Abstract
There are significant gender differences in human brain disease. For example, females are significantly more likely to suffer from Alzheimer's disease (AD) than men (even after correcting for differences in life expectancy), and females on hormone replacement therapy (HRT) are significantly less likely to suffer from Alzheimer's disease than women who do not take HRT. However the neurobiological basis to these differences in clinical brain disease were unknown until relatively recently. In this review we will discuss results of studies that show; (i) gender differences in human brain disease are most likely to be explained by gender differences in brain development and ageing; (ii) sex steroids have a significant effect on the brain; (iii) sex steroids are crucial to the development and ageing of brain regions affected in age-related brain diseases (for example AD); (iv) sex steroids interact with neuronal networks and chemical systems at many different levels; (v) sex steroids affect cognitive function in elderly women. Thus, the current literature supports the hypothesis that sex steroids can modulate brain ageing, and this provides a neurobiological explanation for the significantly higher prevalence of AD in females who do not take HRT, and may lead to new treatment approaches for age-related brain disease including AD.
Keywords: ageing, Alzheimer's disease, cognitive function, oestrogen
Introduction
There are significant age and sex differences in cognitive ability and brain disease. For example, women generally perform better than men in verbal fluency tests, whereas men perform better on tests of spatial ability. Similarly women suffer more often from depression and Alzheimer's disease (AD), whereas autism is seen more often in men. The biological basis for these gender differences is unknown. However, these observations do suggest that oestrogen and/or the gene products of the X chromosome may play a role in brain development and ageing. In this review we will discuss studies investigating the effects of oestrogen on normal brain development and ageing and how they are relevant to Alzheimer's disease.
Gender differences in brain ageing
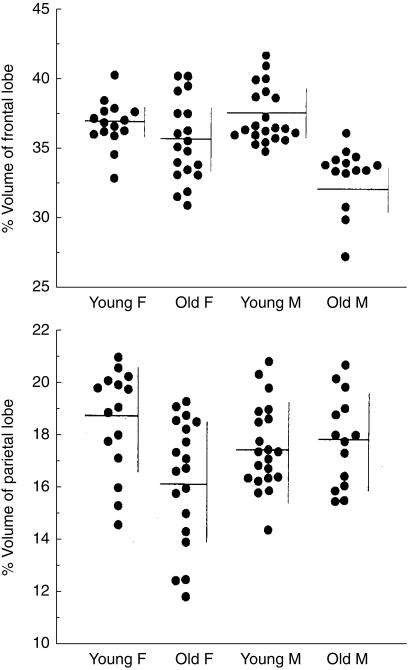
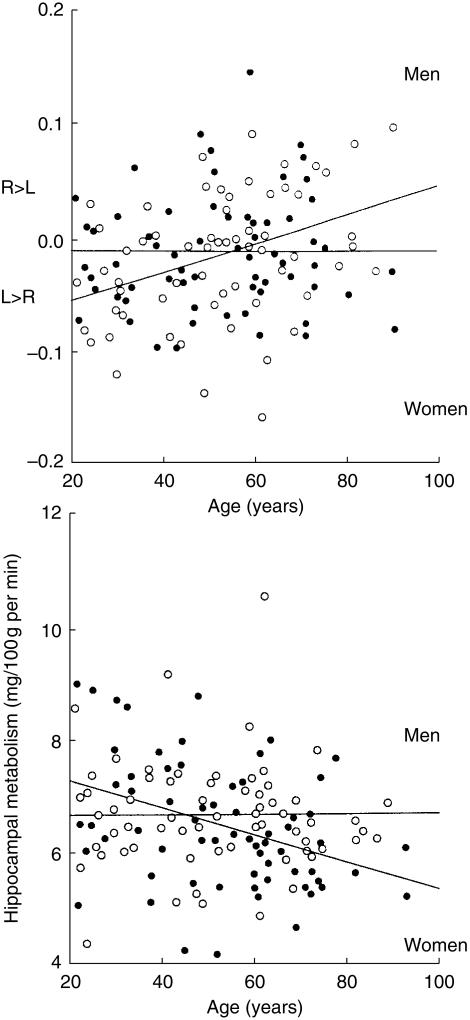
Ageing of the normal brain is accompanied by changes in brain structure, function, and metabolism; but there are significant gender differences in brain ageing. For example, brain atrophy starts earlier in men [1], however, once started, atrophy occurs more rapidly in women [2]. In addition, Murphy et al. [3] reported that age-related loss of brain tissue was significantly greater in males than females in whole brain, frontal, and temporal lobes, whereas the loss was greater in females than males in hippocampus and parietal lobes (Figure 1). A study measuring glucose metabolism and using positron emission tomography (PET) and 18F-2-fluoro-2-deoxy-d-glucose (FDG), showed that age-related decline in brain metabolism is asymmetrical in males, but symmetrical in females, and women have significant age-related decreases in hippocampal glucose metabolism, but men do not (Figure 2) [3]. These gender differences occur in regions that are essential to cognitive function and are implicated in neuropsychiatric disorder. They may therefore underlie gender differences in the prevalence and symptomatology of age-related neuropsychiatric disorders, such as AD. For example, women are more likely to develop AD than men, and this cannot be explained solely by their longer life expectancy as women also have a greater disease severity and a higher age-adjusted prevalence of AD than men.
Figure 1.
Effect on ageing on percentage volume of total frontal and parietal lobe brain matter in females (F) and males (M). (Reproduced with permission [50].)
Figure 2.
Effect of ageing on right-left asymmetry of temporal lobe (a) and absolute glucose metabolism of the hippocampus (b) as measured by positron emission tomography in male (open circles) and female (solid circles) subjects. (Reproduced with permission from Elsevier Science [49].)
Alzheimer's disease
The prevalence of AD increases dramatically with age – from less than 1% at age 65 years to about 15% of people in their eighties [4]. AD is accompanied by progressive cognitive impairment, and this has an enormous impact on the quality of life of patients and their caregivers. Risk factors for AD include a positive family history, presence of Down's syndrome, head injury, female sex, hypothyroidism, depression and the possession of the apolipoprotein E4 gene. In contrast, education, smoking, and nonsteroidal anti-inflammatory agents may be protective factors [5]. On a cellular level, the disease is characterized by neuronal loss, accumulation of intracellular neurofibrillary tangles, and extracellular senile plaques in hippocampus and association neocortex. Much progress has been made in understanding the aetiology and pathology of AD (including the identification of susceptibility genes). Nevertheless no major success has been gained so far in the treatment of AD.
The search for pharmacological treatments of AD has mainly focused on the major deficits in the cholinergic system; including selective loss of basal forebrain cholinergic neurones, a decreased activity of choline acetyltransferase (ChAT), an enzyme involved in the synthesis of acetylcholine, and a decreased activity of acetylcholinesterase (AChE), an enzyme involved in the breakdown of acetylcholine. Trials with precursors of acetylcholine and cholinesterase inhibitors have demonstrated only limited cognitive improvement, and many have significant unwanted side-effects. Also, the cognitive improvement seen in trials with cholinesterase inhibitors in AD was reported to be most evident in women who were also receiving hormone replacement therapy (HRT) [6]. Nevertheless, the first selective cholinesterase inhibitor, donepezil hydrochloride, has now been licensed in the UK as symptomatic treatment for mild to moderate AD. Meanwhile, the search for other possible treatment strategies continues, and more recently scientists have been challenged by the potential therapeutic effects of oestrogens. It is now becoming clear that oestrogens do more than regulate sexual and reproductive behaviour: in addition to their well known effects on bones and heart, oestrogens have significant effects on brain structure and function.
Oestrogen's actions on brain structure
The effects of sex hormones on prenatal, neonatal development and beyond has been succinctly reviewed by Fitch et al. [7]. In their paper they make some proposals which challenge conventional theories, for example, the traditional classification of hormonal effects on the brain into organizational and activational actions and the view that in the absence of androgens the brain is feminized ‘by default’.
Intracellular oestrogen receptors (ER) are widespread and are found in the cerebral cortex, midbrain, hippocampus, brain stem, hypothalamus and pituitary gland. The distribution of ERα is well established, being found in a high concentration in the hypothalamus, pituitary and amygdala. However there is controversy surrounding the localization of the ERβ because the results of the various laboratory techniques which measure the presence of ER indirectly are not consistent with each other [8].
Traditionally oestrogen was thought to exert its effect by binding to intracellular receptors that in turn lead to transcription and translation of proteins. Novel actions of oestrogen have been described, for example, inducing neuronal depolarization and oestrogen's antioxidant effects. Recently oestrogen has been shown to bind to cell membrane receptors, although they have not been well characterized in the brain, and use the same second messenger systems used by growth factors and neurotransmitters [8].
Oestrogens regulate synaptogenesis in the CA1 area of the hippocampus in that they increase synaptic and dendritic spine density in this area of the brain. The CA1 region of the hippocampus is crucial to memory function and spatial and declarative learning and is adversely affected in AD. Synapses are points of contact between axonal endings and tiny branches called dendritic spines on the adjacent neurones. It has been shown that in rats following bilateral oophorectomy there is a significant decrease in dendritic spine density in hippocampal CA1 pyramidal cells. However, this is prevented by administration of oestrogens, and synaptic spine density is significantly related to circulating oestradiol levels [9]. It has been shown that oestradiol levels are positively correlated with superior performance on behavioural memory tasks in rats [10].
Previously, it was unclear how these oestrogen-induced dendritic changes affected neuronal function. However, recently it has been demonstrated that oestrogen induces an increase in N-methyl-d-aspartate (NMDA) receptors in rat hippocampal neurones, in the same region where an increase in dendritic spines is found. This is of importance because the NMDA receptor is a membrane protein that detects incoming signals from the excitatory neurotransmitter glutamate. Thus, the ‘new’ oestrogen-induced spines are thought to be related to NMDA-type synapses [11, 12].
Oestrogens also directly affect neurochemical transmitter systems affected in normal ageing, AD, and other neuropsychiatric disorders. For example, oestrogens can modulate the serotonergic, cholinergic and dopaminergic systems [13–15]. These neurochemical effects of oestrogen may partially explain why depression occurs more often in women, and why AD and very late onset schizophrenia are more common in postmenopausal women, i.e. when levels of circulating oestrogens are low. The oestrogen-induced enhancement of the cholinergic system may be of particular relevance in AD because some of the cognitive impairments in AD are secondary to significant cholinergic deficits. Administration of oestrogens to ovariectomized rats increases the activity of ChAT in the basal forebrain, and in two of its projection areas – the CA1 region of the hippocampus and the frontal cortex. It is thought that the increased ChAT activity is caused by inducing de novo synthesis of the enzyme in the basal forebrain, with subsequent axonal transport to the CA1 region of hippocampus and the frontal cortex [16].
As the brain ages the density of receptors for several neurotransmitters diminishes. This is also seen after oestrogen withdrawal, for example, after oophrectomy and at the menopause and there is evidence that oestrogen replacement can prevent this occurring. The effect of selective oestrogen receptor modulators (SERMs) on brain receptor density has been studied. Cyr et al. compared the effect of 17-β oestradiol, tamoxifen and raloxifene replacement therapy on 5-hydroxytryptamine 2-A (5-HT2A) receptor density in rat brains. They found that after oophrectomy 5-HT2A receptor density was significantly lower in several regions of the rat brain involved with cognition, emotion, neuroendocrine control and motor function. Raloxifene was as effective as oestradiol in restoring receptor levels in the anterior cingulate, anterior frontal cortices, striatum and nuclueus accumbens and superior to tamoxifen. These results suggest that in the brain raloxifene has an agonist effect [17].
In addition to direct effects on neurones, oestrogens also work with neurotrophins (such as nerve growth factor) to stimulate indirectly nerve cell growth. Receptors for oestrogen and neurotrophins are located on the same neurones in rodent basal forebrain, hippocampus and cerebral cortex, and this colocalization may be important for the survival of neurones [18]. Oestrogen also has a neuroprotective action [10] against several toxins that boost production of free radicals; including glutamate (which is toxic in high concentrations) and β amyloid. Recently it has been shown that oestrogen can reduce the neuronal generation of β amyloid by enhancing the cleavage of Alzheimer amyloid precursor protein (Alzheimer APP) into soluble peptides [19, 20]. It has been proposed that 17β-oestradiol elevates protein kinase C activity, an effect possibly mediated by growth factors, and this increases the activity of α-secretase, one of the enzymes that cleaves Alzheimer APP thus prevents deposition of the intact amyloid peptide. It has been shown that oestrogen may also act as an antioxidant [21] and that the neuroprotective antioxidant activity of oestrogens is dependant on the presence of the hydroxyl group in the C3 position on the A ring of the steroid molecule [22]. Furthermore, Green et al. [23] found that the oestrogen molecule also needs a phenolic ring A and at least three rings of the steroid nucleus for its neuroprotective actions. Compared with women, whose serum oestradiol levels drop at the menopause, men have higher brain oestradiol levels compared with age-matched postmenopausal women. This occurs as the testes continue to produce testosterone through out life and testosterone is aromatized to estradiol in neuronal nuclei [24]. This mechanism may also contribute to explaining the gender difference in neuropsychiatric disease.
Oestrogen has been shown to suppress the hypothalamic-pituitary-adrenal axis response to emotional stressors in postmenopausal women [25]. This is of interest as high cortisol levels in the elderly have been associated with loss of hippocampal volume [26] and are implicated in AD. It is unclear how oestrogen affects cortisol levels in normal ageing, but it is known that oestrogen can prevent/reverse glucocorticosteroid-induced damage in the brain [27, 28].
Other indirect beneficial effects of oestrogens on the brain include enhancement of cerebral blood flow [29], and possibly, an interaction with apolipoprotein E4 a protein commonly found in AD [30].
Oestrogen's actions on cognitive function in healthy postmenopausal women
As noted above, sex steroids are crucial to the development and ageing of hippocampus and parietal lobe – brain areas significantly affected in AD [3, 7]. Moreover, animal studies have demonstrated significant effects of oestrogens on neuronal structures and neurochemical systems affected in AD. But what clinical evidence is there that sex steroids may have a significant role in the genesis and treatment of AD?
Several studies have looked at the effects of oestrogen on cognitive function in healthy postmenopausal women. The results of these studies have been conflicting which most likely reflects methodological differences but the majority show that oestrogen improves some memory functions and verbal memory in particular [31, 32]. Sherwin reported that women who had undergone a bilateral oophrectomy had a decrement in cognitive performance postoperatively which was related to a decrease in their plasma levels of circulating sex steroids, and subsequent administration of oestrogens improved scores on cognitive tasks [33]. This study may be confounded by the subjects experiencing symptoms of acute oestrogen deficiency, such as hot flushes, and this may have interfered with their cognitive function. Grodstein et al. conducted a large prospective study of over 2000 healthy elderly women and they found that there were no differences in overall cognitive scores of women who had used HRT compared with never users but that current users scored better than never users on verbal fluency tasks [34]. Jacobs et al. reported in a longitudinal study that a history of oestrogen use during the postmenopausal period was associated with higher scores on verbal memory, language and abstract reasoning [35]. Subsequently, over a 2 year follow-up period oestrogen users increased their scores on verbal memory tasks whereas nonusers showed a decrease in their scores. Similarly, the verbal memory scores of women increased slightly, a significant result, after being treated with raloxifene [36]. Carriers of the apolipoprotein E (APOE) gene are more susceptible to late onset AD. There are three common alleles (ε2, ε3 and ε4) that encode the three common isoforms and the risk for AD is greatest for ε4 heterozygotes and homozygotes. Yaffe et al. have found evidence that oestrogen replacement reduces cognitive decline to a greater extent in elderly women who are ε4-negative compared with ε4-positive women [37]. They have concluded that this supports their hypothesis of a gene–environment interaction. Others did not find any positive effects of oestrogen on cognitive function in a cross-sectional study [38].
Some studies have looked at the effect of oestrogen on nonverbal tasks. A recent longitudinal study reported that healthy, postmenopausal women on long-term HRT performed better than postmenopausal women who had never taken HRT on the Benton Visual Retention Test (a test for short-term visual memory, visual perception, and constructional skills) [39]. In a group of healthy postmenopausal women (mean age 65 years) receiving transdermal oestrogen for 3 weeks, Duka et al. reported improvements in verbal memory and in a task of mental rotation (a test of visuospatial ability) [40]. Further support for oestrogen's effects on nonverbal tasks, comes from a recent functional imaging study which demonstrates oestrogen-induced alterations in brain activation patterns during verbal and nonverbal working memory tasks in frontal and parietal regions in postmenopausal women [41]. Thus, there is recent evidence that oestrogen affects not only verbal but also nonverbal aspects of cognitive function in healthy postmenopausal women.
Studies on HRT and AD
Epidemiological studies have reported that the prevalence of AD is significantly decreased in females on HRT, and that those women with AD who were taking HRT had a milder disease than those who were not [42]. A recent longitudinal study reported that prolonged use of HRT decreases the risk, and delays the onset, of AD (relative risk = 0.40; 95% CI = 0.22, 0.85); moreover use of oestrogen for longer than 1 year reduced the risk of developing AD by 5% annually [43]. An important feature of these epidemiological studies is that the groups of women studied have not been exposed to prolonged periods of oestrogen deprivation as they have either been studied in the perimenopause or after a long time of oestrogen replacement.
Results of early clinical trials of HRT in people with AD were promising. For example, a clinical trial reported that three of seven women with AD improved on measures of attention, orientation, mood and social interaction after 6 weeks of low dosages of oestradiol treatment but with loss of improvement after treatment was discontinued [44]. In another study, women with AD who were using oestrogen had significantly better scores on the Alzheimer's Disease Assessment Scale (ADAS-Cog, a standard instrument used in AD clinical trials), than women with AD who did not take oestrogens [45]. The results of three recent randomised double-blind placebo-controlled trials have less optimistic results. In the trials 97, 50 and 40 subjects with mild to moderate AD took conjugated equine oestrogens for 12, 3 and 4 months, respectively. There were no beneficial effects on cognition, mood or functional outcomes in the patients [46–48]. Thus there is insufficient evidence that oestrogen benefits patients with established AD and its use as a sole agent for AD cannot be justified because of the risks associated with HRT, for example, breast cancer and thromboembolic disease.
Future studies may be able to determine if the route of administration of HRT or treating with combined HRT or androgens result in different therapeutic outcomes.
Conclusions
With the growth in size of the older population, it can be expected that AD will become an increasing public health problem. Women are particularly at risk and the role of sex steroids and its effects on the brain has been a major focus in AD research over the last years. There is evidence that sex steroids modulate brain development and ageing and can affect cognitive function. With regard to AD, epidemiological, neuropsychological, and biological studies appear to support the hypothesis that oestrogens may be implicated in its genesis and treatment. Oestrogens interact with neuronal networks at many different levels, and may affect some of the risk factors for AD. Currently, routine therapeutic use of oestrogens in women with AD is not justified but it may have a role in the prophylaxis of AD.
In the future new oestrogens may be synthesized which have the neuroprotective characteristics of the currently available oestrogens, but which do not carry the same side-effects (e.g. risk of breast cancer and thromboembolic disease). The recent finding that certain subtypes of oestrogens may be more ‘neuroprotective’ than others may help us identify such a compound [16, 17]. The effect of the SERMs on the central nervous system remains to be clarified. In men, the use of such compounds (without feminizing effects), or testosterone and its intraneuronal aromatization to oestradiol could be explored. In conclusion, besides the need for clinical trials in people with AD there is a continuing search for a more selective oestrogen preparation without the unwanted side-effects of current preparations.
References
- 1.Kaye JA, DeCarli CD, Luxenberg JS, et al. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed X-ray tomography. J Am Geriatric Soc. 1992;40:225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 2.Takeda S, Matsuzawa T. Age-related brain atrophy: a study with computed tomography. J Gerentol. 1985;40:159–163. doi: 10.1093/geronj/40.2.159. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DGM, DeCarli C, McIntosh A, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 4.Skoog I, Nilsson L, Palmertz B, et al. A population based study of senile dementia in 85 year olds. N Engl J Med. 1993;328:153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- 5.Burns A, Murphy D. Protection against Alzheimer's disease? Lancet. 1996;348:420–421. doi: 10.1016/S0140-6736(05)64533-3. [DOI] [PubMed] [Google Scholar]
- 6.Schneider LS, Farlow MR, Henderson VW, et al. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer's disease. Neurology. 1996;46:1580–1584. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- 7.Fitch H, Denenberg V. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sciences. 1998;21:311–352. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. The molecular and neuroanatomical basis for oestrogen effects in the central nervous system. J Clin Endocrinol Metab. 1999;84:1790–1797. doi: 10.1210/jcem.84.6.5761. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, Woolley CS, Frankfurt M, et al. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer's disease. Neurobiol Aging. 1994;15(Suppl. 2):S195–S197. doi: 10.1016/0197-4580(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 11.Gazzaley AH, Weiland NG, McEwen BS, et al. Differential regulations of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolley CS, Weiland NG, McEwen BS, et al. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Keane V, Dinan T. G Sex steroid effects on growth hormone response to pyridostigmine throughout the menstrual cycle. J Clin Endocrinol Metab. 1992;75:11–14. doi: 10.1210/jcem.75.1.1618997. [DOI] [PubMed] [Google Scholar]
- 14.O'Keane V, O'Hanlan M, Webb M, et al. d-Fenfluramine/prolactin response throughout the menstrual cycle: evidence for an oestrogen-induced alteration. Clin Endocrinol. 1991;34:289–292. doi: 10.1111/j.1365-2265.1991.tb03768.x. [DOI] [PubMed] [Google Scholar]
- 15.Wieck A, Hirst AD, Kumar R, et al. Growth hormone secretion by human females markedly affected by menstrual cycle phase. Br J Clin Pharmacol. 1989;27:700–701. [Google Scholar]
- 16.Luine V. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- 17.Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23:69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 18.Toran-Allerand C. The estrogen/neurotrophin connection during neural development: is co-localization of estrogen receptor with the neurotrophins and their receptors biologically relevant? Dev Neurosci. 1996;18:36. doi: 10.1159/000111393. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Guoras GK, Greenfield JP, et al. Oestrogen reduces neuronal generation of Alzheimer β amyloid peptides. Nature Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe A, Toran-Allerand D, Greengard P, et al. Estrogen regulates metabolism of Alzheimer amyloid β precursor protein. J Biol Chem. 1994;269:13065–13068. [PubMed] [Google Scholar]
- 21.Behl C, Widmann Trapp T, et al. 17-Beta oestradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Comm. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 22.Behl C, Skutella T, Lezoualc'H F, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 23.Green PS, Gordon K, Simpkins JW. Phenolic ring requirement for the neuroprotective effects of steroids. J Steroid Biochem Mol Biol. 1997;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 24.Beardsworth SA, Purdie DW, Kearney CE. Selective oestrogen receptor modulation: an alternative to conventional oestrogen. Curr Obstet Gynaecol. 1998;8:96–101. [Google Scholar]
- 25.Dayas C, Xu Y, Buller K, et al. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- 26.Lupien SJ, Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi K, Tatshuhide T, De-Hua C, et al. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry. 1990;27:937–952. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 29.Ohkura T, Isse K, Akazawa K, et al. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocrine J. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- 30.Honjo H, Tanaka K, Kashiwagi T, et al. Senile dementia-Alzheimer's type and estrogen. Horm Metab Res. 1995;27:204–207. doi: 10.1055/s-2007-979941. [DOI] [PubMed] [Google Scholar]
- 31.Robinson D, Friedman L, Marcus R, et al. Estrogen replacement therapy and memory in older women. J Am Geriatr Soc. 1994;42:919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 32.Kimura D. Estrogen replacement therapy may protect against intellectual decline in post menopausal women. Hormones Behav. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- 33.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 34.Grodstein F, Chen J, Pollen DA, et al. Postmenopausal hormone therapy and cognitive function in healthy older women. J Am Geriatr Soc. 2000;48:746–752. doi: 10.1111/j.1532-5415.2000.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 36.Nickelsen T, Lufkin E, Riggs L, et al. Raloxifene hydrochloride, a selective estrogen receptor modulator: saftey assessment of effects on cognitive function and mood in postmenopausal women. Psychoendocrinology. 1999;24:115–128. doi: 10.1016/s0306-4530(98)00041-9. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Haan M, Byers A, et al. Estrogen use, APOE, and cognitive decline: evidence of gene–enviroment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 38.Barret-Connor E, Silverstein D. Estrogen replacement therapy and cognitive function in older women. N Engl J Med. 1993;269:2637–2641. [PubMed] [Google Scholar]
- 39.Resnick SM, Metter EJ, Zondermann AB. Estrogen replacement therapy and longitudinal decline in visual memory: a possible protective effect? Neurology. 1997;49:1491. doi: 10.1212/wnl.49.6.1491. 97. [DOI] [PubMed] [Google Scholar]
- 40.Duka T, Tasker R, McGowan JF. The effects of estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- 41.Shaywitz E, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. N Engl J Med. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 42.Henderson V, Paganini-Hill A, Emanuel C, et al. Estrogen replacement therapy in older women. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 43.Tang M, Jacobs D, Stern Y, et al. Effects of oestrogen during menopause on risk and age at onset of Alzheimer’ disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 44.Fillit H, Weinreb H, Cholst I, et al. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer's type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- 45.Doraismay PM, Krishen A, Martin WL, et al. Gender, concurrent oestrogen use and cognition in Alzheimer's disease. Int J Geriatr Psychopharmacol. 1997;40:34–37. [Google Scholar]
- 46.Wang PN, Liao SQ, Liu RS, et al. Effects of estrogen on cognition, mood and cerebral blood flow in AD. A controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 47.Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer's disease in women: randomised, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 48.Mulard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer's disease: a 1-year randomised controlled trial. N Engl J Med. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 49.Murphy DG, DeCarli C, Daly E, et al. X-chromosome effects on female brain: a magnetic resonance imaging study of Turner’s syndrome. Lancet. 1993;342:1188–1189. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- 50.van Amelsvoort T, Murphy D. Development of the human brain, estrogen and the X chromosome. Contemp Rev Obstet Gynecol. 1998;10:281–287. [Google Scholar]