Abstract
Aims
To describe the population pharmacokinetics of tafenoquine in healthy volunteers after receiving tafenoquine for malaria prophylaxis.
Methods
The population consisted of 135 male Thai soldiers (mean age 28.9 years; weight 60.3 kg). All soldiers were presumptively treated with artesunate for 3 days plus doxycycline for 7 days to remove any pre-existing malaria infections. After the treatment regime, 104 soldiers (drug group) received a loading dose of 400 mg tafenoquine base daily for 3 days followed by 400 mg tafenoquine monthly for 5 consecutive months. In the placebo group, 31 soldiers were infected with malaria during the study period. They were re-treated with artesunate for 3 days plus doxycycline for 7 days followed by a loading dose of 400 mg tafenoquine daily for 3 days and then 400 mg tafenoquine weekly for prophylaxis. Blood samples were randomly collected from each soldier on monthly and weekly prophylaxis. Plasma tafenoquine concentrations were measured by h.p.l.c. Population pharmacokinetic modelling was performed using NONMEM.
Results
A one-compartment model was found best to describe the pharmacokinetics of tafenoquine after oral administration. Age and weight influenced volume of distribution (V/F), and subjects who contracted malaria had higher clearance (CL/F), but none of these factors was considered to have sufficient impact to warrant change in dosing. The population estimates of the first-order absorption rate constant (Ka), CL/F and V/F were 0.694 h−1, 3.20 l h−1 and 1820 l, respectively. The intersubject variability in these parameters (coefficient of variation, CV%) was 61.2%, 25.3% and 14.8%, respectively. The absorption and elimination half-lives were 1.0 h and 16.4 days, respectively. The residual (unexplained) variability was 17.9%.
Conclusions
The population pharmacokinetics of orally administered tafenoquine have been determined in Thai soldiers under field conditions. This information, together with its known potent antimalarial activity, portends well for the application of tafenoquine as a useful prophylactic drug or for short-term radical treatment of vivax malaria.
Keywords: malaria, NONMEM, population pharmacokinetics, tafenoquine
Introduction
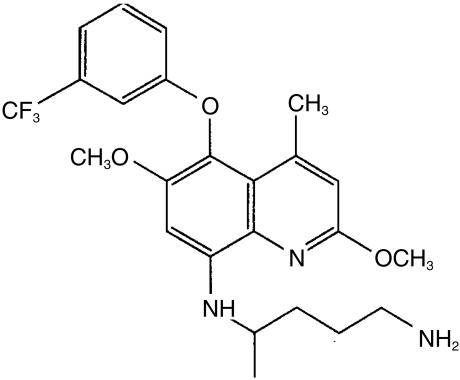
Malaria is a major health problem in many developing or third world countries, with more than 300 million cases and 1–2 million deaths estimated annually [1, 2]. Resistance of malarial parasites to drugs, particularly the quinoline derivatives such as chloroquine and quinine, is increasing at an alarming rate in many regions. The limited options available for the prophylaxis and treatment of malaria necessitate the development of new antimalarial drugs. Tafenoquine (8-[(4-amino-1-methylbutyl) amino]-2,6-dimethoxy-4-methyl-5-(3-trifluoromethyl-phenoxy) quinoline, succinate), also known as WR238 605 (Figure 1) is a new 8-aminoquinoline antimalarial drug being developed by the Walter Reed Army Institute of Research and GlaxoSmithKline Pharmaceuticals as a potential replacement for the tissue schizontocide, primaquine, and as a prophylactic agent. The evolution of tafenoquine has been reviewed recently [3].
Figure 1.
Structure of tafenoquine.
Primaquine is the only drug currently available for the radical cure of Plasmodium vivax and P. ovale malaria. It is active against the secondary exoerythrocytic schizongy tissue stage of both species [4]. However, primaquine has a low therapeutic index and is associated with serious side-effects such as haemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals [4]. Furthermore, gastrointestinal adverse effects such as abdominal pain, nausea and diarrhoea are not uncommon, and leukopaenia and methaemoglobinaemia also can occur. Another limitation of primaquine is its short half-life of 4–6 h, which necessitates daily administration to maintain target concentrations in blood. For the radical cure of vivax malaria, daily administration of 15–22.5 mg primaquine over 14 days is used to prevent relapses.
Tafenoquine was synthesized in response to the deficiencies of primaquine. It possesses greater activity against both the blood and liver stages of malaria than primaquine [5–7]. As a radical curative and causal prophylactic drug, tafenoquine was 7- to 10-fold more active than primaquine in the rhesus monkey malaria model [8, 9]. In vitro studies have further shown tafenoquine to be at least 5-fold more active than primaquine against multidrug-resistant asexual blood stages of P. falciparum [10]. In human studies tafenoquine has been reported to be well tolerated with only mild, transient gastrointestinal effects [5].
A challenge study in which four volunteers were given sporozoites of P. falciparum showed that tafenoquine fully protected three of the subjects and significantly impeded the onset of parasitaemia in the fourth [11]. The prophylactic value of tafenoquine was demonstrated in semi-immune black adult Kenyans against P. falciparum in an holoendemic area [12]. After being treated presumptively with halofantrine (250 mg daily for 3 days), the Kenyans received either of two tafenoquine regimens: 200 mg for 3 days followed by 200 mg weekly or 400 mg for 3 days followed by 400 mg weekly. After 13 weeks of medication the protective effectiveness for weekly 200 mg and 400 mg was 88% and 90%, respectively. Recently, short-term courses of tafenoquine were shown to be effective and well tolerated for radical cure of P. vivax in Thai subjects [13].
Except for a small phase I study in which a single dose was given to fasting subjects [5], there are no published data on the pharmacokinetics of tafenoquine in humans. In contrast to primaquine, the plasma elimination half-life of tafenoquine was reported to be about 14 days which, when coupled with its greater potency, provides distinct advantages for malaria prophylaxis and short-term treatment of vivax malaria. Here we report the results of a population pharmacokinetic study of tafenoquine in Thai soldiers receiving the drug in a field setting as part of a phase II malaria prophylaxis trial.
Methods
Study population, design and dosing
The subjects were male Thai soldiers deployed on security operations along the Thai-Cambodian border in the district of Nam Yun, Ubol Ratchatani province. The data were collected prospectively from April 1998 to October 1998. The volunteers were participating in a larger phase II clinical trial to determine the effectiveness and safety of tafenoquine for malaria prophylaxis, the findings of which will be published elsewhere. The volunteers, who were G6PD normal, were judged healthy based on physical examination, and normal biochemical and haematological indices.
One hundred and thirty-five Thai soldiers participated in the population kinetic study. All were treated presumptively with artesunate (300 mg on day 1, 120 mg daily on days 2 and 3) plus 200 mg daily doxycycline for 7 days to remove any pre-existing malaria infections. After the treatment regime, 104 soldiers (drug group) received a loading dose of 400 mg tafenoquine base daily for 3 days followed by 400 mg tafenoquine monthly for 5 consecutive months. In the placebo group, 31 soldiers contracted malaria during the study period. They were re-treated with the same artesunate/doxycycline regime as outlined above followed by a loading dose of 400 mg tafenoquine base daily for 3 days and then 400 mg tafenoquine weekly for prophylaxis. The characteristics of the 135 study volunteers are shown in Table 1. Tafenoquine, as the succinate salt, was provided by GlaxoSmithKline (United Kingdom) in unmarked gelatin capsules (250 mg salt=200 mg base), and was taken by mouth with water (80–100 ml) and food (cake and biscuits).
Table 1.
Characteristics of study subjects administered tafenoquine.
| Characteristic | Value |
|---|---|
| Number of subjects | |
| Prophylaxis (monthly) | 104 |
| Prophylaxis (weekly) | 31 |
| Age (years)* | 28.9 (21–46) |
| Weight (kg)* | 60.3 (45–90) |
Mean (range).
Ethics
The study was approved by the Royal Thai Army Scientific and Ethical Review Boards, the US Army Scientific and Human Use Review and Regulatory Affairs Division, and the Australian Defence Medical Ethics Committee. The protocol was conducted in accordance with the principles of Good Clinical Practices and prior written, informed consent was obtained from each volunteer before they participated.
Blood sampling
During the 7 month study period (6 months chemosuppression, 1 month follow-up) blood samples were collected randomly in the field from the volunteers. For the 104 soldiers on monthly tafenoquine prophylaxis, blood samples were collected after commencing the loading dose at about 8 h, 24 h, 48 h and 56 h and then at 3–4 day intervals up until the first monthly dose. After each monthly dose, samples were collected at about 8 h post-dose, mid-month and at the end of the monthly dose (trough plasma drug concentration). Following the last monthly dose samples were collected at about 4 h, 8 h, 12 h and 24 h and then at 3–4 day intervals for 2 months. At each blood collection, samples were obtained from 2 to 28 volunteers (mean±s.d., 12.6 ± 7.1). For the 31 soldiers on weekly prophylaxis, blood samples were collected after 2–22 weeks of medication (mean 11.8 ± 6.8). Samples from this group were collected at about 12 h and 168 h post weekly medication and at 14 days, 21 days and 28 days after the last dose.
Blood samples (7 ml) were drawn by venepuncture into EDTA tubes, and transported on ice to the field laboratory within 1 h of collection. Whole blood samples were centrifuged at ∼1200 g for 15 min, the plasma was separated and then stored in dry-ice for up to 1 week pending transport to the Armed Forces Research Institute of Medical Sciences (Bangkok, Thailand) for storage at −70 ° C. The samples were then collectively air freighted on dry-ice to the Australian Army Malaria Institute (Brisbane, Australia) for storage at −70 ° C.
Drug analysis
Tafenoquine concentrations in plasma were measured by reversed-phase high-performance liquid chromatography (h.p.l.c.), with fluorescence detection, as described previously [14]. Briefly, plasma (0.2 ml) was treated with acetonitrile (0.2 ml) and 0.2 m zinc sulphate (0.05 ml) and then centrifuged. A portion (25 µl) of the supernatant fluid was injected onto an S5P Spherisorb phenyl analytical cartridge column (150 mm × 4.6 mm i.d., 5 µm particle size, Waters Australia Ltd, Sydney, Australia) through which was pumped a mobile phase comprising 450 ml of ammonium acetate (pH 4.0, 0.22 mm) and 550 ml of acetonitrile at 1.5 ml min−1. The retention times were 3.5 min for WR VIIIAc (the internal standard) and 7.8 min for tafenoquine. The interday and intraday coefficients of variation (CV) (assay range 20–1000 ng ml−1) were ≤ 8.4% in plasma. Over this range, the mean inaccuracy was 7.3% in plasma and the mean recovery of tafenoquine was 81%. The lower limit of quantification was 10 ng ml−1. The interday CV was 10.8%.
Population modelling
The population pharmacokinetics of tafenoquine were estimated using NONMEM (version 5, level 1.1) [15] on a Compaq Presario 4810, in conjunction with the Microsoft PowerStation Fortran 77 compiler (version 1.00). Using the first-order (FO) approximation, a one-compartment model with first-order absorption and elimination was fitted to the data.
An initial analysis was conducted by permitting NONMEM to estimate the base model parameters (i.e. no covariates). The influence of mean-centred AGE and WT data of the subjects was assessed by adding these to the base model, in turn, and noting the change on the objective function value (OFV). The distributions of weighted residuals and observed plasma tafenoquine concentrations were plotted separately against the population model-predicted concentrations. The inclusion of a covariate improved the fit of the data to the model if there was a decrease in the OFV. Differences between the OFVs when a covariate was included (‘full model’) then excluded (‘reduced model’) were tested for significance at the P = 0.01 level using the chi-squared statistic (χ21,0.01 = 6.6) with 1 degree of freedom [15].
For the intersubject variance model, deviations of tafenoquine clearance (CL/Fj), apparent volume of distribution (V/Fj), and the absorption rate constant (Kaj) of the jth individual from the true (but unknown) typical population values were modelled logarithmically as [15, 16]
where ηj,CL/F and ηj,V/F and ηj,Ka are random variables distributed with zero means and respective variances of ω2CL/F, ω2V/F, ω2Ka and CL/F, V/F, Ka are the population typical values. The residual variability between the observed plasma tafenoquine concentrations and those predicted by the final population model was estimated using an exponential error model
where Cij is the ith observed concentration for the jth individual, Cpred,ij is the plasma tafenoquine concentration predicted by the pharmacokinetic model, and ∈ij is a randomly distributed variable with zero mean and variance of σ2 [15, 16]. These differences (∈ij) are attributed to variability arizing from inaccurate timing of blood collections and doses, variability in the drug assay, and the use of an inappropriate pharmacokinetic model (e.g. one-compartment model instead of a two-compartment model).
Results
The population analysis was conducted using combined plasma data from subjects who received monthly tafenoquine for prophylaxis (n = 104), and from those who contracted malaria and who subsequently received weekly tafenoquine for prophylaxis (n = 31). Summary results of the population model-building are shown in Table 2. Upon individual screening, neither WT (centred by average WT of 60.3 kg, model number 2), nor AGE (centred by average AGE of 28.9 years, model number 3) had any significant influence on CL/F. In contrast, centred WT (model number 4) and centred AGE (model number 5) significantly increased V/F as seen by reductions in the OFV of 12 and 8, respectively (P < 0.01). However, the OFV was reduced by 58 when the presence of malaria on CL/F was added (model number 8). Modelling the combined influence of malaria on CL/F, together with AGE and WT on V/F (model number 8) gave a reduction in the OFV of 83, compared with model number 1. The data did not support the inclusion of an absorption lag-time in any model.
Table 2.
Development of structural model.
| Number/Model | OFVa |
|---|---|
| Forwards stepwise addition | |
| 1 CL/F = θ1; V/F = θ2; Ka = θ3 | |
| 2 CL/F = θ1. (WT-60.3) + θ2; V/F = θ3; Ka = θ4 | −1b |
| 3 CL/F = θ1. (AGE-28.9) + θ2; V/F = θ3; Ka = θ4 | −1b |
| 4 CL/F = θ1; V/F = θ2. (WT-60.3) + θ3; Ka = θ4 | −12b* |
| 5 CL/F = θ1; V/F = θ2. (AGE-28.9) + θ3; Ka = θ4 | −8b* |
| 6 CL/F = θ1. MAL + θ2. (1-MAL); V/F = θ3; Ka = θ4 | −58b |
| 7 CL/F = θ1; V/F = θ2. (WT-60.3) + θ3. (AGE-28.9) + θ4; Ka = θ5 | −18b* |
| 8 CL/F = θ1. MAL + θ2. (1-MAL); V/F = θ3. (WT-60.3) + θ4. (AGE-28.9) + θ5; Ka = θ6 | −83b |
| Backwards stepwise elimination | |
| 9 CL/F = θ1; V/F = θ2 + θ3. (WT-60.3) + θ4. (AGE-28.9); Ka = θ5 | + 53c |
| 10 CL/F = θ1. MAL + θ2. (1-MAL); V/F = θ3 + θ4. (AGE-28.9); Ka = θ5 | + 11c |
| 11 CL/F = θ1. MAL + θ2. (1-MAL); V/F = θ3 + θ4. (WT-60.3); Ka = θ5 | + 2c |
Objective function value
Change in OFV from model no. 1 (OFV = −4556)
Change in OFV from model no. 8 (OFV = −4627).
CL/F: clearance (l h−1), V/F: volume of distribution (l), Ka: absorption rate constant (h−1), (WT-60.3): centred body weight (kg), (AGE-28.9): centred age (years), MAL: presence (1) absence (0) of malaria.
P < 0.01 (full model vs reduced model).
Backwards stepwise elimination indicated that the influence of AGE on V/F could be removed from model number 8, as seen by an increase in the OFV of only 2 (model number 11) when the coefficient for AGE was set to 0. The omission, in turn, of MAL on CL/F (model number 9) or WT on V/F (model number 10) produced increases in the OFV of 53 and 11, respectively. However, the typical population CL/F values for nonaffected and malaria-affected subjects were similar at 3.1 h−1 and 4.0 l h−1, respectively. The typical V/F values for subjects of 45 kg and 90 kg (the extremes of the WT range) were 1633 l and 1947 l, respectively. Accordingly, in view of the relatively small changes in the pharmacokinetic parameter values, the base model (model no. 1) was deemed to be adequate.
A further run was made using model no. 1 in which the off-diagonal element of the Omega matrix, i.e the covariance (covCL/F,V/F), was estimated via the $OMEGA BLOCK(2) function, the underlying premise being that CL/F and V/F may be considered to be correlated in individual subjects. This resulted in a reduction in the OFV from −4556 to −4601. The coefficient of correlation (r) between CL/F and V/F was calculated to be 0.71, from the expression, r=(covCL/F,V/F)(ω2CL/F · ω2V/F)0.5. The structural and variance parameter values and the derived parameters of model no.1 are presented in Table 3. The elimination half-life was 16.4 days, derived from the expression, t½ = (0.693 · V/F)) (CL/F). The typical Ka value of 0.694 h−1 was used to calculate a first-order absorption half-life (tabs) of 1.0 h by the expression, tabs=0.693 ÷ Ka, while the time to reach peak plasma tafenoquine concentration was 8.62 h, calculated from tmax=[1.0 ÷ (Ka−K)] × ln(Ka ÷ K).
Table 3.
Parameter values for population model number 1.
| Parameter | Meaning | Value (CV%)* |
|---|---|---|
| Structural parameters | ||
| θ1 | Clearance (l h−1) | 3.20 (2.7) |
| θ2 | Volume of distribution (l) | 1820 (1.7) |
| θ3 | First-order absorption rate constant (h−1) | 0.694 (12.8) |
| Derived parameters | ||
| t½ | Elimination half-life (days) | 16.4 |
| tabs | Absorption half-life (h) | 1.0 |
| tmax | Time to reach peak concentration (h) | 8.6 |
| Variance parameters | ||
| ωCL/F | Intersubject variability in CL/F | 25.3† (14.0) |
| ωV/F | Intersubject variability in V/F | 14.8† (21.1) |
| covV/F, CL/F | Covariance between CL/F and V/F | 0.0265 (21.5) |
| ωKa | Intersubject variability in Ka | 61.2† (167) |
| σ | Residual variability | 17.9† (8.60) |
Imprecision of estimation (CV%=asymptotic standard error) parameter value. 100%).
Variability expressed as the coefficient of variation (CV%).
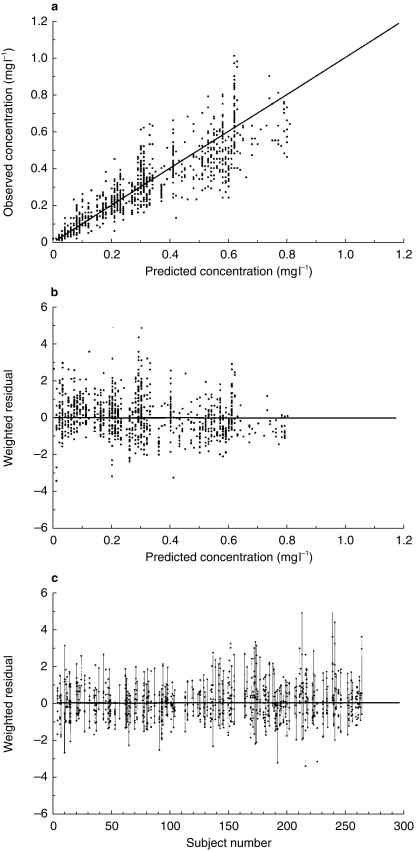
The intersubject variability in the pharmacokinetic parameters and the residual variability, expressed as CV%, is shown in Table 3. Individual plasma concentration time plots for subjects receiving either monthly or weekly tafenoquine are shown in Figure 2a and 2b, respectively. The observed vs model-predicted concentrations are presented in Figure 3a. Weighted residuals vs model-predicted values (Figure 3b) showed points that are symmetrically distributed about the null ordinate indicating a good fit of the model to the data. Similarly, points in the plot of weighted residual vs subject number (Figure 3c) were distributed symmetrically in a band which mostly were within ∼3 units of the null ordinate, indicating that data from any individual did not contribute to marked deviation from the model.
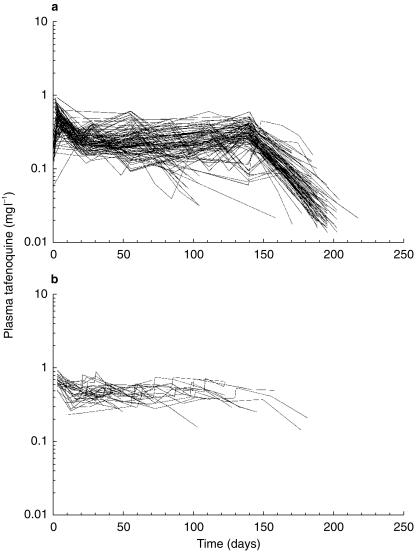
Figure 2.
Plasma tafenoquine concentration vs time plots for individual subjects. a) monthly tafenoquine dosing (n = 104 subjects) and b) weekly tafenoquine dosing (n = 31 subjects).
Figure 3.
Plots of a) observed plasma tafenoquine concentration vs model-predicted tafenoquine concentration, b) weighted residual vs model-predicted tafenoquine concentration and c) weighted residual vs subject number.
Discussion
This is the first report describing the population pharmacokinetics of tafenoquine, the data being obtained from a Phase II clinical trial in Thai soldiers following either monthly or weekly prophylaxis. A one-compartment model with first-order absorption and elimination was found to best characterise the plasma tafenoquine concentration data.
In developing a population model it is useful to determine any relationship between the pharmacokinetic parameters and other factors (i.e. covariates) which can influence the kinetic response. When screened individually, age and weight influenced V/F, but not CL/F. Both factors appeared to act independently on volume since there was very low correlation (r = 0.105) between age and weight. However, age was removed from the model on statistical grounds during stepwise, backwards elimination of covariates. While there was a two-fold range in weight values in the population, the difference between the typical values of V/F at the extremes of weight would not require alteration in loading dose and dosing interval. The large V/F was similar to that reported previously in Caucasians, African-Americans and Hispanics [5], and far exceeded total body water (0.6 l kg−1) indicating extensive tissue binding of tafenoquine, which is known for other aminoquinolines [17]. Unlike primaquine, tafenoquine also accumulates in red blood cells, with a whole blood to plasma concentration ratio of ∼1.8 [5]. Such accumulation may contribute to the greater potency of tafenoquine compared with primaquine. The 16-day elimination half-life compared favourably with those calculated in two previous studies [5, 11], which used either compartmental or noncompartmental analysis.
Subjects who had contracted malaria had an average 28% increase in CL/F, compared with the non-malaria subjects. Both groups had been treated with the same regime of artesunate/doxycycline to eliminate parasitaemia but there are no data published to suggest that eiher drug affects the elimination of tafenoquine. The nature of malaria, particularly the variations in the intensity and duration and the clinical stability of the patient, makes pharmacokinetic comparisons difficult. However, pathophysiological changes associated with malaria have been implicated previously in altering the pharmacokinetics of several other antimalarial drugs [17]. The clearance of mefloquine was reported to be greater in Thai patients with uncomplicated falciparum malaria than in healthy Thai volunteers [18]. On the other hand, the clearance of quinine was decreased and the volume of distribution increased (with little change in elimination half-life) in patients with acute falciparum malaria, compared with those determined during the convalescence period [19]. While the difference in clearance between the two groups remains unexplained it is unlikely to have a major clinical impact especially with the low toxicity of tafenoquine [20]. Accordingly, we adopted the parsimonious approach and resorted to a single population clearance in the model.
The only other published data [5] indicated that peak plasma concentrations of ∼260 ng ml−1 were achieved following a single oral dose (600 mg base) in healthy Caucasian, African-Americans and Hispanics, whereas in the present study similar peak concentrations to this were achieved following the first loading dose of 400 mg. Among other possible factors, this difference may be due to the influence of food on tafenoquine absorption. In contrast with a previous protocol [5], food was provided to soldiers with each dose to minimize possible adverse effects, particularly gastrointestinal disturbances. It is the extent of absorption, F, which is likely to be most affected by food rather than the rate, since the average first-order absorption half-lives from our study and the previous one [5] were less than 100 min. Indeed, further (unpublished) data from those authors [5] indicated that coadministration of tafenoquine with a meal increased oral bioavailability by up to 30% (R.P. Brueckner, personal communication). Moreover, tafenoquine is a promising long-term prophylactic drug and variability in the rate of absorption probably has no major clinical impact other than possible transient adverse effects, perhaps in more rapid absorbers. The intersubject variability in Ka was high, but similar to what has been reported previously for many other orally administered antimalarial drugs [17]. In contrast, there was much less intersubject variability in CL/F and V/F which is not surprising in view of the homogeneous population studied. Modelling the covariance accounted for factors which influenced both CL/F and V/F, one of which would be the oral bioavailability, F, although there are likely to be others.
In conclusion, the population pharmacokinetics of tafenoquine in Thai soldiers have been derived from sparse, oral data obtained in a field setting. Pending the availability of more pharmacokinetic data in other groups, we are cautious about extrapolating these findings to the wider population. Nonetheless, the safety and patient acceptability of tafenoquine, its wide spectrum of antimalarial activity against all stages of the parasite, and its extended half-life provide clinicians with a potentially convenient and useful prophylactic agent against this disease. Phase III trials are currently being planned to evaluate the effectiveness and tolerability of tafenoquine for long-term prophylaxis.
Acknowledgments
We thank Lieutenant Pradith Khaewsathien and Sergeant Naruepon Kutasingdee for their technical excellence in the collection of blood samples. Drug analysis was funded and supported by the Director-General Defence Health Service (Australia).
References
- 1.World Health Organization. Practical chemotherapy of malaria. World Health Organ Tech Rep Series. 1990;805:1–141. [PubMed] [Google Scholar]
- 2.Anonymous. Towards a malaria vaccine [editorial] Lancet. 1992;339:586–587. [PubMed] [Google Scholar]
- 3.Peters W. The evolution of tafenoquine-antimalarial for a new millennium? J Roy Soc Med. 1999;92:345–352. doi: 10.1177/014107689909200705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clyde DF. Clinical problems associated with the use of primaquine as a tissue schizonticidal and gametocytocidal drug. Bull WHO. 1981;59:391–395. [PMC free article] [PubMed] [Google Scholar]
- 5.Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238 605, a new antimalarial. Am J Trop Med Hyg. 1998;58:645–649. doi: 10.4269/ajtmh.1998.58.645. [DOI] [PubMed] [Google Scholar]
- 6.Peters W, Robinson BL, Milhous WK. The chemotherapy of rodent malaria. LI. Studies on a new 8-aminoquinoline, WR 238 605. Ann Trop Med Parasitol. 1993;87:547–552. doi: 10.1080/00034983.1993.11812809. [DOI] [PubMed] [Google Scholar]
- 7.Milhous WK, Brueckner RP, Theoharides AD, Schuster BG. Program and Abstracts of the 31st Interscience Conference on Antimcrobial Agents and Chemotherapy. Washington, DC, USA: American Society of Microbiology; 1992. Preclinical efficacy of WR 238 605. Abstract 376. [Google Scholar]
- 8.Milhous WK, Theoharides AD, Schuster BG, et al. Program and Abstracts of the Xiith International Congress for Tropical Medicine and Malaria. Amsterdam, The Netherlands: 1988. New alternatives to primaquine. Abstract FrS-12–4. [Google Scholar]
- 9.Heisy GE, Milhous WK, Hansuklarita P, Theoharides AD, Schuster BG, Davidson DE. Program and Abstracts of the American Society of Tropical Medicine and Hygiene. Washington, DC, USA: 1988. Radical curative properties of WR 238 605. Abstract 323. [Google Scholar]
- 10.Kyle DE. Symposium on Etaquine Held in Association with the 46th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Florida, USA: 1997. In vitro antimalarial activity of etaquine (WR 238605) pp. 7–11. December. [Google Scholar]
- 11.Brueckner RP, Coster T, Wesche DL, Shmuklarsky M, Schuster BG. Prophylaxis of Plasmodium falciparum infection in a human challenge model with WR 238 605, a new 8-aminoquinoline antimalarial. Antimicrob Agents Chemother. 1998;42:1293–1294. doi: 10.1128/aac.42.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanks D. Symposium on Etaquine Held in Association with the 46th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Florida, USA: 1997. Etaquine (WR 238605) for the prophylaxis of Plasmodium falciparum malaria; pp. 7–11. December. [Google Scholar]
- 13.Walsh DS, Looareesuwan S, Wilairatana P, et al. Randomized dose-ranging study of the safety and efficacy of WR 238 605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in Thailand. J Infect Dis. 1999;180:1282–1287. doi: 10.1086/315034. [DOI] [PubMed] [Google Scholar]
- 14.Kocisko DA, Walsh DS, Eamsila C, Edstein MD. Measurement of tafenoquine (WR 238 605) in human plasma, and venous and capillary blood by high-pressure liquid chromatography. Ther Drug Monit. 2000;22:184–189. doi: 10.1097/00007691-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Boeckmann A, Sheiner L, Beal S. University of California, San Francisco: NONMEM Project Group; NONMEM User's Guide. [Google Scholar]
- 16.Grasela TH, Sheiner LB. Pharmacostatistical modeling for observational data. J Pharmacokinet Biopharm. 1991;19:25S–36S. [Google Scholar]
- 17.White NJ. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokin. 1985;10:187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Karbwang J, Back DJ, Bunnag D, Breckenridge AM. A comparison of the pharmacokinetics of mefloquine in healthy Thai volunteers and in Thai patients with falciparum malaria. Eur J Clin Pharmacol. 1988;35:677–680. doi: 10.1007/BF00637607. [DOI] [PubMed] [Google Scholar]
- 19.White NJ, Looareesuwan S, Warrell DA, Warrell MJ, Bunnag D, Harinasuta T. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med. 1982;73:564–571. doi: 10.1016/0002-9343(82)90337-0. [DOI] [PubMed] [Google Scholar]
- 20.Walsh DS, Sasiprapha T, Sangkharomya S, et al. Program and Abstracts of the 48th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Washington DC, USA,: 1999. Randomized, double-blind, placebo controlled evaluation of monthly WR 238 605 (Tafenoquine) for prophylaxis of Plasmodium falciparum and P. vivax in Royal Thai army soldiers. (abstract no. 845) [Google Scholar]