Abstract
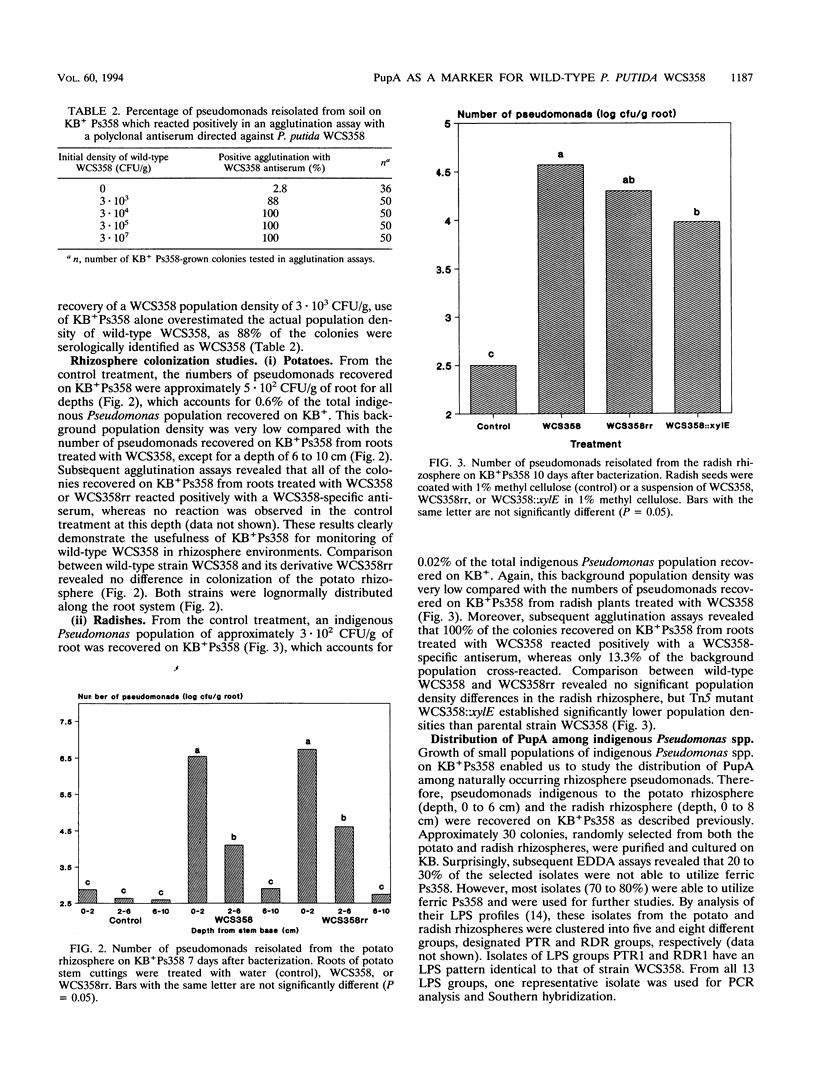
For application of genetically engineered fluorescent Pseudomonas spp., specific markers are required for monitoring of wild-type Pseudomonas strains and their genetically modified derivatives in natural environments. In this study, the specific siderophore receptor PupA of plant growth-promoting Pseudomonas putida WCS358 was used as a marker to monitor wild-type strain WCS358. After introduction into natural soil and rhizosphere environments, strain WCS358 could be recovered efficiently on a medium amended with 300 microM pseudobactin 358. Although low population densisties of indigenous pseudomonads (less than or equal to 10(3)/g of soil or root) were recovered on the pseudobactin 358-amended medium, subsequent agglutination assays with a WCS358-specific polyclonal antiserum enabled accurate monitoring of populations of wild-type strain WCS358 over a range of approximately 10(3) to 10(7) CFU/g of soil or root. Genetic analysis of the background population by PCR and Southern hybridization revealed that natural occurrence of the pupA gene was limited to a very small number of indigenous Pseudomonas spp. which are very closely related to P. putida WCS358. The PupA marker system enabled the study of differences in rhizosphere colonization among wild-type strain WCS358, rifampin-resistant derivative WCS358rr, and Tn5 mutant WCS358::xylE. Chromosomally mediated rifampin resistance did not affect the colonizing ability of P. putida WCS358. However, Tn5 mutant WCS358::xylE colonized the radish rhizosphere significantly less than did its parental strain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Buyer J. S., Leong J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem. 1986 Jan 15;261(2):791–794. [PubMed] [Google Scholar]
- Compeau G., Al-Achi B. J., Platsouka E., Levy S. B. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988 Oct;54(10):2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnadel D., Meyer J. M. Specificity of pyoverdine-mediated iron uptake among fluorescent Pseudomonas strains. J Bacteriol. 1988 Oct;170(10):4865–4873. doi: 10.1128/jb.170.10.4865-4873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte M., Mergeay M., Verstraete W. Marking the rhizopseudomonas strain 7NSK2 with a Mu d(lac) element for ecological studies. Appl Environ Microbiol. 1990 Apr;56(4):1046–1052. doi: 10.1128/aem.56.4.1046-1052.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkevitch E., Hadar Y., Chen Y. Differential siderophore utilization and iron uptake by soil and rhizosphere bacteria. Appl Environ Microbiol. 1992 Jan;58(1):119–124. doi: 10.1128/aem.58.1.119-124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kaniga K., Davison J. Transposon vectors for stable chromosomal integration of cloned genes in rhizosphere bacteria. Gene. 1991 Apr;100:201–205. doi: 10.1016/0378-1119(91)90367-k. [DOI] [PubMed] [Google Scholar]
- Koebnik R., Hantke K., Braun V. The TonB-dependent ferrichrome receptor FcuA of Yersinia enterocolitica: evidence against a strict co-evolution of receptor structure and substrate specificity. Mol Microbiol. 1993 Feb;7(3):383–393. doi: 10.1111/j.1365-2958.1993.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Koster M., van de Vossenberg J., Leong J., Weisbeek P. J. Identification and characterization of the pupB gene encoding an inducible ferric-pseudobactin receptor of Pseudomonas putida WCS358. Mol Microbiol. 1993 May;8(3):591–601. doi: 10.1111/j.1365-2958.1993.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Marugg J. D., de Weger L. A., Nielander H. B., Oorthuizen M., Recourt K., Lugtenberg B., van der Hofstad G. A., Weisbeek P. J. Cloning and characterization of a gene encoding an outer membrane protein required for siderophore-mediated uptake of Fe3+ in Pseudomonas putida WCS358. J Bacteriol. 1989 May;171(5):2819–2826. doi: 10.1128/jb.171.5.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Winstanley C., Morgan J. A., Pickup R. W., Jones J. G., Saunders J. R. Differential regulation of lambda pL and pR promoters by a cI repressor in a broad-host-range thermoregulated plasmid marker system. Appl Environ Microbiol. 1989 Apr;55(4):771–777. doi: 10.1128/aem.55.4.771-777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
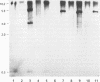
- de Weger L. A., Jann B., Jann K., Lugtenberg B. Lipopolysaccharides of Pseudomonas spp. that stimulate plant growth: composition and use for strain identification. J Bacteriol. 1987 Apr;169(4):1441–1446. doi: 10.1128/jb.169.4.1441-1446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weger L. A., van Boxtel R., van der Burg B., Gruters R. A., Geels F. P., Schippers B., Lugtenberg B. Siderophores and outer membrane proteins of antagonistic, plant-growth-stimulating, root-colonizing Pseudomonas spp. J Bacteriol. 1986 Feb;165(2):585–594. doi: 10.1128/jb.165.2.585-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]