratio was 0.40 (s.d. 0.20, range 0.14–0.72). The study protocol was approved by the Ethics Committee of the Bavarian Medical Association, Munich, Germany, and written informed consent was obtained from all subjects.
Protocol
The study was performed using an open blockwise randomized crossover design with a washout of 1 week. All volunteers received one capsule containing 5.64 mg tropisetron HCl (5.0 mg tropisetron base) orally with 200 ml water or an intravenous bolus injection (30 s) of 2 ml tropisetron HCl (2.0 mg tropisetron base, Novartis Pharma, Nuremberg, Germany) on an empty stomach. Two hours later, a low-fat breakfast was served, with lunch at 4.5 h, a snack 7 h, and dinner 10 h after drug administration. Smoking, alcoholic or caffeine containing beverages were not allowed from 12 h before, and up to 24 h after drug administration. No other medications, besides oral contraceptives, were permitted during the course of the study.
Blood sampling schedule and tropisetron analysis
Venous blood samples were drawn into heparinized tubes before and (5, 10 min, i.v. only) 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 9, 12, 24, 34, 48 h after the dose. Plasma was obtained by centrifugation (10 min, 2300 g) and stored at −70 ° C until assay. Tropisetron was determined by high-performance liquid chromatography (h.p.l.c.) with fluorimetric detection at excitation/emission wavelengths of 283/340 nm. Tropisetron was extracted from alkalinized plasma into tert.-butylmethyl ether [5] and separated on a Spherisorb CN 5 µm column (i.d. 250 × 4 mm, Waters, Eschborn, Germany) with 50 mm ammonium acetate-acetonitrile-methanol (20 : 70 : 10, v/v/v, apparent pH 6.9) as eluent. Tropisetron and the internal standard granisetron (Bristol-Myers-Squibb, Munich, Germany) eluted after 9.5 and 10.5 min, respectively (flow rate 1 ml min−1, column temperature 30 ° C). The limit of quantification was 75 pg ml−1 tropisetron using 1.5 ml plasma. The method was validated over 0.075–25 ng ml−1. Intra- and interassay precision and accuracy were lower than 6% determined with spiked plasma samples over the range of 0.33–16.7 ng ml−1.
Pharmacokinetic analysis
Pharmacokinetic parameters were determined by noncompartmental analysis using the kinetic program TOPFIT 2.0 [6]. Maximum plasma concentration (Cmax) and time of Cmax (tmax) were noted directly after oral administration. To calculate initial peak concentration (Cmax) and initial distribution half-life after intravenous injection, extrapolation by log-linear regression of the first two data points to the time of administration was done. The elimination constant λz was calculated by log-linear regression in the terminal elimination phase, after intravenous injection typically from 1 to 2 h and after oral administration from 4 to 6 h onwards. The terminal half-life was calculated according to t1/2 = ln 2/λz. The area under the plasma concentration-time curve to the last quantifiable concentration (AUC(0,tlast)) and the area under the first moment curve (AUMC) for calculation of the mean residence time (MRT=AUMC/AUC) were calculated by the linear trapezoidal rule. The last measurable concentration (Clast) was used for extrapolation to infinity to determine AUC0–∞ through expression AUC(0,tlast)+Clast/λz. From these parameters total clearance (CL = D/AUC(0,∞)) and steady state distribution volume (Vss = MRT CL) were calculated.
Results
Plasma concentrations and pharmacokinetic parameters
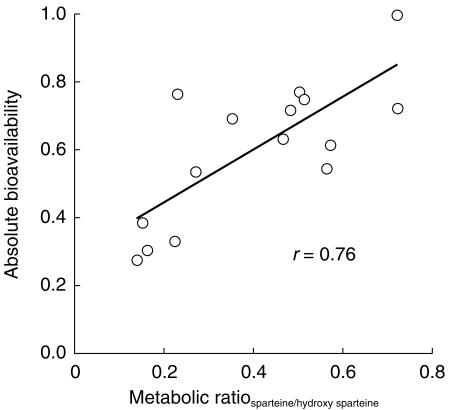
After intravenous injection the plasma concentrations of tropisetron fell rapidly with an initial distribution half-life of about 6 min. The terminal half-life of tropisetron was approximately 6 h after intravenous and oral administration. The mean dose corrected absolute bioavailability of oral tropisetron was 0.60 (range 0.27–0.99, Table 1). The individual bioavailability was correlated with the sparteine metabolic ratio (Pearson r = 0.76, P < 0.001, Figure 1).
Table 1.
Mean (s.d.) pharmacokinetic parameters of tropisetron in 18 healthy volunteers following oral administration of a capsule containing 5 mg tropisetron and intravenous bolus injection of 2 mg tropisetron, respectively.
| Parameter | Ampoule | Capsule | 95% CI on the difference | P value |
|---|---|---|---|---|
| Dose (mg) | 2 | 5 | ||
| Cmax (ng ml−1) | 15.1 (7.3)(1) | 3.46 (1.35) | 8.05, 15.4 | < 0.001 |
| tmax (h) | NA | 2.6 (1.0) | ||
| t1/2 (h) | 5.6 (1.7)(2) | 5.7 (1.9) | −0.29, 0.53 | 0.54 |
| AUC(0,∞) (ng ml−1 h) | 20.7 (7.5) | 32.9 (18.8) | 6.09, 18.4 | < 0.001 |
| Vss (l) | 678 (106) | NA | ||
| CL (ml min−1) | 1800 (579) | NA | ||
| F | 1.00 | 0.60 (0.20) |
1) Obtained by log-linear regression of the first two data points to the time of administration. The concentrations were 7.42 (1.82) ng ml−1 at the first sampling time after 5 min. 2) Initial distribution half-life 5.9 (2.2) min.
Cmax maximum concentration, tmax time of Cmax, t1/2 terminal plasma-half life, AUC(0,∞) area under the plasma concentration-time curve from zero to infinity, Vss steady state volume of distribution, CL total body clearance, NA not applicable, F bioavailability, CI confidence interval on the differences.
Figure 1.
Correlation between absolute bioavailability of oral tropisetron 5 mg and metabolic ratio of sparteine/2-, 5-dehydro sparteine in a 0–12 h urine sample. The data refer to 15 of 18 subjects, because the sparteine test was not performed in three subjects who were known to be extensive metabolizers from previous work.
Discussion
The present study showed that the plasma concentrations of tropisetron 5 min after intravenous bolus injection of 2 mg drug were more than twofold higher and may have been more than fourfold higher immediately after the end of the bolus injection, compared with peak concentrations after administration of the 5 mg capsule (3.5 ng ml−1 after 2.6 h). However, following oral administration all concentrations were higher after 1 h, and the AUC was 50% higher compared with the intravenous route. The consequence of these differences to the pharmacological effects of tropisetron is presently under investigation in clinical trials of patients with fibromyalgia.
Dose corrected mean peak plasma concentrations and AUCs of tropisetron were substantially lower and volume of distribution and total clearance were higher in our study compared with previously published data [1]. However, the latter were performed at higher doses of tropisetron, and the results may not be comparable, because of nonlinear pharmacokinetics. Assuming linear pharmacokinetics at the low doses administered in the present study, the mean absolute bioavailability of oral tropisetron 5 mg was 0.6. However, the individual absolute bioavailability ranged from 0.3 to nearly 1.0 in our subjects. In addition, absolute bioavailability was inversely correlated with CYP2D6 activity.
We conclude that bioavailability of oral tropisetron exhibits a wide range at therapeutic doses. It is determined mainly by CYP2D6 activity, which may influence the magnitude of clinical response to tropisetron therapy.
Acknowledgments
This study was supported by Novartis Pharma, Nuremberg, Germany. K. Mörike and phenotyping with sparteine was supported by Robert Bosch Foundation, Stuttgart, Germany.
References
- 1.Simpson K, Spencer CM, McClellan KJ. Tropisetron. An update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2000;59:1297–1315. doi: 10.2165/00003495-200059060-00008. [DOI] [PubMed] [Google Scholar]
- 2.De Bruijn KM. Tropisetron. A review of the clinical experience. Drugs. 1992;43(Suppl 3):11–22. doi: 10.2165/00003495-199200433-00005. [DOI] [PubMed] [Google Scholar]
- 3.Färber L, Stratz T, Brückle W, et al. Efficacy and tolerability of tropisetron in primary fibromyalgia – a highly selective and competitive 5-HT3 receptor antagonist. Scand J Rheumatol. 2000;29(Suppl 113):49–55. doi: 10.1080/030097400446643. [DOI] [PubMed] [Google Scholar]
- 4.Eichelbaum M, Reetz KP, Schmidt EK, Zekorn C. The genetic polymorphism of sparteine metabolism. Xenobiotica. 1986;16:465–481. doi: 10.3109/00498258609050252. [DOI] [PubMed] [Google Scholar]
- 5.Kees F, Bucher M, Mair G, Grobecker H. Determination of opipramol in human plasma by high-performance liquid chromatography with photometric detection using a cyanopropyl column. J Chromatogr Biomed Appl. 2001;753:237–243. doi: 10.1016/s0378-4347(00)00584-3. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel G, Woloszczak R, Thomann P. TopFit, Version 2.0. Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart: G. Fischer; 1993. [Google Scholar]