Abstract
Aims
To examine whether bile acids such as ursodeoxycholic acid (UDCA) and chenodeoxycholic acid (CDCA) can influence the absorption of nitrendipine, a highly lipophilic calcium channel blocker.
Methods
Six healthy subjects received nitrendipine (10 mg) with and without UDCA (50 mg) and CDCA (200 and 600 mg) with an interval of 1∼2 weeks between study phases.
Results
Bile acids decreased the Cmax (ng ml−1) [control 10.9 ± 5.8 (mean± s.d.), UDCA 5.0 ± 4.7 (95% confidence interval for difference; 3.9, 7.8, P = 0.0006), CDCA (600 mg) 5.0 ± 3.9 (2.6, 9.2, P = 0.0059)] and AUC (ng ml−1 h) [(control; 60 ± 36, UDCA 15 ± 13 (20, 73, P = 0.0064), CDCA (600 mg) 19 ± 19 (21, 63, P = 0.0038)] of nitrendipine, while elimination half-life remained unchanged.
Conclusions
These results suggest that the amount of nitrendipine absorbed was decreased when the drug was administered with UDCA and CDCA.
Keywords: absorption, chenodeoxycholic acid, nitrendipine, ursodeoxycholic acid
Introduction
Bile acids such as ursodeoxycholic acid (UDCA) and chenodeoxycholic acid (CDCA) are used routinely for the dissolution of gallstones and were recently indicated for the treatment of chronic cholestatic liver disease [1]. In addition, these agents may cause the formation of micelles with lipophilic drugs and consequently enhance their absorption from the gastrointestinal tract. For example, UDCA and cholic acid, another bile acid, were reported to increase blood concentrations of cyclosporin, a highly lipophilic drug [2, 3].
Calcium blockers of the dihydropyridine group are frequently prescribed for the treatment of hypertension. As these drugs are generally lipophilic, bile acids might enhance their absorption and lead to an exaggerated reduction in blood pressure. To avoid adverse effects in hypertensive patients with gallstones and chronic cholestatic liver diseases, it is important that the potential for drug interaction is examined. In this study, the effects of UDCA and CDCA on the pharmacokinetics of nitrendipine, a highly lipophilic dihydropyridine derivative [4], were examined in healthy subjects.
Methods
Six healthy male subjects [33.8 (19–43) years old] were assigned to the study after giving informed consent. The investigation was an open trial and approved by the Review Board of Jichi Medical School.
Nitrendipine (10 mg tablet, Baylotensin®, Welfide Corporation), UDCA (5% granule, Urso®, Mitsubishi Tokyo Pharmaceuticals) and CDCA (100 mg capsule, Regalen® Eisai Co.) were used in the study. Subjects were administered single oral doses of the following in random order and separated by 1–2 weeks: nitrendipine 10 mg alone, nitrendipine 10 mg and UDCA 50 mg, or nitrendipine 10 mg and CDCA 200 mg.
Contrary to our expectation, we found that plasma nitrendipine concentrations were significantly decreased by UDCA 50 mg and tended to be decreased by CDCA 200 mg. Therefore, we performed an additional study using CDCA 600 mg, the maximum daily dose, in the same six subjects.
After overnight fasting, subjects received the medication with 100 ml water at 08.00 h. They remained in the supine position, and food and beverages were not allowed for at least 4 h after each dose. Blood samples for measurement of nitrendipine were obtained immediately before and 1, 2, 3, 4, 6, 8 and 12 h after dosing. Blood was centrifuged at 4° C, and the plasma was stored at −80° C until analysis. Plasma nitrendipine concentration was measured by gas chromatography with electron capture detection [5]. The detection threshold of this assay was 0.5 ng ml−1. The coefficient of variation was 2.0% at 5 ng ml−1 (n = 12) and 2.8% at 23 ng ml−1 (n = 18).
Maximum plasma concentration (Cmax) and time to maximum concentration (tmax) were noted directly. Area under the plasma concentration-time curve from time 0–12 h (AUC(0-12 h)) was calculated using the linear trapezoidal rule. The terminal elimination rate constant (k) was obtained by least-squares regression. Elimination half-life (t1/2) was calculated as t1/2 = ln 2/λz.
Data are expressed as mean ± s.d. Statistical analysis was performed by analysis of variance (anova) and paired Student's t-test as appropriate.
Results
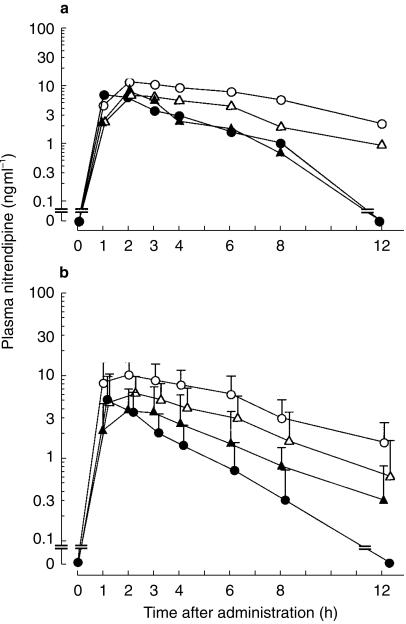
A representative time profile of plasma nitrendipine concentration in one subject is shown in Figure 1a. Plasma nitrendipine concentrations were decreased significantly by UDCA and CDCA administration (P = 0.0069 by anova, Figure 1b) as were Cmax and AUC (0, 12 h) (Table 1). tmax and elimination half-life were not changed significantly by UDCA or CDCA.
Figure 1.
(a) Representative time profile of plasma nitrendipine concentration in one subject.(b) Mean ± s.d. plasma nitrendipine concentration vs time profiles after oral dosing with and without UDCA and CDCA in six healthy subjects. ○ nitrendipine 10 mg, • nitrendipine 10 mg +UDCA 50 mg, ▵ nitrendipine 10 mg +CDCA 200 mg, ▴ nitrendipine 10 mg +CDCA 600 mg.
Table 1.
Influence of UDCA and CDCA on the pharmacokinetics of nitrendipine in healthy subjects (mean ± s.d., (95% confidence interval for the difference), n = 6).
| Treatment | Cmax (ng ml−1) | tmax (h) | AUC(0,12 h) (ng ml−1 h) | t (h) |
|---|---|---|---|---|
| Control | 10.9 ± 5.8 | 1.7 ± 0.5 | 60 ± 36 | 3.7 ± 0.4 |
| UDCA 50 mg | 5.0 ± 4.7 | 1.2 ± 0.4 | 15 ± 13 | 2.7 ± 1.1 |
| (3.9, 7.8, P = 0.0006) | (−0.1, 1.1, P = 0.0756) | (20, 73, P = 0.0064) | (−0.8, 1.8. P = 0.3801) | |
| CDCA 200 mg | 8.7 ± 6.1 | 1.7 ± 0.5 | 49 ± 34 | 3.2 ± 0.9 |
| (0.4, 3.8, P = 0.0230) | (−0.7, 0.7, P = 1) | (−2, 26, P = 0.0850) | (−1.2, 1.1, P = 0.9178) | |
| CDCA 600 mg | 5.0 ± 3.9 | 1.8 ± 0.8 | 19 ± 19 | 2.9 ± 1.1 |
| (2.6, 9.2, P = 0.0059) | (−1.2, 0.9, P = 0.6952) | (21, 63, P = 0.0038) | (−1, 3, P = 0.8849) |
Discussion
Bile acids may increase the gastrointestinal absorption of lipophilic drugs such as cyclosporin, vitamin B12 and tetracycline [2, 3, 6, 7]. The following mechanisms are believed to be involved [8]: (1) enhancement of drug dissolution by micelles formation, (2) alteration of gastrointestinal membrane permeability, and (3) increase in the residence time of the drug at the absorption site. As nitrendipine is highly lipophilic [4], we speculated that its absorption would be enhanced by bile acids. However, contrary to our expectation, Cmax and AUC of nitrendipine were decreased by UDCA and CDCA, but tmax and elimination half-life were unaffected. We suggest that the amount of nitrendipine absorbed was decreased but its rate of absorption was not influenced by UDCA or CDCA.
We have no explanation for these unexpected observations. It is possible that the disintegration or more likely the solubilization, or both, of the nitrendipine tablet used in this study were affected by unknown factors related to the presence of UDCA (Urso®) and CDCA (Regalen®). However, as the presence of different excipients and differing manufacturing processes might result in dosage forms containing the same drug behaving differently in vivo, it is unclear whether a drug interaction between other forms of UDCA and CDCA and nitrendipine might occur.
Previous studies have shown that the S-enantiomer of nitrendipine is 7–8 times more potent than the R-enantiomer [9]. In addition, the bioavailability of the S-enantiomer is greater than that of the R-enantiomer [10, 11]. Therefore, the measurement of plasma concentrations of these enantiomers separately may be relevant to our understanding of the clinical importance of the present drug interaction.
In summary, our studies showed that UDCA (Urso®) and CDCA (Regalen®) decreased the relative bioavailability of nitrendipine, possibly through an effect on the disintegration of the tablet or more likely the solubilization of the drug. Whether such an interaction might diminish the hypotensive effect of nitrendipine remains to be established.
References
- 1.Crosignani A, Stechell KDR, Invernizzi P, et al. Clinical pharmacokinetics of therapeutic bile acids. Clin Pharmacokinet. 1996;30:333–358. doi: 10.2165/00003088-199630050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gutzler F, Zimmermann R, Ring GH, Sauer P, Stiehl A. Ursodeoxycholic acid enhances the absorption of cyclosporine in a heart transplant patient with short bowel syndrome. Transpl Proc. 1992;24:2620–2621. [PubMed] [Google Scholar]
- 3.Lindholm A, Henricsson S, Dahlqvist R. The effect of food and bile acid administration on the realtive bioavalability of cyclosporin. Br J Clin Pharmacol. 1990;29:541–548. doi: 10.1111/j.1365-2125.1990.tb03677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer H, Bossert F, Wehinger E, Scherling D. Chemistry of nitrendipine and its metabolites. In: Scriabine A, Vanov S, Deck K, editors. Nitrendipine. Urban and Schwarzenberg: Baltimore-Munich; 1984. pp. 1–9. [Google Scholar]
- 5.Fujimura A, Ohashi K, Sugimoto K, Kumagai Y, Ebihara A. Chronopharmacological study of nitrendipine in healthy subjects. J Clin Pharmacol. 1989;29:909–915. doi: 10.1002/j.1552-4604.1989.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 6.Teo NH, Scott JM, Neale G, Weir DG. Effect of bile on vitamin B12 absorption. Br Med J. 1980;281:831–833. doi: 10.1136/bmj.281.6244.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aukee S, Venho VMK, Jussila J, Karjalainen PC. Drug absorption in patients with T-tube after cholecystectomy. Ann Clin Res. 1975;7:42–46. 1988. [PubMed] [Google Scholar]
- 8.Mehta MU, Venkataramanan R, Burckart GJ, et al. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988;25:579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltze M, Boer R, Sanders KH, Boss H, Ulrich WR Flockerzi. D. Stereoselective inhibition of thromboxane–induced coronary vasoconstriction by 1,4–dihydropyridine calcium channel antagonists. Chirality. 1990;2:230–240. doi: 10.1002/chir.530020408. [DOI] [PubMed] [Google Scholar]
- 10.Soons PA, Breimer DD. Stereoselective pharmacokinetics of oral and intravenaus nitrendipine in healthy subjects. Br J Clin Pharmacol. 1991;32:11–16. doi: 10.1111/j.1365-2125.1991.tb05606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mast V, Fischer C, Mikus G, Eichelbaum M. Use of pseudoracemic nitrendipine for stereoselective disposition of nitrendipine enantiomers. Br J Clin Pharmacol. 1992;33:51–59. doi: 10.1111/j.1365-2125.1992.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]